Abstract
1-Cys peroxiredoxin (1-cysPrx) is a novel antioxidant enzyme able to reduce phospholipid hydroperoxides in vitro by using glutathione as a reductant. This enzyme is widely expressed and is enriched in lungs. A fusion protein of green fluorescent protein with 1-cysPrx was stably expressed in a lung-derived cell line (NCI-H441) lacking endogenous enzyme. Overexpressing cells (C17 or C48) degraded H2O2 and t-butylhydroperoxide more rapidly and showed decreased sensitivity to oxidant stress as measured by 51Cr release. On exposure to •OH generated by Cu2+-ascorbate (Asc), overexpressing cells compared with H441 showed less increase in thiobarbituric acid-reactive substance and phosphatidylcholine hydroperoxide content. This effect was reversed by depletion of cellular glutathione. Diphenyl-1-pyrenoylphosphonium fluorescence, used as a real-time probe of membrane phospholipid peroxidation, increased immediately on exposure to Cu2+-Asc and was abolished by preincubation of cells with Trolox (a soluble vitamin E) or Tempol (a radical scavenger). The rate of diphenyl-1-pyrenoylphosphonium fluorescence increase with Cu2+-Asc exposure was markedly attenuated in C17 and C48 cells as compared with H441. Annexin V-Cy3 was used to detect phosphatidylserine translocation from the inner to outer leaflet of the plasma membrane. Cu2+-Asc treatment induced phosphatidylserine translocation within 2 h in H441 cells but none was observed in C48 cells up to 24 h. These results indicate that 1-cysPrx can scavenge peroxides but in addition can reduce peroxidized membrane phospholipids. Thus, the enzyme can protect cells against oxidant-induced plasma membrane damage, thereby playing an important role in cellular defense against oxidant stress.
Oxidant-mediated injury to cells likely involves peroxidation of cell membrane-associated phospholipids (1). Thus, the ability to reduce phospholipid hydroperoxides (PLOOH) may be of primary importance to protect cells against oxidative stress (2). Because PLOOH are not reduced by cytosolic glutathione (GSH) peroxidase (type I), the classical concept for repair of peroxidized membrane phospholipids is through excision of the peroxidized fatty acyl moiety by a phospholipase followed by reacylation of the residual lysophospholipid to restore membrane integrity (3). By contrast, a one-step reduction of PLOOH to a less toxic hydroxy derivative would be a more efficient mechanism for repair of membrane lipid peroxidation.
The peroxiredoxins comprise a recently described superfamily of non-seleno peroxidases (4), generally with two reactive cysteines for the redox center that in mammalian systems is reduced by interaction with thioredoxin (5). One member of this family, termed 1-cys peroxiredoxin (1-cysPrx), has a single redox-active cysteine (6). It can reduce a broad range of hydroperoxide substrates including PLOOH (7). Previous studies have demonstrated that overexpression of 1-cysPrx in NIH 3T3 cells enhances their ability to reduce H2O2 and protects their glutamine synthetase against H2O2-mediated oxidation (6). However, protection against cell membrane lipid peroxidation and cytotoxicity have not been evaluated.
In the present study, we used a •OH-generating system to initiate peroxidative cell membrane damage (8). We chose to study a lung cell line because the lungs are uniquely susceptible to oxidant injury and lungs are enriched in 1-cysPrx (9). We established stable cell lines overexpressing 1-cysPrx and demonstrated protection of cells against H2O2 and •OH-mediated oxidative injury.
Materials and Methods
Materials.
We purchased cell culture medium components from Life Technologies (Gaithersburg, MD), Fugene-6 transfection reagent from Roche Molecular Biochemicals, diphenyl-1-pyrenoylphosphine (DPPP) from Dojindo Molecular Technologies (Kumamoto, Japan), 15-lipoxgenase from Cayman Chemicals (Ann Arbor, MI), and annexin V-Cy3 from BioSource International (Camarillo, CA). All other reagents were purchased from Sigma. NCI-H441 cells were obtained from the American Type Culture Collection (ATCC no. HTB-174). N-coumarin-3-carboxylate-labeled phosphatidyldiethanolamine (PE-3CCA) was synthesized from bovine brain phosphatidylethanolamine as reported (10).
Expression of 1-CysPrx in NCI-H441 Cells.
We generated clonal cell lines that stably express a 1-cysPrx-enhanced green fluorescent protein (GFP) fusion protein by transfection of H441 cells. The coding region of rat 1-cysPrx (GenBank accession no. 035244) was amplified by PCR with full-length rat 1-cysPrx cDNA as a template (9). We introduced a XhoI restriction site into the forward primer (5′-CCCTCGAGCCATGCCCGGA GGGCTGCTTCT-3′) and a HindIII restriction site into the reverse primer (5′-CCAAGCTTTTAAGGCTGGGGCGTATAACG-3′). The purified cDNA product was digested with both XhoI and HindIII, ligated into the similarly digested pGFP-C1 vector (CLONTECH), and transformed into competent TOP10F′ Escherichia coli cells (Invitrogen). For transfection, the coding region of the rat 1-cysPrx cDNA was inserted in-frame into the pGFP-C1 vector to encode a fusion protein with GFP fused to the 1-cysPrx N terminus. H441 cells, transfected with the pGFP-C1–1-cysPrx plasmid (4 μg DNA) plus Fugene transfection reagent (6 μl) for 24 h, expressed a fusion protein as determined by fluorescence microscopy and immunoblot analysis (data not shown). To generate stable lines, cells were cultured for 3 weeks in complete medium containing 0.5 mg/ml gentamycin sulfate (Cellgro, Hamden, VA) that was changed every 3–4 days. Colonies with uniform GFP fluorescence were screened and two clonal cell lines (C17 and C48) with approximately similar levels of 1-cysPrx overexpression were chosen for further study.
Cell Culture.
Cells were grown to confluence in RPMI 1640 medium with addition of 10% FBS, 2 mM L-glutamine, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 0.5 mg/ml gentamycin sulfate in 60-mm Petri dishes at 37°C in an atmosphere of 5% CO2 in air.
Peroxidase Activity.
Peroxidase activity of cell sonicates was assayed by oxidation of NADPH in the presence of GSH, GSH reductase, and t-butylhydroperoxide (t-BOOH, 0.75 mM) (4). To evaluate cellular peroxidatic activity, intact cells (2.5 × 105 per well in 24-well culture dishes) were incubated with 5 mM H2O2 for varying times. Remaining H2O2 was determined with o-phenylenediamine (15 mM) in the presence of horseradish peroxidase (5 μg/ml) by absorbance at 490 nm using a Bio-Rad 3550 Microplate Reader (11).
Oxidative Stress.
Oxidative stress to cells was generated by incubation with H2O2, t-BOOH, or Cu2+ (10 μM)-ascorbate (0.2 mM) (Cu-Asc). Cu-Asc generates 0.3 μM •OH during a 100-s exposure (8). •OH interaction with plasma membrane was detected with PE-3CCA, a probe that is nonfluorescent under normal conditions but becomes highly fluorescent with emission maximum at 450 nm (excitation at 395 nm) after hydroxylation of the coumarin ring in position 7 (10). To incorporate the probe into plasma membrane, the cell monolayer was incubated with 10 μM PE-3CCA in PBS, pH 7.4, at 37°C for 45 min, and then cells were washed with saline to remove unbound probe.
For some experiments, intracellular GSH was depleted by 48-h incubation of cells with 10 μM L-buthionine-[S,R]sulfoximine (BSO) (12). Cellular GSH for assay was extracted from cells with ice-cold 5% metaphosphoric acid, derivatized by mixing with 0.1 mM 5,5′-dithiobis(2-nitrobenzoic acid) (1:1, vol/vol) for 1 h at 20°C. in the dark, and analyzed by HPLC (Waters) with a reverse-phase Nova-Pack 3.9 × 300-mm HR C18 (60 Å pore size and 6-μm particle size) column and Aliance 2690 separation unit. Samples were eluted isocratically (1 ml/min) with 23 mM NH4 formate (pH 5)/MeOH (9:1, vol/vol) (13). Eluate absorbance was measured at 412 nm with a PDA 996 UV-VIS detector and data were processed with MILLENIUM software (Waters).
Cell injury was determined by the release of 51Cr (14). To label cells, 51Cr (2 × 105 cpm per well) was added 24 h before the experiment. Cells were washed and incubated with H2O2 or t-BOOH. At intervals, aliquots of the supernatant medium were collected and radioactivity was determined in a Wallac 1470 gamma counter (Wallac, Gaithersburg, MD). At the conclusion of the experiment, total radioactivity remaining in the wells was determined after extraction with 1% Triton X-100 to calculate percent of total counts released.
Thiobarbituric Acid Reactive Substances (TBARS) and Phosphatidylcholine Hydroperoxide (PCOOH).
Lipids were extracted from cells and analyzed for TBARS as described (15) or redissolved in methanol for HPLC reverse-phase chromatography by isocratic elution with a mobile phase of methanol/50 mM NH4Ac, pH 5 (3:2, vol/vol) at 1 ml/min flow rate. Conjugated dienes were detected at 234 nm (extinction coefficient 23,000 M−1cm−1). 1-Palmitoyl-2-linolenoylhydroperoxide-sn-3-glycerophosphocholine was prepared as a standard by peroxidation of 1-palmitoyl-2-linolenoyl-sn-glycero-3-phosphocholine with 15-lipoxygenase (16).
Real-Time Detection of Lipid Peroxidation.
Lipid peroxidation in real time was determined by using DPPP, a fluorescent probe that localizes in plasma membrane (17). Cells grown to confluence on 12 × 25-mm plastic slides (Aclar, Allied Signal, Morristown, NJ) were incubated in the dark with 50 μM DPPP in PBS (pH 7.4) for 10 min at 20°C and washed. The slide with adherent DPPP-labeled cells was placed in a standard quartz cuvette (10 × 10 mm) with fresh PBS at a 45° angle to the excitation beam. Fluorescence at 380 nm (excitation, 350 nm) was measured continuously in a PTI (Photon Technology International, Lawrenceville, NJ) spectrofluorometer equipped with a single photon-counting system and sample holder temperature control with excitation/emission slits of 1 and 3 nm, respectively.
Annexin V Assay.
Annexin V-Cy3 was used to detect phosphatidylserine translocation (18) because the green fluorescence of transformed cells prevented use of FITC. Fluorescence microscopy used a Nikon Diaphot TMD microscope equipped with a charge-coupled device camera, optical filter changer (Lambda 10–2, Sutter Instruments, Novato, CA) and METAMORPH software (Universal Imaging, Downingtown, PA) or a Nikon FE300 microscope equipped with Bio-Rad Radiance 2000 confocal optics and LASERSHARP 2000 software.
Immunoblot Analysis.
Cells were washed, scraped into saline containing a protease inhibitor mixture, and homogenized by ultrasonic disruption. The homogenate was boiled for 2 min, analyzed by SDS/PAGE with 10% acrylamide, then electroblotted to Immobilon-P membranes. The membrane was blocked for 1 h in Tris-buffered saline containing 5% nonfat dry milk, and probed with a polyclonal rabbit 1-cysPrx antipeptide antibody against 196EEAKKLFPKGVFTKEL211 (produced by Strategic Biosolutions, Ramona, CA) at a dilution of 1:3,000 in blocking buffer with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000) as the secondary antibody. Analysis was by enhanced chemiluminescence (Amersham Pharmacia).
Statistical Analysis.
Results with repeated measurements are expressed as mean ± SEM. Statistical analysis was by one-way ANOVA (Sigma Stat, Jandel, San Rafael, CA) with statistical significance set at P < 0.05.
Results
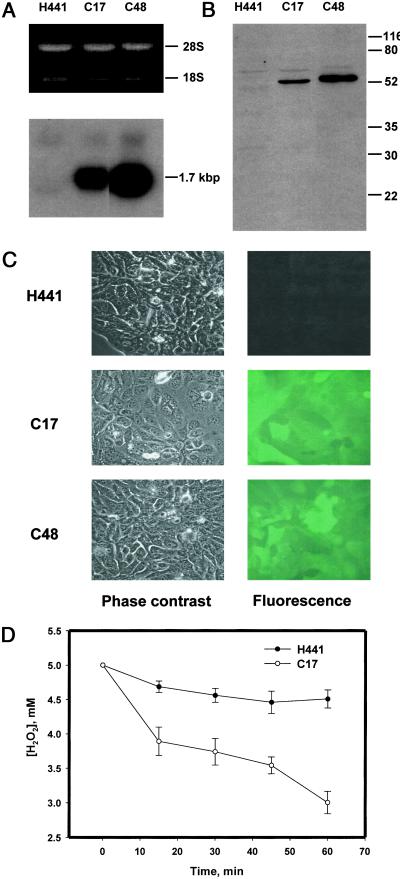
Northern analysis showed the presence of mRNA corresponding to the 1-cysPrx-GFP fusion construct in C17 and C48 cells and its absence in H441 (Fig. 1A). Immunoblot showed a band of immunoreactive protein from C17 and C48 cells at approximately 52 kDa, the predicted molecular mass of the 1-cysPrx-GFP fusion protein (Fig. 1B). Endogenous 1-cysPrx was not detected either in H441 cells or the clonal cell lines. Fluorescence microscopy showed green fluorescence in C17 and C48 cells but not in H441 (Fig. 1C). Peroxidase activity of sonicated cells with t-BOOH substrate was 24 nmol⋅min−1⋅mg−1 protein in H441 and increased to 51 and 43 nmol⋅min−1⋅mg−1 protein in C17 and C48, respectively. Intact C17 cells incubated with H2O2 in the presence of aminotriazole to inhibit endogenous catalase had markedly enhanced H2O2-degrading activity compared with H441 cells (Fig. 1D).
Figure 1.
Expression of a rat 1-cysPrx-GFP fusion product. (A) RNA gel (Upper) and Northern blot (Lower) of H441, C17, and C48 cells. (B) Western blot (25 μg total cellular protein) from H441, C17, and C48 cells. Relative migration of molecular mass markers (in kDa) is indicated on the right. (C) Fluorescence and corresponding phase-contrast images of H441, C17, and C48 cells. (×60.) (D) Time course for degradation of H2O2 (5 mM initial concentration) by 2.5 × 105 H441 or C17 cells in the presence of 50 mM aminotriazole. Data are mean ± SEM for n = 3. For comparison of H441 and C48. P < 0.05 at each time after zero.
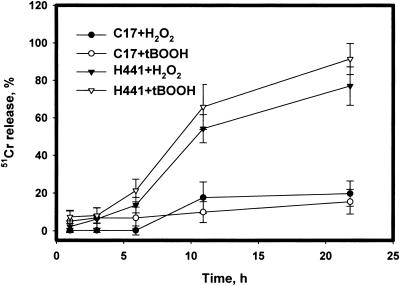
Damage to cells during a 22-h incubation with H2O2 or t-BOOH was evaluated through the release of 51Cr. All values were corrected for nonspecific leakage (<20% of total 51Cr) determined during control incubation. H441 cells released 71 ± 8.0% of their total 51Cr during 22-h treatment with 5 mM H2O2 whereas C17 cells released only 20 ± 6.7% (Fig. 2). Incubation with 0.75 mM t-BOOH gave similar results (Fig. 2). Because purified recombinant 1-cysPrx exhibits both peroxidase and phospholipase A2 enzymatic activities (19), we evaluated whether phospholipase A2 activity contributes to the protection of cells from peroxide-induced cellular injury. Neither MJ33 nor diisopropyl fluorophosphate, inhibitors of 1-cysPrx phospholipase A2 activity (19), increased the sensitivity of C17 cells to t-BOOH (data not shown).
Figure 2.
Cell injury during exposure of H441 or C17 cells to 5 mM H2O2 or 0.75 mM t-BOOH. Cells were preloaded with 51Cr and assayed at the indicated times for 51Cr release. Data are the mean ± SEM for n = 4. Comparing the same peroxide, differences for H441 and C17 are P < 0.05 at each time point beyond 3 h. Comparison of the two peroxides for the same cells are P > 0.05 at all time points.
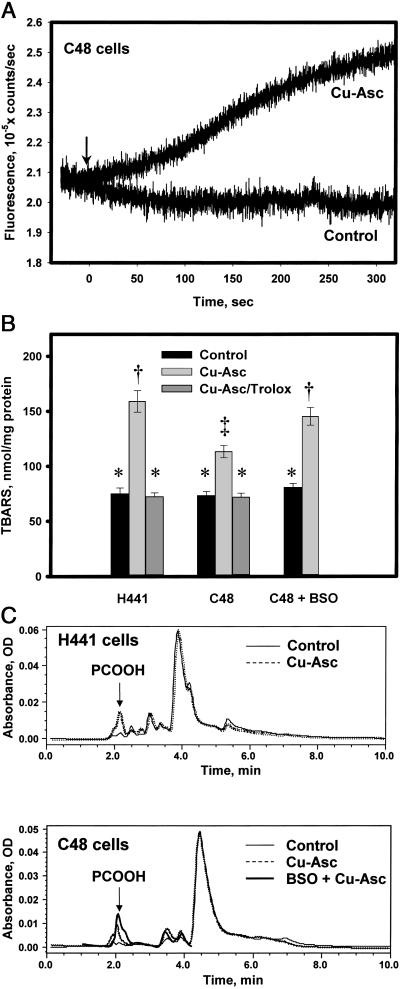
Cu-Asc treatment was used to generate •OH extracellularly. C48 cells prelabeled with PE-3CCA, a probe that intercalates with the plasma membrane and fluoresces when hydroxylated by •OH, demonstrated progressive increase in fluorescence beginning within seconds of exposure to Cu-Asc and continuing for 5 min (Fig. 3A). Qualitatively similar results were observed with H441 cells (not shown), which confirms that •OH interacts with the cellular plasma membrane phospholipids under these experimental conditions.
Figure 3.
Cellular lipid peroxidation with Cu-Asc. (A) Detection of •OH, generated by Cu-Asc, by a plasma membrane-localized probe (PE-3CCA) in C48 cells. A confluent monolayer of C48 cells on a plastic slide was labeled with the probe and fluorescence at 450 nm (excitation at 395 nm) was detected in real time. PE-3CCA fluorescence is shown with and without Cu-Asc addition (indicated by arrow). (B) Analysis of TBARS in lipid extracts of H441 or C48 cells with or without Cu-Asc exposure in the absence or presence of Trolox (50 μM) or C48 cells treated with BSO (10 μM) for 48 h before Cu-Asc exposure. Data are mean ± SEM for n = 3. Bars with different symbols (*, †, ‡) are significantly different (P < 0.05). (C) HPLC analysis for conjugated dienes (absorbance at 234 nm) in lipid extracts of H441 and C48 cells showing control (untreated) cells, Cu-Asc-treated cells (30 min at 37°C), and cells pretreated with BSO (48 h, 10 μM) before addition of Cu-Asc. The arrow indicates the retention time for the phosphatidylcholine hydroperoxide standard. The tracing for Cu-Asc plus BSO is terminated at 4 min because subsequent peaks seemed to be distorted by the BSO treatment.
TBARS were used as an index of cellular lipid peroxidation subsequent to •OH exposure. H441 and C48 cells showed a similar basal level of TBARS that increased 112% (P < 0.05) in H441 cells on exposure to Cu-Asc for 30 min (Fig. 3B). The increase was abolished by pretreatment with Trolox, a water-soluble vitamin E. TBARS increased only 55% (P < 0.05) in C48 cells after Cu-Asc treatment, a value significantly less than for H441 (P < 0.05), suggesting more rapid reduction of peroxidized cellular phospholipids in the 1-cysPrx-expressing cells. To investigate whether protection against lipid peroxidation depends on GSH, C48 cells were pretreated for 48 h with BSO, an inhibitor of γ-glutamylcysteine synthetase that decreased cellular GSH by 66% (control, 25.4 ± 3.0 nmol/mg protein; BSO-treated, 8.6 ± 1.2 nmol/mg protein; mean ± SEM; n = 3). BSO treatment reversed the protective effect of 1-cysPrx overexpression on cellular TBARS generation because of Cu-Asc treatment (P < 0.05) (Fig. 3B).
The cellular content of PCOOH after •OH exposure was analyzed by HPLC (Fig. 3C). PCOOH was not detected in control H441 or C48 cells. With Cu-Asc treatment of H441 cells for 30 min, a peak at 2.1 min was observed (Fig. 3C) that corresponded to the elution profile of standard PCOOH (not shown). Calculation of peak area indicated a PCOOH content of 220 pmol/mg protein in H441 cells compared with 125 pmol/mg protein for similarly exposed C48 cells. Pretreatment of H441 or C48 cells with Trolox before Cu-Asc exposure abolished the increase in PCOOH (not shown). C48 cells pretreated with BSO showed increased PCOOH with Cu-Asc treatment, significantly greater than for GSH-sufficient cells and similar to H441 cells (Fig. 3C). The major phospholipid peak eluting at 4–4.5 min was similar under all conditions.
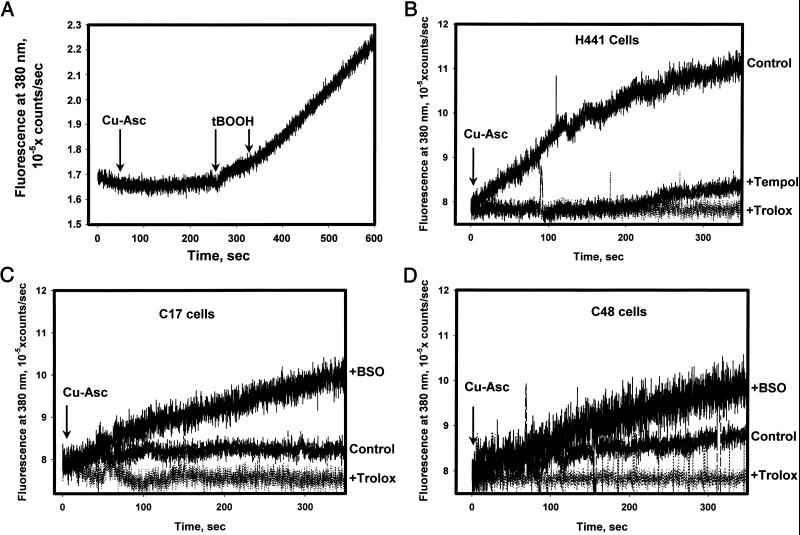
DPPP fluorescence was used for real-time detection of membrane lipid peroxidation. Fluorescence of this probe in vitro was not altered directly by •OH generated by Cu-Asc but increased significantly on exposure to t-BOOH (Fig. 4A). Thus, DPPP fluorescence seems to be specific for detection of hydroperoxides as reported (17). Cu-Asc treatment of DPPP-labeled H441 cells resulted in a progressive increase in fluorescence that was linear for approximately 200 s indicating •OH-mediated generation of hydroperoxides in the cell membrane (Fig. 4B). Pretreatment of cells with Trolox or Tempol, a nitroxide probe that scavenges lipid peroxyl radicals, abolished the •OH-mediated increase in DPPP fluorescence. With either C17 or C48 cells, the increase in DPPP fluorescence with Cu-Asc treatment was approximately 25% of that seen in H441 cells and was abolished by pretreatment of cells with Trolox (Fig. 4 C and D). The protective effect of 1-cysPrx overexpression was reversed in part by GSH depletion (Fig. 4 C and D).
Figure 4.
Fluorescence detection with DPPP of •OH-mediated lipid peroxidation in cellular plasma membrane. (A) DPPP oxidation in vitro. Fluorescence of DPPP (15 nmol/ml PBS in suspension) measured after addition of Cu-Asc (indicated by first arrow) or two additions of 40 μM t-BOOH (indicated by second and third arrows). (B) Real-time kinetics of oxidized DPPP fluorescence in H441 cells after Cu-Asc addition (indicated by arrow). Tracings indicate control (no pretreatment) or pretreatment with Trolox (50 μM) or Tempol (30 μM) for 30 min before Cu-Asc addition. (C and D) DPPP fluorescence in C17 (C) or C48 (D) cells after Cu-Asc addition under control conditions or in cells pretreated with Trolox for 30 min or with BSO for 48 h.
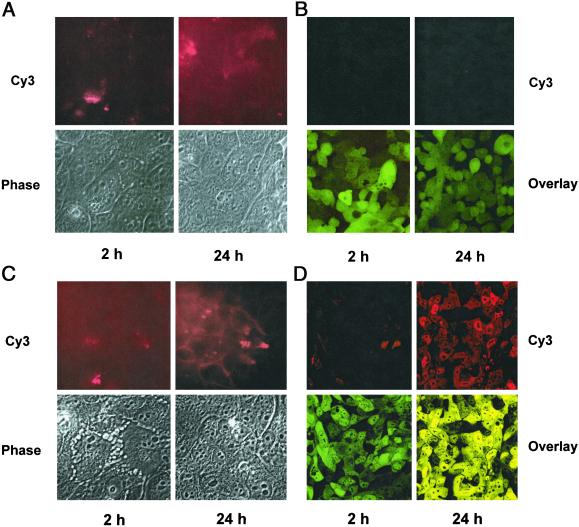
Annexin V binding was used as an index of plasma membrane perturbation caused by phospholipid peroxidation. H441 cells showed low levels of annexin V-Cy3 binding at 2 h after exposure to •OH and a marked increase at 24 h (Fig. 5A). Similar results were obtained with an annexin V-FITC probe (data not shown). By contrast, essentially no annexin V binding by C48 cells occurred at 24 h after Cu-Asc (Fig. 5B). Increased propidium iodide staining was not observed under any condition (not shown). Thus, C48 cells are relatively resistant to •OH-induced membrane bilayer perturbation as indicated by annexin V binding. To evaluate whether these results were specific for oxidative stress, the response to tumor necrosis factor-α (TNF-α) was determined. With this treatment, increased annexin V binding was observed at 2 h with both H441 and C48 cells and increased significantly at 24 h (Fig. 5 C and D). Thus, C48 and H441 cells had similar potential to respond to a cytokine-induced stress and the relative resistance of C48 cells seems to be specific for the oxidant-mediated membrane lipid peroxidation pathway.
Figure 5.
Annexin V-binding assay at 2 and 24 h in H441 and C48 cells after exposure to •OH or TNF-α (50 ng). (A) H441 and (B) C48 cells exposed to Cu-Asc. (C) H441 and (D) C48 cells exposed to TNF-α. (Upper) Annexin V-Cy3 fluorescence. (Lower) Phase contrast for H441 cells and overlap (red and green fluorescence) for C48 cells. (Magnifications: ×60.)
Discussion
1-CysPrx is a recently described member of the peroxiredoxin family that is expressed at relatively high levels in the lungs, eyes, and olfactory epithelium (3, 9, 20, 21). Overexpression of the protein in 3T3 cells has been shown to increase the ability of these cells to degrade H2O2 and protects them against H2O2-mediated inactivation of glutamine synthetase (6). However, protection by 1-cysPrx against H2O2 or hydroperoxide-mediated cytotoxicity has not been evaluated. In the present study, overexpression of 1-cysPrx augmented the H2O2-degrading activity of the cells and protected them from peroxide-mediated cell membrane damage as determined by 51Cr release. These results are compatible with a general peroxidatic role for this enzyme in antioxidant defense.
This study further evaluated the antioxidant role of 1-cysPrx by determining protection against plasma membrane phospholipid peroxidation. We confirmed delivery of Cu-Asc-generated •OH to plasma membrane phospholipids through the use of PE-3CCA and DPPP, two plasma-membrane-localized fluorescent probes. Evidence for membrane phospholipid peroxidation under these conditions is the •OH-mediated generation of TBARS and PCOOH and its inhibition by pretreatment with Trolox or Tempol. Compared with control cells, 1-cysPrx-overexpressing cells challenged with •OH showed less lipid peroxidation compatible with the increased reduction of PLOOH by the enzyme. Further evidence was obtained with DPPP, a membrane-localized probe that is oxidized during Cu-Asc treatment by hydroperoxides generated by the interaction of membrane phospholipids with •OH. A decreased rate of change in DPPP fluorescence in overexpressing cells exposed to •OH indicates more rapid reduction of peroxidized membrane phospholipids.
Previous studies in vitro with 1-cysPrx have provided conflicting results concerning the physiological reductant for this enzyme. Initial studies indicated that GSH could function as a reductant with 1-cysPrx isolated from bovine eye (20) or rat olfactory epithelium (21), although the latter result subsequently could not be reproduced (22). Studies with recombinant protein also have been inconclusive because GSH has been effective in some studies (7, 23) but not in others (6). All reports agree that thioredoxin, a physiological reductant for other members of the peroxiredoxin family, does not reduce 1-cysPrx (7, 24). Recently, cyclofilin A was shown to bind 1-cysPrx and was suggested to be the physiological reductant although no direct evidence was presented (25). In the present study, depletion of intracellular GSH by pretreatment of cells overexpressing 1-cysPrx with BSO markedly reduced their ability to prevent lipid peroxidation. Thus, these results with intact cells provide evidence that GSH can serve as a physiological reductant for 1-cysPrx.
Annexin V was used as an indicator of plasma membrane bilayer perturbation. This protein binds preferentially to negatively charged phospholipids, principally phosphatidylserine, on the outer surface of the cell (18). Under our experimental conditions, lipid peroxidation initiated by •OH in H441 resulted in plasma membrane perturbation without significant effect on its integrity as indicated by propidium iodide fluorescence. C48 cells overexpressing 1-cysPrx were protected against this membrane perturbation induced by Cu-Asc treatment. As a control, overexpression of 1-cysPrx had no effect on annexin V binding after treatment with TNF-α, indicating a similar response in both cell types and suggesting that the TNF-α effect is not a manifestation of membrane lipid peroxidation (26). 1-cysPrx presumably protects against oxidant-induced plasma membrane perturbation by reduction of oxidized cell membrane phospholipids.
H441 cells treated with high concentrations of H2O2 or t-BOOH showed evidence of cellular disruption as indicated by release of 51Cr. Cytotoxicity was inhibited by overexpression of 1-cysPrx. The mechanism for 1-cysPrx protection against H2O2 or t-BOOH-mediated damage may be primarily by reduction of these exogenous peroxides. This latter function is also provided by cytosolic GSH peroxidase which is generally present in cells at significantly greater concentrations than 1-cysPrx and under usual conditions should be responsible for the bulk of peroxidative activity (27). We postulate that the unique role of 1-cysPrx in oxidant stress is protection against plasma membrane bilayer perturbation and lipid peroxide-mediated radical generation by reduction of membrane-associated phospholipid hydroperoxides to lipid alcohol derivatives. Full restoration of membrane function may then require activity of an alcohol reductase or phospholipase.
Acknowledgments
We thank Drs. Sharon Sweitzer, Michael Koval, and Frank Branco for helpful discussions, Dr. Vladimir Bezuglov for help in synthesis of the PE-3CCA probe, Chandra Dodia, Kathy Notarfrancesco, Anu Thomas, and Kristine DeBolt for technical assistance, and Elaine Primerano for typing the manuscript. This research was supported by Grant HL64553 from the National Heart, Lung and Blood Institute. T.D.S. was supported by an Institutional National Research Service Award in Lung Cell and Molecular Biology (HL07748).
Abbreviations
- 1-cysPrx
1-cys peroxiredoxin
- PLOOH
phospholipid hydroperoxides
- GSH
glutathione
- t-BOOH
t-butylhydroperoxide
- DPPP
diphenyl-1-pyrenoylphosphine
- PE-3CCA
N-coumarin-3-carboxylate-labeled phosphatidyldiethanolamine
- GFP
green fluorescent protein
- Cu-Asc
Cu2+-ascorbate
- BSO
l-buthionine-[S,R]sulfoximine
- TBARS
thiobarbituric acid reactive substances
- PCOOH
phosphatidylcholine hydroperoxide
- TNF-α
tumor necrosis factor-α
References
- 1.Anzai K, Ogawa K, Gotto Y, Senzaki Y, Ozawa T, Yamamoto H. Antitoxid Redox Signal. 1999;1:339–347. doi: 10.1089/ars.1999.1.3-339. [DOI] [PubMed] [Google Scholar]
- 2.Wang H P, Qian S Y, Schafer F Q, Domann F E, Oberly L W, Buettner G R. Free Radical Biol Med. 2001;30:825–835. doi: 10.1016/s0891-5849(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 3.van Kuijk F J G M, Sevanian A, Handelman G J, Dratz E A. Trends Biochem Sci. 1987;12:31–34. [Google Scholar]
- 4.Knoops B, Clippe A, Bogard C, Arsalane K, Wattez R, Hermans C, Duconseille E, Falmagne P, Bernard A. J Biol Chem. 1999;274:30451–30458. doi: 10.1074/jbc.274.43.30451. [DOI] [PubMed] [Google Scholar]
- 5.Fujii T, Fujii J, Taniguchi N. Eur J Biochem. 2001;268:218–224. doi: 10.1046/j.1432-1033.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 6.Kang S W, Baines I C, Rhee S G. J Biol Chem. 1998;273:6303–6311. doi: 10.1074/jbc.273.11.6303. [DOI] [PubMed] [Google Scholar]
- 7.Fisher A B, Dodia C, Manevich Y, Chen J-W, Feinstein S I. J Biol Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 8.Manevich Y, Held K D, Biaglow J E. Radiat Res. 1997;148:580–591. [PubMed] [Google Scholar]
- 9.Kim T S, Dodia C, Chen X, Hennigan B B, Jain M, Feinstein S I, Fisher A B. Am J Physiol. 1998;274:L750–L761. doi: 10.1152/ajplung.1998.274.5.L750. [DOI] [PubMed] [Google Scholar]
- 10.Bezuglov V V, Biaglow J E, Manevich Y. Bioorg Chem. 1997;23:310–313. [Google Scholar]
- 11.Gow A J, Branco F, Christofidou-Solomidou M, Black-Schultz L, Albelda S M, Muzykantov V R. Am J Physiol. 1999;277:L271–L281. doi: 10.1152/ajplung.1999.277.2.L271. [DOI] [PubMed] [Google Scholar]
- 12.Piwien-Pilipuk G, Galigniana M D. Biochim Biophys Acta. 2000;1495:263–280. doi: 10.1016/s0167-4889(99)00166-4. [DOI] [PubMed] [Google Scholar]
- 13.Reeve J, Kuhlenkamp J. J Chromatogr. 1980;194:424–428. doi: 10.1016/s0021-9673(00)81435-1. [DOI] [PubMed] [Google Scholar]
- 14.Muzykantov V R, Sakharov D, Domagatsky S, Goncharov N, Danilov S. Am J Pathol. 1987;128:226–234. [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Mehdi A B, Dodia C, Jain M K, Fisher A B. Biochim Biophys Acta. 1993;1167:56–62. doi: 10.1016/0005-2760(93)90217-w. [DOI] [PubMed] [Google Scholar]
- 16.Maiorino M, Gregolin C, Ursini F. Methods Enzymol. 1990;186:448–458. doi: 10.1016/0076-6879(90)86139-m. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Shibata M, Niki E. Free Radical Biol Med. 2001;31:164–174. doi: 10.1016/s0891-5849(01)00575-5. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanian K, Bevers E M, Willems G M, Shroit A J. Biochemistry. 2001;40:8672–8676. doi: 10.1021/bi010841y. [DOI] [PubMed] [Google Scholar]
- 19.Chen J-W, Dodia C, Feinstein S I, Jain M K, Fisher A B. J Biol Chem. 2000;276:28421–28427. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- 20.Shichi H, Demar J C. Exp Eye Res. 1990;50:513–520. doi: 10.1016/0014-4835(90)90040-2. [DOI] [PubMed] [Google Scholar]
- 21.Peshenko I V, Novoselov V I, Evdokimov V A, Nikolaev Y-V, Shuvaeva T M, Lipkin V M, Fesenko E E. FEBS Lett. 1996;381:12–14. doi: 10.1016/0014-5793(96)00071-3. [DOI] [PubMed] [Google Scholar]
- 22.Peshenko I V, Novoselov V I, Evdokimov V A, Nikolaev Y V, Kamzalov S S, Shuvaeva T M, Lipkin V M, Fesenko E E. Free Radical Biol Med. 1998;25:654–659. doi: 10.1016/s0891-5849(98)00111-7. [DOI] [PubMed] [Google Scholar]
- 23.Singh A K, Shichi H. J Biol Chem. 1998;273:26171–26178. doi: 10.1074/jbc.273.40.26171. [DOI] [PubMed] [Google Scholar]
- 24.Rhee S G, Kang S W, Netto L E, Seo M S, Stadman E R. Biofactors. 1999;10:207–209. doi: 10.1002/biof.5520100218. [DOI] [PubMed] [Google Scholar]
- 25.Lee S P, Hwang Y S, Kim Y J, Kwon K-S, Kim H J, Kim K, Chae H Z. J Biol Chem. 2001;276:29826–29832. doi: 10.1074/jbc.M101822200. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Manzano V, Ishikawa Y, Lucio-Cazana J, Kitamura M. J Biol Chem. 2000;275:12684–12691. doi: 10.1074/jbc.275.17.12684. [DOI] [PubMed] [Google Scholar]
- 27.Cheng W H, Ho Y-S, Ross D A, Valentine B A, Combs G F, Lei X G. J Nutr. 1997;127:1445–1450. doi: 10.1093/jn/127.8.1445. [DOI] [PubMed] [Google Scholar]