Abstract
Gonocytes are a transient population of male germ-line stem cells that are derived from primordial germ cells in the embryo and give rise to spermatogonial stem cells, which establish and maintain spermatogenesis in the postnatal testis. In contrast to spermatogonial stem cells, gonocytes can be identified easily in neonatal rat testis cell suspensions based on their large size and distinct morphology. Furthermore, histological analysis of testes from neonatal transgenic rats demonstrated that gonocytes are the only cells that express a lacZ reporter transgene. Two gonocyte subpopulations, designated pseudopod and round, were identified and isolated from neonatal (0–4 days postpartum) rat testis cell suspensions. Male germ-line stem cells, identified by their ability to produce and maintain colonies of spermatogenesis upon transplantation into infertile recipient testes, were present almost exclusively in the pseudopod gonocyte subpopulation. In contrast, annexin V staining indicated that the majority of round gonocytes undergo apoptosis. These results indicate that a nearly pure population of male germ-line stem cells can be prospectively identified in neonatal rat testis cell suspensions by morphological criteria. Together, the pseudopod and round gonocyte populations will provide powerful tools for the study of cellular mechanisms that control cell fates and the establishment of spermatogenesis in the postnatal testis.
Spermatogenesis is initiated in the testes of postnatal mammals when quiescent gonocytes resume proliferation, migrate to the seminiferous tubule basement membrane, and give rise to stem cell spermatogonia. Maintenance of spermatogenesis and other self-renewing systems in postnatal animals depends on the activity of resident stem cells that have the capacity to both self-renew and produce progenitors that give rise to the specified cell lineage. Male germ-line stem cells are unique among self-renewing systems of postnatal animals because they can pass genes, through the germ line, to subsequent generations. Despite their critical position in mammalian physiology, there is a paucity of information regarding the molecular and biochemical characteristics of male germ-line stem cells. This results, in part, from the fact that these cells are extremely rare, comprising 1 in 3,333 cells of the adult mouse testis (1) and 1 in 500 cells of the adult rat testis (2, 3). Establishment of pure or significantly enriched populations of male germ-line stem cells is a critical first step that will facilitate biological and molecular analyses.
The development of a functional transplantation assay for hematopoietic stem cells over 40 years ago (4, 5) enabled investigators to identify biochemical markers (6), develop enrichment strategies, and eventually purify hematopoietic stem cells (7, 8). Spermatogenesis is the only other self-renewing system for which a stem cell functional assay is available, and male germ-line stem cells are defined by their ability to generate and maintain colonies of spermatogenesis upon transplantation into infertile recipient testes (9–11). Using this assay as a functional endpoint, we have developed methods for enriching spermatogonial stem cells from adult testis cell populations by taking advantage of physical binding properties (3, 12, 13), surgical manipulation (13), and immunoselection (14). In the best case, a 166-fold enrichment of spermatogonial stem cells was achieved by using fluorescence-activated cell sorting (FACS) to isolate a subpopulation of cells from adult mouse cryptorchid testes (14). This testis cell population, characterized as cryptorchid/side scatterlo (SSClo)/α6-integrinhi/αv-integrin(−), has a stem cell concentration of about 1 in 30 and provides a valuable resource for further characterization of spermatogonial stem cells. Continued development of enrichment strategies eventually will lead to the establishment of a pure population of spermatogonial stem cells.
During development, germ-line stem cells first can be identified as a distinct population of primordial germ cells (PGCs) that arises from the embryonic ectoderm (15). PGCs proliferate and migrate to the genital ridge where they associate with somatic cells of the presumptive gonad (16). In females, PGCs form oocytes, which stop dividing and enter meiosis. In males, the germ cells are enclosed in the sex cords, become gonocytes, and cease dividing until after birth (17). The differentiation of PGCs into gonocytes marks the transition from a cell with multiple potentials, because pluripotent embryonic germ cells can be derived from PGCs (18, 19), to one that has the restricted potential to develop the male germ cell lineage (17, 20). Therefore, gonocytes are the first stem cells committed to male germ-line development and the only germ cells in the neonatal testis. Shortly after birth, some gonocytes resume proliferation, migrate to the basement membrane of seminiferous tubules (21–25), presumably differentiate into spermatogonial stem cells, and initiate spermatogenesis (21, 22, 26, 27). However, gonocytes of the immature testis are a complex population from which only a portion are destined to become stem cells (17). A significant number of gonocytes (30–75%) degenerate (22, 23, 28, 29), whereas some appear to differentiate directly into type A1 spermatogonia (17). The molecular and biochemical characteristics that determine whether a gonocyte will become a stem cell, differentiate, or die are not known. Histological and in vitro studies demonstrate that gonocytes can be readily identified in immature testes and testis cell cultures based on distinct morphological characteristics (27), they can be isolated to homogeneity by micromanipulation (30, 31), and they can be maintained in culture (24, 25, 30–34).
We took advantage of the morphological characteristics of gonocytes, their purity as the only germ cells in the neonatal testis, and their unique expression of a transgene to identify two subpopulations of gonocytes (pseudopod and round) from dispersed neonatal rat testis cell suspensions. These two populations have distinct developmental potential, which is already established at the time of birth; pseudopod cells become stem cells and round cells undergo apoptosis. The availability of nearly pure subpopulations of gonocytes with specific destinies has enormous potential for identification of their biological and molecular characteristics.
Methods
Donor Rats and Cell Collection.
Donor testis cells were obtained from neonatal Sprague–Dawley rats of 0–4 days postpartum (dpp, day of birth is 0 dpp) carrying a fusion transgene composed of the mouse metallothionein I (MT) promoter and the Escherichia coli lacZ (lacZ) structural gene encoding a nuclear localized β-galactosidase protein (35, 36). The lacZ transgene is expressed in germ cells derived from MT-lacZ donor rats (36), facilitating the identification of donor-derived spermatogenesis after transplantation into recipient seminiferous tubules by staining with the β-galactosidase substrate, 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal, ref. 37). Testes of MT-lacZ neonatal rats were decapsulated and cell suspensions were prepared by digestion of seminiferous tubules in Hank's balanced salt solution containing trypsin (0.17%) and DNase I (2.3 mg/ml) for 5 min at 37°C followed by strong pipeting. Enzymatic reactions were stopped by addition of FBS (10% vol/vol) and cell aggregates were removed by straining through a 40-μm nylon mesh. Dispersed cells were suspended in DMEM containing 10% FBS and plated in 35-mm Petri dishes at a density (0.5 × 106 cells/dish) that allowed visualization of single cell characteristics.
Selection of Gonocytes by Micromanipulation.
Two subpopulations of gonocytes (pseudopod and round) were identified in dispersed cell suspensions from neonatal rat testes (0–4 dpp) by using a phase-contrast microscope, based on size (12–15 μm) and distinct morphological characteristics (low intracellular complexity, prominent round nuclei, and in some cases pseudopods) that have been described previously by others (25). Individual gonocytes with smooth round or pseudopod morphologies were selected with a micromanipulator and a glass capillary pipette (tip diameter ≈20 μm), during the first 1–2 hr after testis cell collection, and deposited into a second 35-mm Petri dish or on poly-l-lysine-coated slides for transplantation or staining, respectively. To assess β-galactosidase activity, unselected and selected populations were fixed in 0.5% glutaraldehyde for 5 min at 22°C, washed three times with PBS, and stained with X-Gal.
Annexin V Staining and Detection of Apoptosis.
Annexin V staining (Apoptosis Detection Kit, Annexin V-CY3, Sigma) was used to identify cells in early stages of apoptosis and exhibiting loss of plasma membrane asymmetry. Selected round or pseudopod gonocyte subpopulations (from 0 to 4 dpp rat testes) were placed in drops (50 μl) of PBS on poly-l-lysine-coated slides and allowed to adhere to the slide by incubating for 10 min at 22°C. Cells were then washed three times in binding buffer (10 mM Hepes, pH 7.5/140 mM NaCl/2.5 mM CaCl2) and double-stained with CY3-labeled annexin V (1 μg/ml) and 6-carboxyfluorescein diacetate (6-CFDA, 100 μM) in binding buffer for 10 min at 22°C. After the incubation, cells were washed five times with binding buffer and observed immediately with an epifluorescent microscope.
Recipient Mice and Transplantation Procedure.
Unselected donor testis cell populations (107 cells per ml) and selected gonocytes (from 0 to 4 dpp rat testes) were transplanted into immunodeficient NCr nude (nu/nu, Taconic Farms) recipient mice that were treated with busulfan (44 mg/kg, Sigma) at 4–6 weeks of age (36, 38). Busulfan treatment destroys the majority of endogenous germ cells and creates space for donor-derived spermatogenesis. Selected rat gonocytes (12–40 gonocytes/testis) were injected with carrier cells (107 cells per ml) obtained from the testes of Wv/W54 or W/Wv mutant mice that are incapable of producing spermatogenesis because of a mutation in the c-kit receptor tyrosine kinase (39). Approximately 10 μl of donor cell suspension was introduced through the efferent ducts of each recipient testis, about 6 weeks after busulfan treatment (40). Recipient mice were anesthetized by Avertin injection (640 mg/kg, i.p.).
Analysis of Recipient Testes.
Three months after transplantation, recipient mouse testes were collected and stained with X-Gal to visualize donor-derived spermatogenesis (37). Donor spermatogonial stem cells are defined by their ability to produce and maintain colonies of spermatogenesis in recipient testes, and each colony is thought to be clonally derived from a single spermatogonial stem cell (37, 41). Expression of the lacZ transgene allows unequivocal identification of donor stem cell-derived colonies because differentiated germ cells cannot produce and maintain colonies of spermatogenesis and endogenous germ cells do not express the transgene.
Statistical Analyses.
χ2 analyses were used to compare the relative frequencies, stem cell potential, and apoptotic activity of pseudopod and round gonocytes in neonatal rat testes (SPSS, Chicago). Pearson and Mantel–Haenszel χ2 analyses were used to test for linear trends in cell frequencies, stem cell potential, and apoptotic activity across 0, 1, 2, 3, and 4 dpp.
Results
Quantification and Morphological Characterization of Gonocytes in Neonatal Rat Testis Cell Suspensions.
Histological examination and staining of neonatal MT-lacZ rat testes demonstrated that lacZ expression was in large cells located in the seminiferous tubule lumen, but no staining was observed in other cells (Fig. 1). Classical morphological studies have established clearly that gonocytes are a distinct population of relatively large cells located in the seminiferous tubule lumen of newborn rats (27). Therefore, blue staining correlates well with gonocyte morphology, and this feature provided a rapid assay to identify gonocytes in neonatal rat testes. When cell suspensions were prepared from neonatal MT-lacZ rat testes and stained with X-Gal, 1.41% (571/40,428) were blue (Fig. 2, Table 1). Two distinct gonocyte morphologies, designated pseudopod (Fig. 2C) and round (Fig. 2D), were evident when stained testis cell suspensions were examined at high magnification, and these subpopulations were equally represented in the neonatal rat testis (P = 0.78). From 571 total gonocytes (blue cells) counted, 45.0% were classified as pseudopod (257/571) and 42.6% were classified as round (243/571). The remaining 12.4% of blue cells (71/571) could not be readily identified as pseudopod or round (other, Table 1). The relative numbers of pseudopod, round, and other gonocytes was consistent for all replicates analyzed from 0 to 4 dpp (P = 0.54).
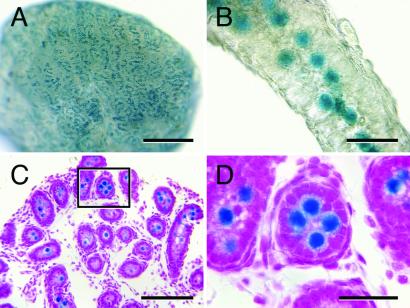
Figure 1.
MT-lacZ neonatal rat testis (2 dpp) stained with X-Gal to detect lacZ-encoded β-galactosidase activity. (A) Macroscopic image showing that lacZ expressing cells are evenly distributed in the neonatal rat testis. (B) Higher magnification image of an individual seminiferous tubule from the testis shown in A demonstrating that lacZ expressing cells are located in the center of the seminiferous tubule. (C) Histological analysis confirms that lacZ-expressing cells are located in the seminiferous tubule lumen, the characteristic location of gonocytes in neonatal testes. (D) Higher magnification of the seminiferous tubule boxed in C reveals the large size of gonocytes relative to other cells in the neonatal rat testis. Counterstain (C and D), hematoxylin and eosin. [Bars = 0.5 mm (A), 40 μm (B), 100 μm (C), and 30 μm (D).]
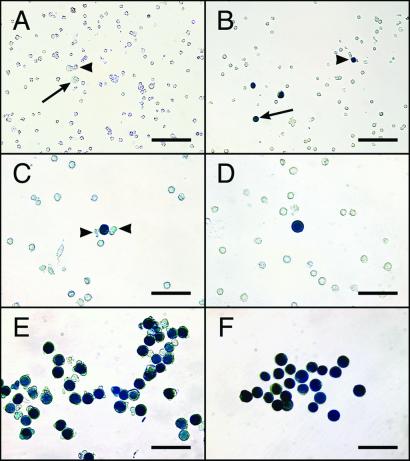
Figure 2.
Dispersed cell suspensions from MT-lacZ neonatal rat testes. (A) Unstained and unselected cell suspension. Pseudopod (arrowhead) and round (arrow) gonocytes can be recognized by their large size and distinct morphology. (B) Stained, unselected cell suspension. Pseudopod (arrowhead) and round (arrow) gonocytes express the lacZ transgene and stain blue in the presence of X-Gal. The majority of gonocytes (blue cells) in MT-lacZ neonatal rat testes can be categorized as pseudopod or round and these two phenotypes are equally represented in the gonocyte population (see Table 1). Higher magnification pictures of pseudopod (C) and round (D) gonocytes demonstrating large size and unique morphology. Distinct pseudopod extensions associated with a single gonocyte are indicated by arrowheads in C); note that because β-galactosidase activity is localized in the nucleus, pseudopod extensions do not stain blue. Homogeneous populations of pseudopod (E) and round (F) gonocytes were selected from unstained, live testis cell suspensions, placed in a second Petri dish, and stained with X-Gal to assess selection accuracy. [Bars = 200 μm (A and B) and 50 μm (C–F).]
Table 1.
Classification of gonocytes in neonatal MT-lacZ rat testis cell populations
| Blue cells*
|
Total cells counted | ||||
|---|---|---|---|---|---|
| Pseudopod | Round | Other | Total† | ||
| No. of cells‡ | 257 | 243 | 71 | 571 | 40,428 |
| Percent of total§ | 0.64 | 0.60 | 0.18 | 1.41 | |
Single cell suspensions were prepared from the testes of neonatal MT-lacZ transgenic rats (0–4 dpp) and stained with the β-galactosidase substrate, X-Gal. Blue cells (gonocytes) were morphologically classified as pseudopod, round, or other.
Sum of pseudopod, round, and other gonocytes.
Results are combined from six replicate experiments, encompassing 0–4 dpp.
Percent of total cells counted (40,428).
Isolation of Gonocyte Subpopulations from Neonatal Rat Testis Cell Suspensions.
To determine whether viable pseudopod and round gonocytes could be identified and isolated at high purity from unstained neonatal rat testis cell suspensions, individual cell populations were selected based on size and morphological characteristics, using phase-contrast microscopy and a micromanipulator. Relative to other cells in neonate rat testis cell suspensions, gonocytes can be recognized readily by their large size (12–15 μm), prominent nuclei, and low intracellular complexity (few organelles are revealed by light diffraction). In addition, round gonocytes have an unusually smooth surface, whereas pseudopod gonocytes have distinct cytoplasmic extensions (Fig. 2 C and D). Cells selected by using these characteristics were deposited on poly-l-lysine-coated slides, fixed, and stained with X-Gal to assess the accuracy of gonocyte selection. Blue staining indicates that nearly homogeneous populations of pseudopod (>99%) and round (≈90%) gonocytes can be isolated routinely from neonatal rat testis cell suspensions (Fig. 2 E and F). Occasionally (<1%), a gonocyte that appears round will develop a pseudopod after selection. The gonocytes, characterized as other, were morphologically heterogeneous and difficult to identify and select prospectively from unstained testis cell suspensions.
Transplantation and Functional Analysis of Stem Cell Activity in Selected Gonocyte Subpopulations.
In vitro and in vivo morphologic studies suggest that gonocytes in neonatal rat testes are a functionally heterogeneous population from which some will develop into spermatogonial stem cells (17), some will differentiate (17), and some will degenerate (22, 23, 28, 29). Results described in the present study indicate that newly isolated neonatal rat gonocytes are also phenotypically heterogeneous. To ascertain whether cell phenotype predicts function, homogeneous populations of pseudopod and round gonocytes were transplanted into nude mouse recipient testes to determine their spermatogonial stem cell activity. Recipient animals were analyzed 3 months after transplantation, and the results demonstrate that the majority of spermatogonial stem cell activity was found in the pseudopod subpopulation of gonocytes (Table 2, P < 0.01). Fifty of 1,001 transplanted pseudopod gonocytes (5%) produced and maintained colonies of spermatogenesis in recipient seminiferous tubules (Table 2 and Fig. 3). In contrast, round gonocytes produced only one colony of spermatogenesis from 579 transplanted cells (Table 2) and, as mentioned above, it is possible that this one colony resulted from the selection of a pseudopod gonocyte that was mistakenly identified as round. Transplantation results were consistent for all replicates analyzed from 0 to 4 dpp (P = 0.2).
Table 2.
Colonization of recipient testes after transplantation of pseudopod, round, or unselected neonatal rat testis cells
| Donor cells* | Testes analyzed | Cells transplanted | Colonies† | Percent‡ |
|---|---|---|---|---|
| Pseudopod | 48 | 1,001 | 50 | 5 |
| Round | 26 | 579 | 1 | 0.17 |
| Unselected | 58 | 6.4 × 106 | 769 | 0.012 |
Single cell suspensions were prepared from the testes of neonatal MT-lacZ transgenic rats (0–4 dpp), and pseudopod, round, or unselected cells were transplanted into immunodeficient nude mouse recipient testes.
Results are combined from 5–8 replicate experiments, encompassing 0–4 dpp.
Percent of cells in the transplanted rat donor population that produced and maintained colonies of spermatogenesis in nude mouse recipient testes.
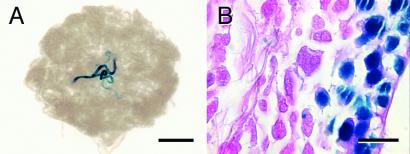
Figure 3.
Donor-derived spermatogenesis from pseudopod gonocytes selected from neonatal MT-lacZ rats and transplanted into nude mouse recipient testes. (A) Macroscopic picture of nude mouse recipient testis 3 months after transplantation showing a typical colony of donor-derived spermatogenesis. (B) Histological analysis demonstrates that donor pseudopod gonocytes produced normal colonies of spermatogenesis with multiple germ cell layers and spermatozoa in the tubular lumen. Blue staining in differentiated germ cells in the testes of MT-lacZ rats is variable and may reflect stage of the spermatogenic cycle. Counterstain: eosin (B). [Bars = 2 mm (A) and 20 μm (B).]
Analysis of Apoptotic Activity in Selected Gonocyte Subpopulations by Annexin V Staining.
Because the pseudopod phenotype indicates germ-line stem cell potential, we hypothesized that the round phenotype might represent the gonocyte subpopulation that is destined to undergo apoptosis and degenerate. To test this hypothesis, homogeneous populations of pseudopod or round gonocytes were stained with annexin V. Viable cells maintain an asymmetric arrangement of phospholipids in the plasma membrane such that phosphatidylserine (PS) normally is located on the inner leaflet, oriented toward the cell cytoplasm. Annexin V is one of a family of proteins that binds with high affinity to anionic phospholipids, such as PS, in the presence of calcium. One of the early cellular changes associated with apoptotic cell death is the loss of plasma membrane asymmetry without loss of membrane integrity (reviewed in refs. 42 and 43). Consequently, PS is exposed on the cell surface of apoptotic and necrotic cells and is available for binding by annexin V-CY3 that gives red fluorescence (42). Apoptosis can be distinguished from necrosis when the nonfluorescent compound 6-CFDA is hydrolyzed to produce the green fluorescent compound, 6-carboxyfluorescein (6-CF) in viable cells (44). Three results are possible with this double-staining procedure: (i) live cells stain only with 6-CFDA (green), (ii) necrotic cells stain only with annexin V-CY3 (red), and (iii) cells in the early stages of apoptosis stain with 6-CFDA (green) and annexin V-CY3 (red). The results in Fig. 4A demonstrate that few pseudopod gonocytes exhibit annexin V staining (Upper Center), whereas the majority of round gonocytes displayed red fluorescence (Lower Center). Green fluorescence demonstrated that most cells (>92%) in both subpopulations were alive (Fig. 4A Right), although round gonocytes contained significantly more necrotic cells (red only) than pseudopod gonocytes (7.9% vs. 1.0%, P < 0.01, data not shown). Overlapping red and green in the majority of round gonocytes produced yellow fluorescence, demonstrating apoptotic activity (Fig. 4A Lower Right). Fig. 4B provides a quantitative analysis of the results shown in Fig. 4A; 2.2% of viable pseudopod gonocytes were undergoing apoptosis, compared with 66.7% of viable round gonocytes (P < 0.01). Necrotic cells were not included in this analysis. Although apoptosis staining in pseudopod cells was consistently low for all replicates analyzed (P = 0.9, 0–4 dpp), the apoptotic activity of round cells tended to increase from approximately 55% to 75% between 0 and 4 dpp (P < 0.01).
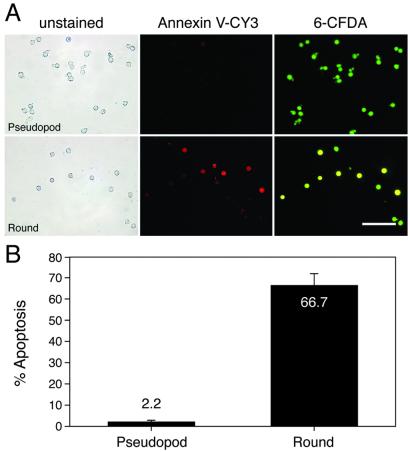
Figure 4.
Evaluation of apoptotic activity in selected pseudopod and round gonocyte subpopulations by annexin V staining. (A) Selected pseudopod (Upper) and round (Lower) gonocytes were double-stained with CY3-labeled annexin V (red) and 6-CFDA (green) to identify apoptotic and viable cells, respectively. Phase-contrast microscopic images show homogeneous populations of pseudopod (A Upper Left) and round (A Lower Left) gonocytes. Pseudopod gonocytes rarely exhibit annexin V staining (A Upper Center), but many round gonocytes bind annexin V (A Lower Center), indicating that they are dying. Staining with 6-CFDA demonstrates that the majority of selected pseudopod (99%, A Upper Right) and round (92% A Lower Right) are viable. Overlapping red and green produces yellow fluorescence in most round gonocytes (A Lower Right), demonstrating that the annexin V staining can be attributed to apoptosis (a regulated event) rather than necrosis. The fluorescence results (A Center and Right) were obtained by using an epifluorescent microscope and Leitz N2 (Center, red: excitation, 530–560 nm; emission, >580 nm) and H3 (Right, green + red: excitation, 420–490 nm; emission, >515 nm) filter cubes. (Bar = 100 μm.) (B) Quantitative analysis of annexin V staining results indicate that only 2.2 ± 0.9% of viable pseudopod gonocytes are undergoing apoptosis compared with 66.7 ± 5.8% of viable round gonocytes. Necrotic cells were excluded from this analysis. Values (means ± SEM) were from eight replicate experiments encompassing 0–4 dpp. An average of 42.6 ± 4.0 cells was evaluated for each replicate.
Discussion
Gonocytes represent a transient population of male germ-line stem cells that bridge the developmental gap between primordial germ cells in the embryo and spermatogonial stem cells in the postnatal testis. At the time of birth in rats, quiescent gonocytes appear uniformly round and located in the center of the seminiferous tubules (ref. 27, Fig. 1). Within a few days (≈2–5 dpp) important changes in the gonocyte population occur that are essential for the establishment of spermatogenesis in the postnatal testis. Histological observations demonstrate that some gonocytes develop cytoplasmic extensions, which may presage migration to the seminiferous tubule basement membrane (23–25) to a position important for establishing the germ line. This relocation event is critical because gonocytes that fail to relocate and remain centrally located in the seminiferous tubule eventually degenerate (21, 23). Whether gonocytes at birth are a homogeneous population, whose developmental fate will be determined subsequently by location in the seminiferous tubule at a critical time postpartum, or a heterogeneous population, in which cells are intrinsically programmed for migration or degeneration, cannot be determined histologically.
Gonocytes have unique characteristics (e.g., large size) that allow them to be identified in and isolated from heterogeneous testis cell populations (refs. 20, 32, and 45, current study). They can be maintained in gonocyte/Sertoli cell cocultures and can, in some measure, recapitulate their in vivo activities, including the development of cytoplasmic extensions and migratory activity (24, 32–34). However, these activities were not observed until after 1–3 days in culture (24, 32–34), and the extent to which results from these complex culture systems reflect gonocyte biology in vivo is not known. In the current study, the demonstration that gonocytes are the only cells in the MT-lacZ neonatal rat testis that express the lacZ transgene (Figs. 1 and 2) was critical to confirm the accuracy of gonocyte selection from dispersed testis cell suspensions. This feature allowed us unequivocally to identify and characterize gonocytes and provided assurance that large cells selected from unstained viable cell suspensions were gonocytes and not large somatic cells. These cells were isolated at a time (0–4 dpp) preceding and concurrent with the observed onset of gonocyte migration and degeneration, in vivo. Our studies revealed that gonocytes comprise 1.41% of freshly collected neonatal rat testis cell suspensions (Table 1), which is in excellent agreement with previous reports from van Dissel-Emiliani et al. (1%, ref. 20) and Li et al. (1–2%, ref. 45) and lower than the estimates of Orth and Boehm (32), who used older rat pups (3–6%, 6 dpp). Examination of isolated gonocytes during the first 1–2 hr after collection indicated that the majority (87.6%, 500/571, Table 1) could be assigned to one of two distinct morphological categories, pseudopod or round. It is possible that these two subpopulations are analogous to the migrating and degenerating gonocytes described by others (22–25, 30, 32–34). The relative number of migrating vs. degenerating gonocytes is difficult to assess in histological section, but in vitro studies found that whereas most gonocytes were round at the beginning of culture, 2.3–22% of gonocytes developed cytoplasmic extensions by 2–4 days (24, 32, 33). In contrast, our analysis of fresh neonatal rat testis cell suspensions indicates that pseudopod and round gonocytes are equally represented (45% vs. 42.6% of total gonocytes) and this relationship did not change during the first 4 days of postnatal life. The discrepancy in the proportion of pseudopod to round gonocytes between this and previous in vitro studies might be attributed to differences between fresh and cultured testis cells. For example, identification and selection, particularly of pseudopod gonocytes, becomes increasingly difficult after 1–2 hr in vitro. Furthermore, the presence of feeder cells and the culture environment in previous studies likely affected cell morphology. Finally, approximately 70% of rat pup male germ-line stem cells are lost during the first 12 hr of culture (46).
Using the spermatogonial transplantation system to evaluate germ-line stem cell activity, we found that stem cells reside almost entirely in the pseudopod gonocyte population. Approximately 5% of pseudopod gonocytes generated colonies of spermatogenesis after transplantation, suggesting that this population of cells is identical or very similar functionally to adult spermatogonial stem cells. Based on morphological estimates, there are approximately 35,000 stem cells in an adult mouse testis, which constitutes about 0.03% of total germ cells in the testis, or one stem cell in 3,333 total cells (1). When adult mouse testis cells are transplanted, about 19 colonies of spermatogenesis are generated per 106 cells transplanted, or one colony in 52,632 total cells (3, 37, 41). Therefore, about one colony of spermatogenesis is produced for every 16 stem cells (3,333/52,632), resulting in a transplantation efficiency of 6.3%. A similar efficiency was observed (8.5%) when adult rat testis cells were transplanted into nude mouse recipient testes (3). Thus, the production of spermatogenic colonies by 5% of transplanted pseudopod gonocytes in the present study suggests that these cells represent a nearly homogeneous population of male germ-line stem cells. Furthermore, to produce colonies of spermatogenesis in the transplantation assay, pseudopod gonocytes had to migrate from the lumen of the recipient seminiferous tubule to establish residence on the basement membrane. These results strongly suggest that pseudopod gonocytes represent the migrating population, described in histological studies, which are purported to give rise to stem cell spermatogonia in the postnatal testis (23).
Considering these results, if 0.64% of cells in the neonatal rat testis are stem cells (pseudopod gonocytes, Table 1) and 5% of these produce colonies in the transplantation assay, we would expect 0.032% (0.64% × 5%) of unselected neonatal rat testis cells to produce and maintain colonies of spermatogenesis upon transplantation. In fact, the number of stem cells predicted based on our gonocyte data are nearly 3-fold higher than actually observed when unselected neonatal rat testis cells were transplanted into nude mouse recipient tubules (0.032% vs. 0.012%, Table 2). Thus, the selected gonocyte population appears to colonize more efficiently than the unselected population. Perhaps the W mutant mouse carrier cells, which contain many mouse Sertoli cells, enhanced the colonizing efficiency of selected rat pseudopod gonocytes in nude mouse recipient seminiferous tubules. The colonization performance of pseudopod gonocytes and the absence of stem cell activity in round gonocytes suggest that the pseudopod gonocyte population can account for the entire male germ-line stem cell activity found in neonatal rat testes.
Apoptosis plays an essential role in all stages of male germ-line development and disrupting the balance of survival vs. death signals in genetic models results in spermatogenic dysfunction (47–49). Morphological studies revealed that between 30% and 75% of gonocytes are lost during the first few postnatal days (22, 23, 28), which can be attributed in large part to apoptotic cell death starting on 2 dpp in the rat (50). Our results are in agreement with these observations because annexin V staining demonstrated that the majority of round gonocytes are apoptotic (Fig. 4), consistent with their failure to produce colonies of spermatogenesis, and these cells comprise 42.6% of the total gonocyte population (243/571, Table 1). Our ability to detect apoptotic activity in round gonocytes starting on the day of birth, at least 2 days earlier than previous in situ observations (50), may be related to the use of annexin V staining, which reflects very early stages of apoptosis (42). Therefore, the round gonocytes identified in fresh neonatal rat testis cell suspensions probably represent the cells that fail to migrate, remain centrally located in the seminiferous tubule and degenerate in vivo. Interestingly, Wang et al. (29) found that in the early postnatal period in the mouse, a significant number of degenerating germ cells exhibited features of necrotic rather than apoptotic cell death. Although we observed more necrosis in the round than pseudopod population of testis cells, the majority of round gonocytes were alive (>92%, 6-CFDA staining) and exhibited early signs of apoptosis (annexin V staining, Fig. 4). These results may reflect a difference between the rat and mouse during the time when spermatogenesis is established in the postnatal testis.
The spermatogonial transplantation system provides an essential biological assay to retrospectively characterize stem cell activity in donor testis cell populations, although there is an inherent 2- to 3-month delay between transplantation and analysis. In the current study, we have demonstrated that phenotype predicts function in the heterogeneous gonocyte population of neonatal rat testes. Based on these results it is now possible to prospectively identify and isolate nearly homogeneous populations of male germ-line stem cells (pseudopod gonocytes) and cells destined for programmed cell death (round gonocytes) using morphological criteria. This progress was possible because (i) our analyses were restricted to testis cell isolates during the first 1–2 hr after donor cell collection, (ii) lacZ staining provided a rapid assay to evaluate strategies for gonocyte selection, and (iii) the spermatogonial transplantation system allowed definitive identification/confirmation of spermatogonial stem cell activity in selected gonocyte populations.
Although gonocyte migration and degeneration are not observed until after postnatal day 2 in the rat (23, 25, 28, 50), the present investigation suggests that developmental fate is already determined at the time of birth. This conclusion follows from the consistent relationship between the number and biological activity of pseudopod and round gonocytes from 0 to 4 dpp. It seems unlikely that significant numbers of cells changed morphology/function from pseudopod to round, or the reverse, because we rarely observed a round cell develop a pseudopod (<1%), round cells almost never made colonies, and the fraction of pseudopod cells that produced colonies (5%) remained constant. The disparate developmental destinies (stem cell vs. death) are undoubtedly orchestrated by divergent genetic programs. Thus, the pseudopod and round gonocyte cell populations will provide valuable tools for identifying molecular mechanisms controlling cell fate and the establishment of spermatogenesis in the postnatal testis. In addition, examination of these cell populations may provide general insight into stem cell biology and apoptotic processes that are critical for the development and maintenance of a variety of self-renewing systems.
Acknowledgments
We thank Drs. R. Behringer, H. Kubota, and E. Sandgren for critical evaluation of the manuscript and helpful comments. We appreciate the assistance of C. Freeman and R. Naroznowski with animal maintenance and experimentation, C. Brensinger for statistical assistance, and J. Hayden for help with photography. The MT-lacZ transgenic rat line was a gift from R. Hammer. Microscopic sections were produced in the Institute for Human Gene Therapy, Cellular Morphology Core, University of Pennsylvania (5-P30-DK-47747-07). Financial support for the research was from the National Institutes of Health Institute of Child Health and Human Development Grant 36504; the Commonwealth and General Assembly of Pennsylvania; and the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
Abbreviations
- 6-CFDA
6-carboxyfluorescein diacetate
- dpp
days postpartum
- MT
metallothionein I
- PGC
primordial germ cell
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Tegelenbosch R A J, de Rooij D G. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 2.Huckins C. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 3.Orwig K E, Shinohara T, Avarbock M R, Brinster R L. Biol Reprod. 2002;66:944–949. doi: 10.1095/biolreprod66.4.944. [DOI] [PubMed] [Google Scholar]
- 4.Till J E, McCulloch E A. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 5.Harrison D E. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 6.Spangrude G J. Immunol Today. 1989;10:344–350. doi: 10.1016/0167-5699(89)90192-8. [DOI] [PubMed] [Google Scholar]
- 7.Smith L G, Weissman I L, Heimfeld S. Proc Natl Acad Sci USA. 1991;88:2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 9.Brinster R L, Avarbock M R. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinster R L, Zimmermann J W. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinster R L. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinohara T, Avarbock M R, Brinster R L. Proc Natl Acad Sci USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara T, Avarbock M R, Brinster R L. Dev Biol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara T, Orwig K E, Avarbock M R, Brinster R L. Proc Natl Acad Sci USA. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsburg M, Snow M H, McLaren A. Development (Cambridge, UK) 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 16.Tam P P L, Snow M H L. J Embryol Exp Morph. 1981;64:133–147. [PubMed] [Google Scholar]
- 17.de Rooij D G. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnick J L, Bixler L S, Cheng L, Donovan P J. Nature (London) 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 19.Matsui Y, Zsebo K, Hogan B L. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 20.van Dissel-Emiliani F M F, de Rooij D G, Meistrich M L. J Reprod Fertil. 1989;86:759–766. doi: 10.1530/jrf.0.0860759. [DOI] [PubMed] [Google Scholar]
- 21.Clermont Y, Perey B. Am J Anat. 1957;100:241–260. doi: 10.1002/aja.1001000205. [DOI] [PubMed] [Google Scholar]
- 22.Beaumont H M, Mandl A M. J Embryol Exp Morph. 1963;11:715–740. [PubMed] [Google Scholar]
- 23.Roosen-Runge E C, Leik J. Am J Anat. 1968;122:275–299. doi: 10.1002/aja.1001220208. [DOI] [PubMed] [Google Scholar]
- 24.McGuinness M P, Orth J M. Eur J Cell Biol. 1992;59:196–210. [PubMed] [Google Scholar]
- 25.McGuinness M P, Orth J M. Anat Rec. 1992;233:527–537. doi: 10.1002/ar.1092330406. [DOI] [PubMed] [Google Scholar]
- 26.Sapsford C S. Aust J Zool. 1962;10:178–192. [Google Scholar]
- 27.Orth J M. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing L L, editors. New York: Oxford Univ. Press; 1993. pp. 3–42. [Google Scholar]
- 28.Huckins C, Clermont Y. Arch Anat Histol Embryol. 1968;51:341–354. [PubMed] [Google Scholar]
- 29.Wang R A, Nakane P K, Koji T. Biol Reprod. 1998;58:1250–1256. doi: 10.1095/biolreprod58.5.1250. [DOI] [PubMed] [Google Scholar]
- 30.Hasthorpe S, Barbic S, Farmer P J, Hutson J M. J Reprod Fertil. 1999;116:335–344. doi: 10.1530/jrf.0.1160335. [DOI] [PubMed] [Google Scholar]
- 31.Hasthorpe S, Barbic S, Farmer P J, Hutson J M. J Reprod Fertil. 2000;119:85–91. doi: 10.1530/jrf.0.1190085. [DOI] [PubMed] [Google Scholar]
- 32.Orth J M, Boehm R. Endocrinology. 1990;127:2812–2820. doi: 10.1210/endo-127-6-2812. [DOI] [PubMed] [Google Scholar]
- 33.van Dissel-Emiliani F M F, de Boer-Brouwer M, Spek E R, van der Donk J A, de Rooij D G. Cell Tissue Res. 1993;273:141–147. doi: 10.1007/BF00304621. [DOI] [PubMed] [Google Scholar]
- 34.Orth J M, Qiu J, Jester W F, Jr, Pilder S. Biol Reprod. 1997;57:676–683. doi: 10.1095/biolreprod57.3.676. [DOI] [PubMed] [Google Scholar]
- 35.Rhim J A, Sandgren E P, Degen J L, Palmiter R D, Brinster R L. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 36.Clouthier D E, Avarbock M R, Maika S D, Hammer R E, Brinster R L. Nature (London) 1996;381:418–421. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagano M, Avarbock M R, Brinster R L. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell L D, Brinster R L. J Androl. 1996;17:615–627. [PubMed] [Google Scholar]
- 39.Geissler E N, Ryan M A, Housman D E. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa T, Aréghaga J M, Avarbock M R, Brinster R L. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 41.Dobrinski I, Ogawa T, Avarbock M R, Brinster R L. Mol Reprod Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.van Engeland M, Nieland L J, Ramaekers F C, Schutte B, Reutelingsperger C P. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Bedner E, Li X, Gorczyca W, Melamed M R, Darzynkiewicz Z. Cytometry. 1999;35:181–195. doi: 10.1002/(sici)1097-0320(19990301)35:3<181::aid-cyto1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Rotman B, Papermaster B W. Proc Natl Acad Sci USA. 1966;55:134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Endocrinology. 1997;138:1289–1298. doi: 10.1210/endo.138.3.5021. [DOI] [PubMed] [Google Scholar]
- 46. Orwig, K. E., Avarbock, M. R. & Brinster, R. L. (2002) Biol. Reprod., in press. [DOI] [PubMed]
- 47.Knudson C M, Tung K S, Tourtellotte W G, Brown G A, Korsmeyer S J. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 48.Furuchi T, Masuko K, Nishimune Y, Obinata M, Matsui Y. Development (Cambridge, UK) 1996;122:1703–1709. doi: 10.1242/dev.122.6.1703. [DOI] [PubMed] [Google Scholar]
- 49.Rucker E B, III, Dierisseau P, Wagner K U, Garrett L, Wynshaw-Boris A, Flaws J A, Hennighausen L. Mol Endocrinol. 2000;14:1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- 50.Boulogne B, Olaso R, Levacher C, Durand P, Habert R. Int J Androl. 1999;22:356–365. doi: 10.1046/j.1365-2605.1999.00191.x. [DOI] [PubMed] [Google Scholar]