Abstract
The protective nature of memory immune responses is attributed largely to terminally differentiated memory T cells that retain memory of the antigen via the antigen receptor and memory of the effector functions that initially cleared the pathogen. It is not known whether a given population of antigen-specific memory T cells is endowed with functional flexibility to provide protective responses against antigens reencountered in different immunological contexts. Here, we examine functional properties of influenza hemagglutinin (HA)-specific memory CD4 T cells recovered from adoptive hosts that received in vitro-activated HA-specific T cell receptor-transgenic CD4 T cells 2 months to 1 year previously. We demonstrate that this HA-specific memory CD4 T cell population bearing a clonal T cell receptor can produce predominantly T helper 1 or T helper 2 effector cytokines depending on the nature of the recall stimulus. Our findings reveal remarkable functional plasticity within an antigen-specific memory T cell population and have direct implications for modulating memory T cell function in vaccine design and treatments for autoimmune diseases.
The immune response to pathogens previously encountered is more effective than the primary immune response because of an expanded population of antigen-specific “memory” T lymphocytes that efficiently elicit effector functions for antigen clearance. The protective nature of memory responses is attributed largely to terminally differentiated memory T cells that retain memory of the antigen via the antigen receptor and memory of the effector functions that initially cleared the pathogen (1). It is unknown, however, whether a given memory T cell population can exhibit functional flexibility to potentially provide protective responses against antigens that may be reencountered in an altered immunological context (2).
CD4 T lymphocytes orchestrate an immune response primarily through the types of effector cytokines they produce. After activation, naive CD4 T cells differentiate into T helper types I (Th1) or II (Th2) effector cells producing predominantly IFN-γ or IL-4 for initiation of inflammatory or humoral responses, respectively (3). A number of factors such as alterations in antigen dose (4), antigen affinity for the T cell receptor (TCR) (5), polarizing cytokines (1), costimulation (6), and entry into the cell cycle (7) all have been shown to affect generation of Th1 and Th2 effector cells from naive CD4 T cell precursors. By contrast, little is known concerning regulation of effector cytokine production from memory T cells. Although numerous studies have demonstrated that restimulation of memory CD4 or CD8 T cells with cognate antigen yields the same cytokine profile observed in the primary response (8–11), none of these previous studies explored the capacity of the antigen-specific memory T cell population to alter its cytokine profile.
Effector T cells generated by in vitro or in vivo activation have been shown to exhibit varying degrees of functional commitment and flexibility. Bulk populations of Th1 and Th2 effector cells generated in vitro under strongly polarizing conditions (exogenous cytokines) maintain their polarity of cytokine production when restimulated with cognate antigen or nonspecifically with phorbol 12-myristate 13-acetate/ionomycin (12, 13). However, both in vitro- and in vivo-activated effector Th1 and Th2 cells can switch their pattern of cytokine production when restimulated in conditions that drive the opposing polarity (14, 15). Irreversible commitment has been shown to occur only after repeated antigenic stimulation in vitro (15, 16) or as a result of chronic diseases such as allergy and long-term infections in vivo (17). Although it has been shown that memory T cells derive from activated/effector cell precursors (18), it is not known whether the resultant memory T cell population is irreversibly committed for effector cytokine production.
In this study, we asked whether a given population of antigen-specific memory T cells could modify their effector response. To address this question, we used an in vivo adoptive transfer system well characterized in this laboratory to generate influenza hemagglutinin (HA)-specific memory T cells bearing a TCR clonotype specific for a single HA peptide/MHC class II complex. We demonstrate that this HA-specific memory T cell population can alter its pattern of cytokine production in response to changes in recall antigen dose and TCR-mediated stimuli. Our results suggest that a proportion of memory CD4 T cells are not terminally differentiated in their ability to produce effector cytokines.
Materials and Methods
Mice.
BALB/c mice were obtained from the National Cancer Institute Biological Testing Branch, and HA-TCR-transgenic mice (19) were maintained as heterozygotes in the animal facility at the University of Maryland School of Medicine. RAG2−/− mice (20) on a BALB/c genetic background purchased from Taconic Farms were bred and maintained in the animal facility under sterile and pathogen-free conditions.
Antibodies and Reagents.
The following antibodies were purified from culture supernatants from hybridomas maintained in the laboratory: C363.29B (anti-CD3ɛ; ref. 21), GK1.5 (anti-CD4; ref. 22), anti-CD8 (TIB105, American Type Culture Collection), 212.A1 (anti-I-Ad), anti-Thy1 (TIB238, American Type Culture Collection), anti-Mac-1α (TIB128, American Type Culture Collection), and 6.5 (anticlonotype HA-TCR; ref. 19) conjugated to biotin (Pierce) according to manufacturer recommendations. The following monoclonal antibodies were purchased from BD PharMingen (San Diego): FITC- and phycoerythrin (PE)-conjugated anti-CD25 (clones 7D4 and PC61, respectively), purified anti-mouse Ly-6G (clone RB6–8C5), PE-conjugated anti-CD4 (clone GK1.5), purified anti-CD16/CD32 (clone 2.4G2), FITC- and PE-conjugated anti-IFN-γ (clone XMG1.2), PE- and allophycocyanin-conjugated anti-IL-4 (clone 11B11), PE- and allophycocyanin-conjugated anti-IL-2 (clone JES6-SH4), and FITC-, PE-, and allophycocyanin-conjugated streptavidin. The HA peptide 110–119 of the sequence, SFERFEIFPK, was synthesized by the Biopolymer Laboratory, University of Maryland.
Cell Purification.
CD4 cells (>90% pure) were isolated from HA-TCR spleen by using immunomagnetic depletion as described (23). Mitomycin C-treated antigen-presenting cells (APCs) were from BALB/c splenocytes by complement-mediated depletion of T cells as described (23).
In Vitro Generation of Effector Cells.
Effector CD4 T cells were generated from HA-TCR CD4 T cells (1 × 106 cells per ml) by incubating with 5 μg/ml HA peptide in the the presence of APC (3 × 106 cells per ml) in complete Clicks medium (Irvine Scientific) containing 5% FCS (Gemini Biological Products, Calabasas, CA), 50 units/ml penicillin/streptomycin (GIBCO/BRL), 2 mM glutamine (GIBCO), 10 mM Hepes (GIBCO), and 50 μM β-mercaptoethanol in 24-well plates for 3 days at 37°C in a 5% CO2 humidified atmosphere as described (23, 24). The resultant HA-TCR activated/effector CD4 T cells were >95% pure with no residual APC.
Proliferation and Cytokine Assays.
CD4 T cells (50,000 per well) and antigen-presenting cells (150,000 per well) were incubated with titrated amounts of HA peptide or anti-CD3ɛ antibody in flat-bottomed 96-well plates in complete Clicks medium. Proliferation was assessed after 72 h by the addition of 1 μCi (1 Ci = 37 GBq) of [3H]thymidine (6.7 Ci/mmol) per well and harvested after 18 h by using a Tomtec 96-well plate harvester (Wallac, Gaithersburg, MD). Radioactivity was quantitated by using a Microbeta Tri-luxe plate scintillation counter (Wallac). The level of IFN-γ and IL-4 in 48-h supernatants from duplicate cultures was measured by specific ELISA (Endogen, Cambridge, MA) as done previously (23, 24). ELISA results were analyzed by using MICROPLATE MANAGER software (Bio-Rad).
Adoptive Transfers and Cell Purification.
Equal numbers of purified effector CD4 T cells and resting HA-TCR CD4 T cells from naive HA-TCR mice (107 cells per 0.5 ml of PBS) were injected into the tail vein of RAG2−/− mice as done previously (24). Adoptive transfer recipient mice were killed 2–12 months posttransfer, and the resultant memory CD4 T cells were isolated by immunomagnetic depletion with anti-Mac-1α, anti-FcγR, anti-Ly6G, and anti-MHC II antibodies followed by anti-rat IgG-, anti-mouse IgG-, and anti-mouse IgM-coupled magnetic beads as described (24).
Intracellular Cytokine Staining (ICS).
Purified naive or memory HA-TCR CD4 T cells (106 cells per ml) were cultured with HA peptide or anti-CD3 antibody (5 μg/ml) and APC (3 × 106 cells per ml) in 1-ml total volume for 30–40 h at 37°C. Monensin (Golgi Stop, BD PharMingen) was added (4 μl/ml) for an additional 6 h of culture. Cells were harvested, centrifuged through Ficoll, washed with medium to remove dead cells and residual APC, and resuspended in 100–150-μl staining buffer (PBS/5% FCS/0.05% sodium azide) containing Fc-Block (CD16/32, PharMingen) followed by surface staining for 6.5 and CD25. For intracellular staining, cells were washed and resuspended in Cytoperm/Cytofix solution (BD PharMingen) for 20 min on ice and subsequently incubated on ice for 30–60 min with appropriate dilutions of anticytokine or isotype control antibodies in Permwash solution. Cells were washed in Permwash solution before flow-cytometric analysis with the FACScallibur equipped with two lasers for three-color analysis using FITC, PE, and allophycocyanin, and analyzed by using CELLQUEST software (BD, PharMingen).
Results
To address the question of functional flexibility of an antigen-specific memory population, we used an adoptive transfer system to generate long-lived memory T cells from TCR-transgenic CD4 T cells bearing a clonotypic TCR (6.5) specific for influenza HA and MHC class II I-Ed (HA-TCR; ref. 19). As we previously demonstrated, adoptive transfer of antigen-activated HA-TCR effector CD4 T cells into T cell- and B cell-deficient RAG2−/− mice yields substantial numbers of memory T cells that express the 6.5 TCR, are small in size, lose CD25 expression, and mediate potent recall responses (24). These HA-TCR memory T cells are similar to those generated in both sublethally irradiated and unmanipulated BALB/c mice (refs. 23 and 24, and data not shown), yet the use of RAG2−/− hosts has the advantage that all of the T cells recovered after time represent memory cells generated from the input activated/effector T cells.
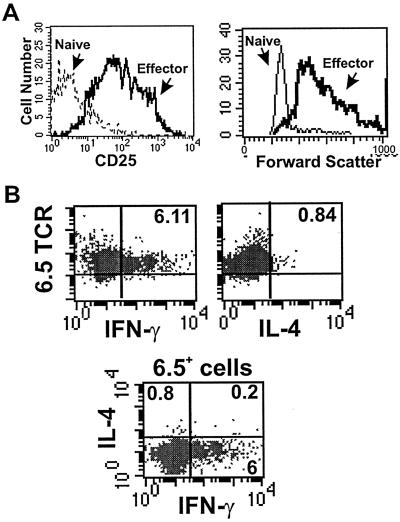
We initially assessed the cytokine profile and activation state of HA-TCR effector CD4 T cells before transfer into adoptive hosts. Effector cells were generated by activating HA-TCR CD4 T cells with HA peptide and splenic APCs for 3 days (23), in the absence of exogenous IL-2 or polarizing cytokines, by using peptide and APC doses previously determined to optimally stimulate HA-TCR CD4 T cells (23). The resultant effector T cells were >90% 6.5+, uniformly large in size, and exhibited up-regulation of CD25 (IL-2R) expression when compared with naive 6.5+ precursors (Fig. 1A; ref. 23). Functionally, these HA-TCR effector cells produced high levels of IFN-γ and low levels of IL-4 as assessed by ELISA (ref. 23 and data not shown). To examine effector cytokine production on the cellular level during effector generation, we used ICS to determine the proportion of activated 6.5+ cells producing IFN-γ and/or IL-4. Fig. 1B shows that during the 6-h assay, 6.11% of the activated 6.5+ T cells produced IFN-γ, 0.84% produced IL-4, and 93% did not produce effector cytokines. These data indicate that the majority of activated HA-TCR CD4 T cells had not fully differentiated to produce effector cytokines in levels sufficient to be detected by ICS. However, the 6.5+ cytokine-producing cells were predominantly Th1-like and produced IFN-γ with a low proportion of 6.5+ IL-4-producing Th2-like cells. A negligible fraction (<0.2%) of 6.5+ cells were Th0-like, producing both IFN-γ and IL-4 (Fig. 1B Lower, FACS plot), consistent with the lack of Th0 generation after antigenic stimulation of CD4 T cells derived from another TCR-transgenic strain (13).
Figure 1.
Phenotype and cytokine profile of HA-TCR effector CD4 T cells. (A) CD25 expression profile (Left) and size (forward scatter, Right) of 6.5+ HA-TCR naive and effector CD4 T cells. (B) Production of IFN-γ and IL-4 during generation of HA-TCR effector CD4 T cells. Intracellular cytokine analysis of IFN-γ (Upper Left) and IL-4 production (Upper Right) by 6.5+ HA-TCR effector CD4 T cells during their generation in vitro is shown. Monensin was added 2–3 days after activation of HA-TCR CD4 T cells with HA peptide and APC. IFN-γ versus IL-4 production is gated on 6.5+ cells (Lower).
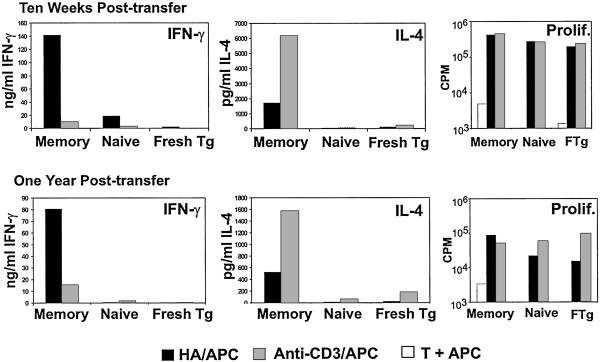
We transferred similarly activated HA-TCR effector CD4 T cells intravenously into RAG2−/− mice and recovered antigen-specific memory T cells at time points between 8 weeks and 1 year in vivo. We had determined previously that the resultant memory population produced high levels of IFN-γ and low levels of IL-4 in response to restimulation by antigen (23, 24). To assess whether the persisting HA-TCR memory CD4 T cells were fixed in their ability to produce high levels of IFN-γ, we compared the cytokine profile that resulted from activation with HA peptide antigen versus anti-CD3 antibody in the presence of APC (HA/APC versus anti-CD3/APC). Persisting 6.5+ memory T cells proliferated well to both HA/APC and anti-CD3/APC (ref. 24; see also Fig. 2) yet exhibited strikingly different patterns of effector cytokine production (Fig. 2). In response to HA/APC, HA-TCR memory T cells persisting 10 weeks to 1 year posttransfer produced predominantly IFN-γ with low levels of IL-4 (IFN-γ > IL-4, Fig. 2), similar to the pattern of cytokine production by the input HA-TCR effector cells. By contrast, in response to anti-CD3 stimulation, HA-TCR memory cells produced very low levels of IFN-γ yet substantial levels of IL-4 (IL-4 > IFN-γ, Fig. 2). As expected, CD4 T cells recovered from adoptive hosts that had received naive HA-TCR CD4 T cells 10 weeks or 1 year previously, and freshly isolated naive HA-TCR CD4 T cells proliferated but did not produce significant levels of IFN-γ or IL-4 in response to either antigen or antibody stimulation (Fig. 2). These results indicate that collectively, HA-TCR memory T cells exhibit a Th1-like pattern of cytokine production in response to antigen and a Th2-like pattern in response to anti-CD3 stimulation.
Figure 2.
Functional responses of differentially stimulated HA-TCR memory CD4 T cells. HA-TCR memory CD4 T cells were recovered 10 weeks and 1 year posttransfer of HA-TCR effector cells into RAG2−/− adoptive hosts. For controls, naive HA-TCR CD4 T cells were transferred into RAG2−/− hosts in parallel. Memory and naive CD4 T cells were restimulated with 1 μg/ml HA peptide (black bars) or 1 μg/ml anti-CD3 antibody (gray bars) in the presence of mitomycin C-treated APC. Freshly purified HA-TCR CD4 T cells (marked Fresh Tg or FTg) were cultured in parallel. IFN-γ and IL-4 content in 48-h culture supernatants was measured by specific ELISA, and proliferation was assessed by measuring [3H]thymidine incorporation after 72 h (see Materials and Methods). These results are representative of five different experiments.
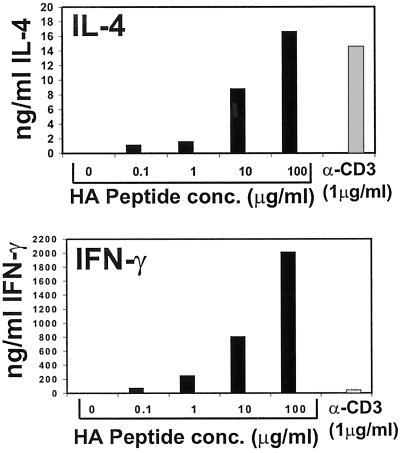
Because anti-CD3 antibody is a potent activator of T cells (25), the biased production of IL-4 by anti-CD3-stimulated memory T cells could be caused by increased activation strength. We asked whether activating HA-TCR memory CD4 T cells with increased antigen doses would result in a Th2-like pattern of cytokine production, analogous to findings demonstrating that stimulation of naive CD4 T cells with increased antigen dose or affinity favored Th2 effector generation (26, 27). When stimulated with the optimal dose of 1 μg/ml HA peptide (23, 24), HA-TCR memory cells produced high levels of IFN-γ (250 ng/ml) and low levels of IL-4 (<2 ng/ml) (Fig. 3); however, when stimulated with supranormal HA peptide concentrations (100 μg/ml), HA-TCR memory cells produced high levels of IL-4 comparable with amounts produced by anti-CD3 stimulation (17 ng/ml and 15 ng/ml, respectively) (Fig. 3 Upper). High antigen dose also led to greatly increased production of IFN-γ by HA-TCR memory cells (Fig. 3), whereas all doses of anti-CD3 antibody led to biased IL-4 production (data not shown). These data suggest that although memory T cells seem to have varying thresholds for cytokine synthesis, the biased IL-4 production revealed by anti-CD3-mediated stimulation is not caused merely by activation strength.
Figure 3.
Cytokine production from HA-TCR memory CD4 T cells in response to increasing antigen doses. Memory CD4 T cells were isolated from adoptive hosts 6 months posttransfer and activated with the indicated doses of HA peptide or 1 μg/ml anti-CD3 antibody in the presence of APC. IFN-γ and IL-4 content in culture supernatants was quantitated by specific ELISA as described for Fig. 2.
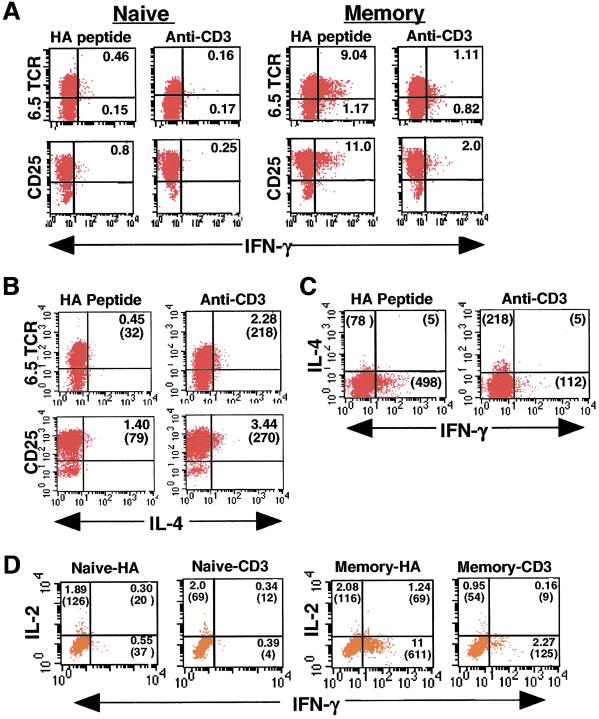
We therefore asked whether the change in cytokine production by the HA-TCR memory T cell population in response to anti-CD3 was caused by preferential stimulation of a persisting nonclonotype (6.5−) population or a reduced ability of anti-CD3 to activate memory T cells. We thus used ICS to analyze cytokine production on the cellular level in conjunction with TCR and activation markers. Representative ICS results are shown in Fig. 4, and a summary of ICS results from memory CD4 T cells isolated 2–5 months posttransfer is shown in Table 1. Because memory CD4 T cells require restimulation to elicit effector function (28, 29), we cultured HA-TCR memory T cells before adding a Golgi protein transport inhibitor for intracellular entrapment of cytokines (see Materials and Methods).
Figure 4.
ICS analysis of differentially stimulated naive and memory HA-TCR CD4 T cells. HA-TCR memory CD4 T cells isolated from adoptive hosts 3 months previously were reactivated with HA peptide (antigen) or anti-CD3 in the presence of APC followed by incubation with monensin, surface staining for 6.5 and CD25, and intracellular staining for IFN-γ, IL-4, and IL-2. (A) Production of IFN-γ from 6.5 TCR-expressing and CD25+ memory T cells gated on large, activated cells. Unactivated T cells showed no IFN-γ production, and quadrants are designated based on staining with isotype-matched control antibodies for each cytokine. Numbers in quadrants refer to the percentage of total activated cells. (B) Production of IL-4 from 6.5+ and CD25+ HA-TCR memory CD4 T cells. The numbers in parentheses refer to the absolute number of cells in each quadrant. Activation of naive HA-TCR CD4 T cells yielded negligible numbers of IL-4-producing cells (data not shown). (C) IL-4+ and IFN-γ+ memory T cells shown together. The numbers in parentheses indicates absolute cell number. (D) IL-2 and IFN-γ production from 6.5+ activated naive and memory CD4 T cells. The numbers in parentheses refer to the absolute numbers of 6.5+ T cells, and data shown are gated on 6.5+ CD4+ T cells.
Table 1.
Summary of ICS analysis of memory CD4 T cells isolated from RAG2−/− adoptive hosts that had received HA-TCR 6.5+ effector T cells 2–5 months previously
| Time posttransfer* | Stimulation† | % 6.5+IFN-γ+ | % 6.5+IL-4+ | Ratio IFN-γ+/IL-4+ |
|---|---|---|---|---|
| 3 months | Antigen | 25.02 | 3.32 | 7.5 |
| Anti-CD3 | 2.22 | 6.19 | 0.36 | |
| 5 months | Antigen | 9.04 | 0.45 | 20.1 |
| Anti-CD3 | 1.11 | 2.28 | 0.48 | |
| 2 months | Antigen | 16.55 | 2.9 | 5.7 |
| Anti-CD3 | 2.05 | 3.61 | 0.57 | |
| 3.5 months | Antigen | 9.85 | 1.00 | 9.85 |
| Anti-CD3 | 1.32 | 1.47 | 0.9 |
Results are from four separate experiments.
Stimulation was achieved by culturing 106 purified memory CD4 T cells with 5 μg/ml antigen or anti-CD3 antibodies in the presence of 3 × 106 mitomycin C-treated antigen-presenting cells as described for Fig. 4.
By ICS analysis, striking differences are seen in the proportion of activated 6.5+ memory CD4 T cells that produce IFN-γ or IL-4 in response to antigen versus anti-CD3 stimulation in the presence of APC (Fig. 4). Stimulation with HA peptide resulted in a much higher proportion of 6.5+ IFN-γ-producing memory T cells compared with anti-CD3 stimulation (9.04 versus 1.11%, Fig. 4A), with an average of 8.6-fold more 6.5+/IFN-γ+ cells generated by antigen compared with anti-CD3 stimulation (see Table 1). Naive 6.5+ T cells, as expected, produced negligible IFN-γ in response to either stimulus (Fig. 4A), as did unstimulated memory T cells cultured with APC alone (data not shown). To ensure that these differences in IFN-γ production were not caused by differences in the extent of activation, we analyzed the activated CD25+ population for IFN-γ production. As shown in the second row of Fig. 4A, both antigen and anti-CD3 activated the vast majority of HA-TCR naive and memory CD4 T cells as assessed by CD25 up-regulation. In response to HA peptide, 11% of these activated CD25+ memory cells produced IFN-γ+; however, in response to anti-CD3 stimulation, only 2% of CD25+ memory cells produced IFN-γ+ (Fig. 4A). As expected, CD25+ T cells generated by activation of naive CD4 T cells with either stimulus produced negligible IFN-γ+ (Fig. 4A).
We also observed differences in the proportion of 6.5+ IL-4-producing cells in response to antigen versus anti-CD3 stimulation. Although a low fraction of 6.5+ IL-4-producing cells were generated in response to antigen (0.45%, Fig. 4B), a greater proportion of 6.5+ IL-4+ cells (2.28%, Fig. 4B) were generated in response to anti-CD3 stimulation. This increased IL-4 production derived primarily from 6.5+ memory CD4 T cells, because very few nonclonotype 6.5−IL-4+ T cells were observed (Fig. 4B). As shown in Table 1, we consistently observed an increase (average of 2.4-fold) in the proportion of 6.5+/IL-4+ activated memory T cells produced by anti-CD3 compared with antigen stimulation. Similarly, we found an increase in CD25+/IL-4+ memory T cells generated in response to anti-CD3 stimulation (3.44% or 270 cells, Fig. 4B) versus HA stimulation (1.4% or 79 cells, Fig. 4B). Few dual-producing IFN-γ+/IL-4+ memory T cells are observed in response to either stimulus (Fig. 4C), consistent with enzyme-linked immunospot results demonstrating single cytokine production from polyclonal mouse memory CD4 T cells generated in vivo (30).
The ICS results shwon in Fig. 4 and Table 1 collectively demonstrate that HA-specific memory T cells can produce different patterns of effector cytokines depending on the recall stimulus: In response to antigen, the ratio of HA-specific IFN-γ versus IL-4-producing memory cells ranged from 5.7 to 20 (Table 1), indicating a Th1-like profile, and in response to anti-CD3 stimulation, the ratio of IFN-γ- to IL-4-producing memory T cells was always less than 1.0 (Table 1), indicating the predominance of IL-4-producing memory cells and a Th2-like profile.
Given the functional flexibility of the HA-TCR memory population, we asked whether “nonpolarized” memory T cells producing IL-2 (31) were present within this memory T cell pool. As shown in Fig. 4D, the proportion of IL-2-producing 6.5+ cells was low for both anti-CD3 and antigen-activated memory T cells (1–2%). Dual-producing IFN-γ+/IL-2+ 6.5+ memory T cells occurred in response to antigen at a low frequency (1.24%) but not in response to anti-CD3 stimulation (Fig. 4D). Activation of naive CD4 T cells resulted in similar proportions of 6.5+ IL-2+ in response to antigenic or anti-CD3-mediated stimulation (2% for both). These results show that IL-2 producers do not represent a large proportion of the HA-TCR memory T cell pool.
Discussion
Our results demonstrate that a memory CD4 T cell population derived from a common activated/effector T cell pool and bearing a clonotype TCR specific for influenza HA can produce either Th1 or Th2 effector cytokines in response to different recall stimuli. In this system, antigen activation led to the predominant production of IFN-γ, anti-CD3 activation led to the predominant production of IL-4, and high-dose antigen activation stimulated production of both IFN-γ and IL-4. Our results indicate remarkable heterogeneity and plasticity in cytokine production within a population of influenza-specific memory T cells and suggest that memory T cell function can be modulated in antipathogen immune responses and autoimmune diseases.
The overall pattern of effector cytokine production by antigen-activated 6.5+ HA-TCR memory T cells was similar to that of the input activated/effector cells from which they derived, with a higher proportion of IFN-γ producers than IL-4 producers. This result is consistent with results of others demonstrating that restimulation of memory T cells with cognate antigen leads to the same pattern of cytokine production as the precursor effector cells (8, 9). In response to anti-CD3 stimulation, known to differ qualitatively from antigen stimulation (21, 24, 32), there was a dramatic curtailment in IFN-γ production by activated memory cells, concomitant with an increase in IL-4-producing memory T cells. This biased IL-4 production by anti-CD3-stimulated memory T cells is consistent with findings by Bluestone and coworkers showing that anti-CD3 promotes increased IL-4 production by activated Th2 cells while it triggers inactivation or deletion of activated Th1 cells (33, 34). A therapeutic potential for using anti-CD3 for in vivo modulation of memory T cell function in diseases is suggested by the recent demonstration that anti-CD3 treatment of patients with autoimmune diabetes [known to involve Th1 effector/memory T cells (35)] lessened disease severity (36).
When stimulated with high doses of antigen, there was a dramatic increase in both IL-4 and IFN-γ production, suggesting heterogeneity in activation threshold in this clonotypic 6.5+ memory T cell population. Heterogeneity in cytokine production by polyclonal human memory T cells has been demonstrated in response to different antigen doses (37, 38), most likely because of memory cells expressing TCR with different antigen affinities. Our findings that high-dose antigen stimulation leads to increased IL-4 production from clonotypic memory T cells are reminiscent of findings that stimulation of TCR-transgenic naive CD4 T cells with supranormal antigen doses leads to increased IL-4 secretion (26), and suggests that the functional fate of resting memory T cells may be subject to similar influences of antigen dose and signal strength.
Our demonstration that the cytokine profile of HA-TCR memory T cells could be altered by the recall stimulus strongly suggests that a proportion of memory cells are uncommitted for cytokine production and therefore not terminally differentiated. Undifferentiated human and mouse memory phenotype cells that produce IL-2 have been identified and can be generated under suboptimal antigen stimulation conditions (31, 39, 40). Although we did not detect a significant fraction of HA-TCR memory cells solely producing IL-2 (Fig. 4D), it is likely that the majority of the input activated/effector cells had not fully differentiated to produce effector cytokines despite having acquired an activated cell-surface phenotype. Therefore, we hypothesize that the HA-TCR memory T cell population consists of cells in various stages of functional commitment depending on the differentiation state of the activated/effector T cell from which they derived. For example, the memory 6.5+ cells that produced IFN-γ in response to both antigen and anti-CD3 stimuli (1–2%, see Table 1) may derive from irreversibly committed effector cells, whereas the activated memory 6.5+ cells that produce IFN-γ in response to antigen but not anti-CD3 stimulation may derive from uncommitted activated cells. It will be necessary to sort activated T cells expressing different levels of effector cytokines before transfer to precisely determine the origin of these “uncommitted” memory T cells.
Mechanisms controlling memory T cell cytokine production are not known, although the molecular basis of Th1 and Th2 effector generation from naive T cells has been elucidated (1). The transcription factors T-bet and GATA-3 have been found necessary and sufficient for Th1 and Th2 generation, respectively (41, 42), and transcription of the genes encoding IFN-γ and IL-4 is marked by reconfiguring of chromatin structure in these gene loci (43). Assessing the maintenance or loss of these molecular changes during the activated/effector-to-memory transition in vivo is likely to provide insight into mechanisms for the functional commitment of memory T cells.
In summary, we have identified both functional heterogeneity and plasticity in a single antigen-specific memory CD4 T cell population. We and others have also identified memory T cell heterogeneity in tissue distribution, homing, and chemokine receptor expression that correlates with functional differences (24, 31, 44, 45). This memory cell heterogeneity on several levels suggests that the greater effectiveness of the anamnestic response may depend, in part, on the plasticity of the memory T cell population in its ability to home to multiple tissue sites and fine-tune functional responses. A greater understanding of memory T cell complexity can lead to potential therapies for manipulation of the memory immune response in vaccine development and autoimmune, infectious, and malignant diseases.
Acknowledgments
We thank Drs. Stephen Bartlett, Gregg Hadley, and Sandeep Krishnan from the Department of Surgery, University of Maryland, and Hyam Levitsky from the Department of Oncology, Johns Hopkins University School of Medicine (Baltimore), for critical reading of this manuscript. This research was supported by National Institutes of Health Grant AI42092 (to D.L.F.).
Abbreviations
- Th
T helper
- HA
hemagglutinin
- TCR
T cell receptor
- PE
phycoerythrin
- APC
antigen-presenting cell
- ICS
intracellular cytokine staining
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Murphy K M, Ouyang W, Farrar J D, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy T L. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 2.Gray D. Nat Immunol. 2000;1:11–12. doi: 10.1038/76862. [DOI] [PubMed] [Google Scholar]
- 3.Mosman T R, Coffman R L. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blander J M, Sant'Angelo D B, Bottomly K, Janeway C A., Jr J Exp Med. 2000;191:2065–2074. doi: 10.1084/jem.191.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C, Juedes A E, Temann U-A, Shresta S, Allison J P, Ruddle N H, Flavell R A. Nature (London) 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 7.Bird J J, Brown D R, Mullen A C, Moskowitz N H, Mahowald M A, Sider J R, Gajewski T F, Wang C-R, Reiner S L. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 8.Swain S L. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 9.Cerwenka A, Carter L L, Reome J B, Swain S L, Dutton R W. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 10.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 11.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 12.Tao X, Grant C, Constant S, Bottomly K. J Immunol. 1997;158:4237–4244. [PubMed] [Google Scholar]
- 13.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O'Garra A. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle A G, Buttigieg K, Groves P, Johnson B J, Kelso A. J Exp Med. 1999;190:1081–1092. doi: 10.1084/jem.190.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez V L, Lederer J A, Lichtman A H, Abbas A K. Int Immunol. 1995;7:869–875. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- 17.O'Garra A. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 18.Opferman J T, Ober B T, Ashton-Rickardt P G. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 19.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M, et al. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 21.Portoles P, Rojo J, Golby A, Bonneville M, Gromkowski S, Greenbaum L, Janeway C A, Jr, Murphy D B, Bottomly K. J Immunol. 1989;142:4169–4175. [PubMed] [Google Scholar]
- 22.Dialynas D P, Wilde D B, Marrack P, Pierres A, Wall K A, Havran W, Otten G, Loken M R, Pierres M, Kappler J, Fitch F W. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadzadeh M, Hussain S F, Farber D L. J Immunol. 1999;163:3053–3063. [PubMed] [Google Scholar]
- 24.Ahmadzadeh M, Hussain S F, Farber D L. J Immunol. 2001;166:926–935. doi: 10.4049/jimmunol.166.2.926. [DOI] [PubMed] [Google Scholar]
- 25.Samelson L E, O'Shea J J, Luong H, Ross P, Urdahl K B, Klausner R D, Bluestone J. J Immunol. 1987;139:2708–2714. [PubMed] [Google Scholar]
- 26.Hosken N A, Shibuya K, Heath A W, Murphy K M, O'Garra A. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constant S L, Bottomly K. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Dutton R W, Bradley L M, Swain S L. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed R, Gray D. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 30.Karulin A Y, Hesse M D, Tary-Lehmann M, Lehmann P V. J Immunol. 2000;164:1862–1872. doi: 10.4049/jimmunol.164.4.1862. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature (London) 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Farber D L, Luqman M, Acuto O, Bottomly K. Immunity. 1995;2:249–259. doi: 10.1016/1074-7613(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 33.Smith J A, Tang Q, Bluestone J A. J Immunol. 1998;160:4841–4849. [PubMed] [Google Scholar]
- 34.Smith J A, Bluestone J A. Curr Opin Immunol. 1997;9:648–654. doi: 10.1016/s0952-7915(97)80044-1. [DOI] [PubMed] [Google Scholar]
- 35.Katz J D, Benoist C, Mathis D. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 36.Herold K C, Hagopian W, Auger J A, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman S E, Harlan D M, Xu D, Zivin R A, Bluestone J A. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 37.Picker L J, Singh M K, Zdraveski Z, Treer J R, Waldrop S L, Bergstresser P R, Maino V C. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 38.Waldrop S L, Davis K A, Maino V C, Picker L J. J Immunol. 1998;161:5284–5295. [PubMed] [Google Scholar]
- 39.Wang X, Mosmann T. J Exp Med. 2001;194:1069–1080. doi: 10.1084/jem.194.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D H, Cohn L, Ray P, Bottomly K, Ray A. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 42.Szabo S J, Kim S T, Costa G L, Zhang X, Fathman C G, Glimcher L H. Cell. 2000;100:655–669. [Google Scholar]
- 43.Agarwal S, Rao A. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 44.Masopust D, Vezys V, Marzo A L, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 45.Farber D L, Ahmadzadeh M. Immunol Res. 2002;25:247–259. doi: 10.1385/IR:25:3:247. [DOI] [PubMed] [Google Scholar]