Abstract
Certain IFN regulatory factor (IRF) transcription factors indirectly influence T helper (Th) cell differentiation by regulating the production of IL-12. Here, we show that IRF4 directly regulates Th cell differentiation in vitro and in vivo during murine leishmaniasis. In the absence of IRF4, IL-12-induced Th1 cell differentiation was compromised, while IL-4 failed to induce Th2 cell differentiation. Instead, IL-4 tended to induce Th1 cells, defined by production of IFN-γ and TNF. Although early IL-4 signaling was normal in IRF4−/− Th cells, the protein GATA-3, a transcription factor critical for Th2 development, was not up-regulated following IL-4 treatment. Retroviral overexpression of GATA-3 rescued Th2 differentiation. Therefore, IRF4 deficiency manifests itself as severely dysregulated Th cell differentiation.
The IFN regulatory factor (IRF) family of transcription factors includes the IRF1–IRF7 proteins, IFN-stimulated gene factor 3 γ (ISGF3γ), and IFN consensus sequence-binding protein (ICSBP) (1). IRF proteins bind to IFN-stimulated regulatory elements (ISREs) found in the promoters of IFN-inducible genes. IRF1, IRF2, and ICSBP were shown to be mandatory for normal T helper (Th)-1 cell differentiation because of their ability to induce expression of the Th1 differentiation factor IL-12 (2–6).
In several of the above-cited studies (2, 5, 6), the well established model infection of mice with the protozoan parasite Leishmania major (7) was used for the analysis. In mouse strains resistant to this disease (e.g., C57BL/6), L. major-specific Th1 cells expand and control the infection. In susceptible mouse strains (e.g., BALB/c), the immune response eventually becomes dominated by Th2 cells, which are responsible for a fatal outcome. However, 1 week after infection, resistant and susceptible mice contain L. major-specific Th1 and Th2 cells (8), which allows for their simultaneous analysis within the same animal.
In the present study we apply the murine model of leishmaniasis to investigate the role of IRF4 in the differentiation of Th1 and Th2 cells. In gene-deficient mice, this factor has been demonstrated to be essential for the function of B cells and CD8+ T cells (9). Herein, we demonstrate that this factor is also irreplaceable for appropriate Th cell differentiation. In contrast to the respective effects of IRF1, IRF2, and ICSBP on Th cell development, IRF4 acts directly on Th cells and not via regulation of IL-12 production by APC.
Materials and Methods
Mice.
IRF4-deficient mice (9) were used at the seventh backcross generation to C57BL/6. Wild-type (WT) C57BL/6 mice were purchased from Charles River Breeding Laboratories, Sulzfeld, Germany. C57BL/6 mice transgenic for green fluorescent protein (GFP) (10) were kindly provided by T. Mosmann, Max Planck Institut, Freiburg, Germany. RAG-1-deficient mice (11) were purchased from Taconic Farms. All mice were used between 6 and 12 weeks of age.
Cell Purification and Transfer.
C57BL/6 B cells and IRF4+/− or IRF4−/− CD4+ cells, as well as GFP+CD4+ T cells, were purified by magnetic cell sorting using the MACS system (Miltenyi, Bergisch Gladbach, Germany), as described (2). The purity of these cells was always between 93 and 98%. GFP+CD4+ T cells (8 × 106) were transferred i.p. into IRF4-deficient mice (three mice per experiment). B cells (9 × 106) and IRF4+/− or IRF4−/− CD4+ cells (6 × 106) were transferred i.p. into RAG-1−/− mice (three to four mice per experimental group). Mice were infected with L. major promastigotes, as described below, and killed for the respective analysis either 4 (GFP+ cells) or 6 (RAG-1−/− mice) weeks later. All experiments were performed twice with similar results. For the analysis of Th cell differentiation in vitro, naive CD4+62L+ Th cells from IRF4+/+, IRF4+/− or IRF4−/− mice were purified using the multisort kit (Miltenyi), as described (6).
Leishmania Infection.
Mice (three to four per group) were infected in the right hind footpad with 2 × 107 stationary-phase promastigotes of L. major strain MHOM/IL/81/FEBNI and the increase in footpad thickness (%) was calculated, as described (2). Parasite burden was determined by limiting dilution analysis (2). The number of parasites per cell number plated was determined for each mouse. Popliteal LN cells or spleen cells were restimulated with the leishmania antigen preparation LmAg in vitro and the secreted cytokines were measured by ELISA, as described (2). In two experiments using GFP+ Th cells, the LmAg-specific cytokine production was tested by intracellular cytokine staining (ICS) using LmAg-pulsed dendritic cells as described (8).
Th Cell Differentiation in Vitro.
Purified naive CD4+62L+ Th cells from IRF4+/+, IRF4+/−, or IRF4−/− mice were stimulated in vitro with immobilized anti-CD3 Ab in the presence or absence of anti-IL-4 and IL-12 or anti-IL-12 plus IL-4, for 96 h, as described (6). Where indicated, anti-CD28 Ab 37.51 (PharMingen, 2.5 μg/ml) was also added. After a resting period of 48–72 h in the presence of IL-2 (6), cells were restimulated with anti-CD3 and cytokine production was tested by ELISA or ICS, as described (2).
Western Blot Analysis.
To measure GATA-3 protein, naive IRF4−/− and IRF4+/− Th cells and control cells of the B10BI (Th1) and L1/1 (Th2) clones (12) were primed with anti-CD3 in the presence and absence of IL-4, rested, and restimulated with anti-CD3 as described above. After 6 h, cells were harvested and processed for determination of GATA-3 by Western blotting (20 μg/ml protein lysate per lane for GATA-3), using anti-GATA-3 ab HG3–31 (Santa Cruz Biotechnology) as described (13). The blots were stripped and reprobed with anti-β-actin antibody as a loading control. To analyze STAT6 phosphorylation, naive IRF4−/− and IRF4+/− Th cells were primed with anti-CD3 and IL-4. Seventy-two hours later, the cells were washed, recultured overnight in IL-2, restimulated for 15 min with or without IL-4, and processed for determination of STAT6 phosphorylation by Western blot, as described (14). STAT6 was immunoprecipitated using anti-STAT6 M20 (1:100; Santa Cruz Biotechnology). Phosphorylated STAT6 was detected by the rabbit antiserum Tyr.641 (1:1,000; New England Biolabs) developed by POX conjugated donkey anti-rabbit antiserum (1:10,000; Dianova, Hamburg, Germany) and the ECL system (Amersham Pharmacia). The blots were stripped and reprobed with M20 antibody to determine total STAT6. All results shown are representative of two independent experiments with similar results.
Retroviral Overexpression of GATA-3.
Naive IRF4−/− (KO) and IRF4+/+ (WT) Th cells were stimulated with anti-CD3, as above. Forty-eight hours later, they were infected with retrovirus containing either bicistronic GATA-3 and GFP or GFP alone (provided by K. Murphy, Howard Hughes Medical Institute, St. Louis), as described (13). Twenty-four hours after infection, the cells were washed, rested and restimulated with anti-CD3, as above, and processed for ICS staining 6 h later.
Results and Discussion
Defective in Vivo Th Differentiation of IRF4−/− Cells During Leishmaniasis.
To test whether IRF4 was relevant for Th differentiation in vivo, IRF4-deficient mice (9) were infected with L. major promastigotes in their footpads. One week later, popliteal lymph node cells (LNC) of these mice were isolated and restimulated in vitro with the L. major antigen preparation LmAg to investigate cytokine production. At this early time point, LNC from IRF4+/− mice secreted IL-4 and IFN-γ (Table 1), as expected (8). In contrast, LmAg-stimulated IRF4−/− LNC produced neither IL-4 nor IFN-γ. However, L. major-specific production of IL-2, a cytokine secreted by WT Th1, as well as precursor Th cells (15), was normal in IRF4−/− LNC, indicating that responses to L. major antigens were not globally impaired. The percentage of CD4+ Th cells was comparable in IRF4+/− and IRF4−/− LNC (data not shown).
Table 1.
Differentiation of CD4+ Th cells after infection of IRF4-deficient mice with L. major
| Stimulus | IL-2, pg/ml
|
IL-4, pg/ml
|
IFN-γ, pg/ml
|
|||
|---|---|---|---|---|---|---|
| IRF4+/− | IRF4−/− | IRF4+/− | IRF4−/− | IRF4+/− | IRF4−/− | |
| Medium | 19 ± 5 | 9 ± 2 | <4 | <4 | 180 ± 36 | <70 |
| LmAg | 60 ± 8 | 40 ± 17 | 237 ± 48 | <4 | 12,500 ± 6,600 | 250 ± 300 |
IRF4+/− and IRF4−/− mice (four per group) were infected with 2 × 107 L. major promastigotes in the right hind footpad. Mice were killed 1 week later and cell suspensions of the popliteal LN of individual mice were restimulated in vitro with LmAg (2). Cytokine production in 24 h culture supernatants was measured by ELISA. Data are the mean concentration of cytokines ± SD for each group of mice. The results are representative of three independent experiments.
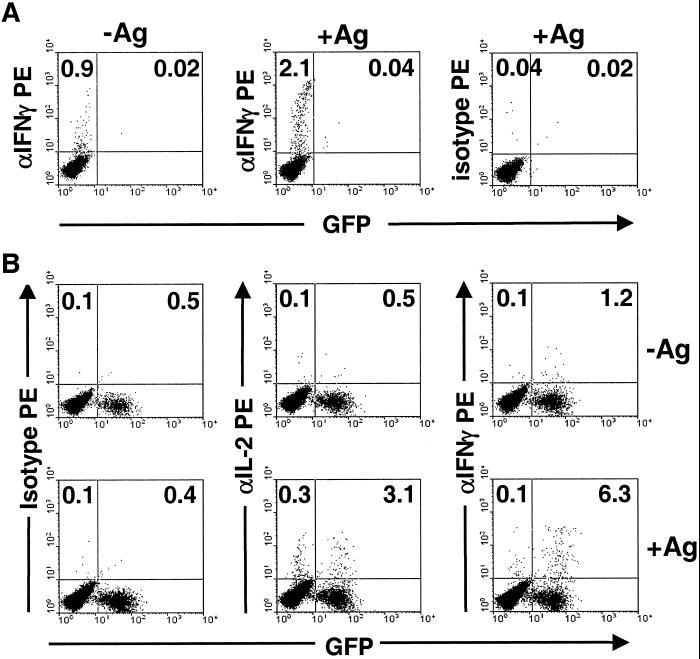
To investigate whether the cytokine secretion defect was intrinsic to Th cells, CD4+ T cells of C57BL/6 mice transgenic for GFP (10) were transferred into IRF4−/− mice and the recipients infected with L. major. After 4 weeks (to ensure grafting of the transferred cells and expansion of L. major-reactive cells), the draining LNC were restimulated in vitro with LmAg presented by dendritic cells according to a recently described protocol (8). The frequencies of CD4+ T cells of donor or host origin producing either IL-2 or IFN-γ were determined by intracellular cytokine staining (ICS) and flow cytometric analysis. In infected WT mice without cell transfer (Fig. 1A), LmAg-specific production of IFN-γ was readily detectable. In IRF4−/− mice that had received IRF4+/+ CD4+ T cells, the relative frequency of LmAg-reactive IRF4+/+ (donor, GFP positive) CD4+ T cells that produced IFN-γ was dramatically increased compared with IRF4−/− (host, GFP negative) CD4+ T cells (Fig. 1B). The difference in the percentage of cells producing IL-2 was less pronounced. Because IRF4+/+ and IRF4−/− CD4+ T cells behaved differently within the same animal, the defect in Th1 differentiation of IRF4−/− Th cells was intrinsic to the Th cells themselves.
Figure 1.
Defective differentiation of IRF4−/− CD4+ Th cells in vivo. (A) Differentiation of WT Th cells in IRF4+/+ mice. C57BL/6 mice were infected with L. major and the frequency of IFN-γ-producing, LmAg-specific CD4+ Th cells in popliteal LN was determined 4 weeks later by intracellular staining after in vitro restimulation with or without LmAg. Numbers on the left of the dot plots indicate the percentages of cytokine-positive CD4+ T cells. Numbers on the right confirm the absence of GFP+ cells in these control mice (see B for comparison). (Left and Center) IFN-γ production. (Right) Isotype control. For A and B, all dot plots were gated on CD4+ cells, identified by staining with anti-CD4-Tri-Color. (B) Differentiation of WT Th cells in IRF4−/− mice. Purified IRF4+/+ CD4+ cells from GFP transgenic mice were transferred i.p. into IRF4−/− mice. Mice were infected as in A and the frequencies of IFN-γ- or IL-2-producing LmAg-specific CD4+ Th cells in popliteal LN were detected. Numbers indicate cytokine-positive IRF4−/− cells as a percentage of GFP− (host) CD4+ T cells (Left), or cytokine-positive IRF4+/+ cells as a % of GFP+ (donor) CD4+ T cells (Right). The ratio of GFP− to GFP+CD4+ T cells in recipient mice was 95:5. Restimulation in the absence (Upper) or presence (Lower) of LmAg. (Left) Isotype control. (Center) IL-2 production. (Right) IFN-γ production.
We went on to analyze whether defective Th1 differentiation during leishmaniasis would be followed by an increase in disease susceptibilty. To exclude any effects that IRF4 might also have at the level of the APC, RAG1-deficient mice (11) of the genetic C57BL/6 background were used in another cell-transfer experiment. These mice, which contain no B and T cells and are therefore susceptible to L. major, were reconstituted with WT B cells from C57BL/6 mice, as well as with purified CD4+ T cells from either IRF4+/− or IRF4−/− mice. The mice were infected with L. major and killed 6 weeks later. Lesion size and parasite burden (Table 2) were then determined. When compared with RAG1−/− mice without cell transfer, mice containing IRF4+/− CD4+ T cells were clearly protected from leishmaniasis according to both parameters. In contrast, transfer of IRF4−/− CD4+ T cells did not mediate a significant change in either of the two parameters. In support of the above-described data, LmAg-specific IFN-γ was produced only from splenic T cells of mice that had received IRF4+/− CD4+ T cells (data not shown). From these studies, it can be concluded that IRF4−/− CD4 Th cells have an intrinsic defect to differentiate into Th1 cells in vivo, and that this defect is relevant for protection during leishmaniasis. In support of this conclusion, production of NO and IL-12 by IRF4−/− peritoneal macrophages was normal (data not shown).
Table 2.
IRF4-deficient CD4+ Th cells are nonprotective during leishmaniasis
| Transferred cells
|
|||
|---|---|---|---|
| None | IRF4+/+ B cells + IRF4+/− CD4+ | IRF4+/+ B cells + IRF4−/− CD4+ | |
| Increase in lesion size, % ± SD | 83 ± 24 | 29 ± 6 | 127 ± 23 |
| Parasite burden per spleen | 1.3 × 106 ± 1.0 × 106 | 2.7 × 102 ± 0.8 × 102 | 1.6 × 105 ± 0.2 × 105 |
RAG1−/− mice were injected i.p. with the indicated purified cells from IRF4+/+, IRF4+/−, or IRF4−/− mice and infected with L. major into the right hind footpad. Six weeks later, the mice were killed and the relative increase in footpad thickness compared to the uninjected footpad (%), as well as the splenic parasite burden, were determined.
Severely Altered Th Differentiation of IRF4−/− Th Cells in Vitro.
To further corroborate that the differentiation defect indeed resides intrinsically within the Th cells, we next examined the in vitro differentiation of IRF4−/− Th cells in the absence of APC. Naive CD62L+CD4+ T cells from IRF4+/+ and IRF4−/− mice were induced to differentiate in vitro into either Th1 or Th2 cells by treatment with either anti-CD3 Ab plus IL-12 and anti-IL-4 Ab, or anti-CD3 plus IL-4 and anti-IL-12 Ab, respectively. After restimulation with anti-CD3, culture supernatants were tested for the presence of cytokines (Table 3). In the absence of either IL-4 or IL-12 during primary stimulation, WT CD4+ T cells developed into Th1 cells (defined as producing IFN-γ but not IL-4) at a relatively low rate. The presence of IL-12 during primary stimulation greatly enhanced WT Th1 differentiation, consistent with previous data (16). Similarly, WT CD4+ T cells stimulated with IL-4 led to a Th2 phenotype defined by a strong relative increase in IL-4 over IFN-γ production (17–19). However, IRF4 deficiency dramatically changed the differentiation profile of Th cells. In the absence of IL-4 or IL-12 during primary stimulation, IRF4−/− Th cells failed to differentiate into either Th1 or Th2 cells (Table 3), but did produce IL-2 (data not shown). Addition of IL-12 provoked the generation of Th1 cells, but at considerably lower levels than in WT cells. Addition of IL-4 resulted in only minimal production of IL-4, indicating a failure in Th2 differentiation. These data are compatible with the in vivo findings described above. In addition, however, we found that IL-4 induced significant levels of IFN-γ production by IRF4−/− Th cells. Thus, in anti-CD3 triggered IRF4−/− CD4+ T cells, IL-4 promoted Th1 rather than Th2 cell differentiation, while IL-12-induced Th1 cell differentiation was defective. These effects of IRF4 were most likely due to alterations in the Th differentiation program itself and not to a direct effect on the promoters of the IL-4 or IFN-γ genes, because production of the Th1 cytokine TNF and the Th2 cytokine IL-5 was influenced in a similar way (Table 3).
Table 3.
Cytokine production by CD4+ T cells after short-term Th1/Th2 differentiation in vitro
| Primary stimulus | IFN-γ, pg/ml
|
IL-4, pg/ml
|
TNF, pg/ml
|
IL-5, pg/ml
|
||||
|---|---|---|---|---|---|---|---|---|
| IRF4+/− | IRF4−/− | IRF4+/− | IRF4−/− | IRF4+/− | IRF4−/− | IRF4+/− | IRF4−/− | |
| Anti-CD3 | 4,000 | <40 | 16 | <2 | <4 | <4 | 20 | <5 |
| Anti-CD3, anti-IL-4, IL-12 | 300,000 | 12,000 | 2 | <2 | 550 | <4 | <5 | <5 |
| Anti-CD3, IL-4, anti-IL-12 | 50,000 | 500,000 | 50,000 | 150 | 180 | 1,500 | 18,000 | <5 |
CD62L+CD4+ T cells from IRF4+/− and IRF4−/− mice purified by magnetic cell sorting were stimulated with immobilized anti-CD3 (5 μg/ml) and/or cytokines for 96 h, as indicated. Thereafter, they were rested for 72 h in the presence of IL-2 and restimulated in triplicate cultures with anti-CD3. Twenty-four-hour culture supernatants were tested for cytokines by ELISA. Data shown give the mean of the triplicates; the SD was in all cases <10%. Results shown are representative of six (IFN-γ, IL-4) and three (TNF, IL-5) different experiments. ND, not determined.
To investigate the effects of costimulation via CD28 on Th cell differentiation, cells were cultured, as described for Table 3, but received anti-CD28 in addition to anti-CD3 ab. This treatment did not change the inability of IRF4−/− CD4+ T cells to produce IL-4, nor did it change the reduced capacity to produce IFN-γ in response to IL-12 (Table 4). However, anti-CD28 cotreatment did induce Th1 cells in the absence of either IL-4 or IL-12, and this Th1 cell development could not be further increased by IL-4. Thus, in the presence, but not the absence of anti-CD28, IL-4 had no more overt effect on IRF4−/− CD4+ T cell differentiation at all. This intriguing similarity of effects created by anti-CD28 and IL-4 is a matter of our current analysis.
Table 4.
Differential effect of IRF4 deficiency on Th cells triggered with or without anti-CD28
| Primary stimulus | IFN-γ (pg/ml)
|
IL-4 (pg/ml)
|
||
|---|---|---|---|---|
| IRF4+/+ | IRF4−/− | IRF4+/+ | IRF4−/− | |
| Anti-CD3 | 400 | <120 | 20 | 20 |
| Anti-CD3, anti-IL-4, IL-12 | 25,000 | 5,700 | 20 | 20 |
| Anti-CD3, IL-4, anti-IL-12 | 1,200 | 22,000 | 4,200 | 40 |
| Anti-CD3/28 | 75,000 | 13,000 | 240 | 20 |
| Anti-CD3/28, anti-IL-4, IL-12 | 840,000 | 54,000 | 30 | 20 |
| Anti-CD3/28, IL-4, anti-IL-12 | 600 | 13,000 | 27,000 | 200 |
Cultures were set up as described for Table 3. Where indicated, cultures received in addition anti-CD28 Ab 37.51 (2.5 μg/ml).
Normal STAT6 Phosphorylation, but Dysregulated Expression of GATA3 in IL-4-Triggered IRF4−/− Th Cells.
To determine whether the disturbance in cytokine production in the absence of IRF4 was due to abnormalities in IL-4 signaling, we examined the status of IL-4R expression and STAT6 (20) phosphorylation in IRF4−/− Th cells. In anti-CD3-triggered IRF4−/− and IRF4+/− Th cells, the expression levels of both IL-4Rα and the common γ-chain were similar (data not shown). Likewise, IL-4-induced STAT6-signaling was normal and comparable to that of a control Th2 cell clone, as determined in naive IRF4−/− and IRF4+/− Th cells that had been stimulated with anti-CD3 plus IL-4 for 72 h, rested in IL-4-free medium overnight, and stimulated for 15 min with or without IL-4 (Fig. 2A). Despite this finding, it cannot be totally excluded that STAT6 contributes to the phenotype of IRF4−/− Th cells in a way described for human B cells (21). In these cells, IRF4 interferes with the interplay of STAT6 and the repressor BCL-6; as a consequence, IRF4 deficiency in human B cells leads to a dominant-negative effect of BCL-6 on transcription of CD23. However, a similar dominant effect of BCL-6 is not likely to account for the IRF4−/− Th phenotype in our study, because a lack of BCL-6 has no impact on Th cell differentiation in vitro (22).
Figure 2.
STAT6 phosphorylation and GATA-3 protein expression. (A) Naive IRF4−/− and IRF4+/− Th cells, as well as L1/1 control cloned Th2 cells, were primed with anti-CD3 and IL-4. Seventy-two hours later, the cells were harvested, washed, recultured overnight in IL-2, restimulated for 15 min with or without IL-4 as indicated, and processed for determination of phosphorylated and total STAT6 by Western Blot, as described in Materials and Methods. Blot is representative of two experiments with similar results. (B) Naive IRF4−/− and IRF4+/− Th cells and control cells of B10BI (Th1) and L1/1 (Th2) clones were primed with anti-CD3 in the presence and absence of IL-4, cultured for 96 h, rested, and restimulated with anti-CD3 as described in Table 2. After 6 h, cells were harvested and processed for determination of GATA-3 expression by Western blotting. The blots were stripped and reprobed with anti-β-actin antibody as a loading control. Results shown are representative of two independent experiments. (C) Retroviral overexpression of GATA-3. Naive IRF4−/− (KO) and IRF4+/+ (WT) Th cells were stimulated with anti-CD3, as above. Forty-eight hours later, they were infected with retrovirus containing either bicistronic GATA-3 and GFP or GFP alone, as indicated. Twenty-four hours after infection, the cells were washed, rested, and restimulated with anti-CD3, as above, and processed for intracellular cytokine staining 6 h later. x axis, GFP-staining; y axis, IL-4 staining (Upper and Lower Right) or IFN-γ staining (Lower Left). Numbers give the percentage of positive cells in the respective quadrants.
Recent studies have demonstrated a decisive role for the transcription factor GATA-3 in Th2 cell differentiation (23). To analyze GATA-3 expression, naive IRF4−/− and IRF4+/− Th cells were stimulated with anti-CD3 with and without IL-4 as above, rested, and restimulated for only 6 h to avoid activation-induced cell death. Expression of GATA-3 protein in cell lysates was detected by Western blotting (Fig. 2B). Culture of IRF4+/− Th cells with IL-4 induced high levels of GATA-3 protein similar to those observed in control-cloned long-term Th2 cells. In contrast, the amounts of GATA-3 in IL-4-treated IRF4−/− cells were as low as those observed in control-cloned long-term Th1 cells. This result strongly suggests that the lack of IL-4 production of IRF4−/− Th cells in response to IL-4 is secondary to an inability of these cells to up-regulate GATA-3. This defect explains the phenotype, because GATA-3 alone is sufficient to force Th2 differentiation even under Th1-inducing conditions (13). To directly test whether increased expression of GATA-3 would restore the Th2 phenotype in IRF4−/− Th cells, we performed retroviral overexpression of GATA-3 (Fig. 2C). Overexpression of GATA-3 in conjunction with GFP, but not of GFP alone, led to an increase in frequency of IL-4, but not IFN-γ-producing IRF4−/− cells. This increase was restricted to GFP-positive retrovirally transformed cells was equivalent to that of WT cells. Thus, GATA-3 overexpression rescued the Th2 phenotype in IRF4−/− cells.
While our manuscript was in preparation, a different publication appeared which also reported defective Th2 cytokine production in IRF4−/− Th cells (24). Although the authors did not test the expression of GATA-3, it was suggested that the defective IL-4 production observed might be the result of an absence of the reported strong synergism between NFATc2 and IRF4 in IL-4 gene transcription. We consider it unlikely that this interaction explains all effects of IRF4 deficiency on cytokine synthesis, however, because it is difficult to explain why a gene deficiency in NFATc2 could lead to enhanced IL-4 production (25), while IRF4 deficiency has the reverse phenotype. This issue, as well as the relationship between NFATc2 and the lack of GATA3 induction presented in our paper, are current issues of our investigation.
Acknowledgments
We thank Mary Saunders for scientific editing, Dr. J. Zerrahn (Max Planck Institute, Berlin) for support with retroviral infections, and Dr. K. Murphy for providing GATA-3-overexpressing retrovirus. This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations
- GFP
green fluorescent protein
- IRF
IFN regulatory factor
- LNC
lymph node cells
- Th
T helper
- WT
wild type
References
- 1.Mamane Y, Heylbroeck C, Genin P, Algarte M, Servant M J, LePage C, DeLuca C, Kwon H, Lin R, Hiscott J. Gene. 1999;237:1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 2.Lohoff M, Ferrick D, Mittrucker H W, Duncan G S, Bischof S, Rollinghoff M, Mak T W. Immunity. 1997;6:681–689. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 3.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, Suzuki G, Mitsuyama M, Shin E H, Kojima S, et al. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 4.Scharton Kersten T, Afonso L C, Wysocka M, Trinchieri G, Scott P. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 5.Giese N A, Gabriele L, Doherty T M, Klinman D M, Tadesse H L, Contursi C, Epstein S L, Morse H C. J Exp Med. 1997;186:1535–1546. doi: 10.1084/jem.186.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohoff M, Duncan G S, Ferrick D, Mittrucker H W, Bischof S, Prechtl S, Rollinghoff M, Schmitt E, Pahl A, Mak T W. J Exp Med. 2000;192:325–336. doi: 10.1084/jem.192.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiner S L, Locksley R M. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 8.Sommer F, Meixner M, Mannherz M, Ogilvie A L, Rollinghoff M, Lohoff M. Int Immunol. 1998;10:1853–1861. doi: 10.1093/intimm/10.12.1853. [DOI] [PubMed] [Google Scholar]
- 9.Mittrucker H W, Matsuyama T, Grossman A, Kundig T M, Potter J, Shahinian A, Wakeham A, Patterson B, Ohashi P S, Mak T W. Science. 1997;275:540–543. [PubMed] [Google Scholar]
- 10.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 11.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 12.Lohoff M, Schmitt E, Reske Kunz A B, Rollinghoff M. Eur J Immunol. 1990;20:653–658. doi: 10.1002/eji.1830200328. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang W, Ranganath S H, Weindel K, Bhattacharya D, Murphy T L, Sha W C, Murphy K M. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Paul W E. J Exp Med. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg A D, English M, Swain S L. J Immunol. 1990;144:1800–1807. [PubMed] [Google Scholar]
- 16.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 17.Swain S L, Weinberg A D, English M, Huston G. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 18.Le Gros G, Ben Sasson S Z, Seder R, Finkelman F D, Paul W E. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt E, Van Brandwijk R, Fischer H G, Rude E. Eur J Immunol. 1990;20:1709–1715. doi: 10.1002/eji.1830200813. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Schindler U, Henzel W J, Ho T C, Brasseur M, McKnight S L. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Jiang M, Anthony A, Pernis A B. J Exp Med. 1999;190:1837–1848. doi: 10.1084/jem.190.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent A L, Hu-Li J, Paul W E, Staudt L M. Proc Natl Acad Sci USA. 1998;95:13823–13828. doi: 10.1073/pnas.95.23.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng W, Flavell R A. Cell. 1997;89:587–596. [Google Scholar]
- 24.Rengarajan J, Mowen K A, McBride K D, Smith E D, Singh H, Glimcher L H. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodge M R, Ranger A M, Charles d l B, Hoey T, Grusby M J, Glimcher L H. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]