Abstract
Neurons in a restricted zone in the precentral gyrus of macaque monkeys respond to tactile, visual, and auditory stimuli. The tactile receptive fields of these multimodal cells are usually located on the face, arm, or upper torso. In the present study, in awake monkeys sitting in a primate chair, the neurons responded to a tactile probe touching the skin within the tactile receptive field. However, the same neurons did not respond when the tactile receptive field was touched by the primate chair, to which the monkey was habituated.
The precentral gyrus of monkeys contains a restricted zone in which the neurons respond with short latency to tactile, visual, and sometimes auditory stimuli (1–4). Here, we refer to it as the polysensory zone (PZ). Most neurons in this cortical region have a tactile receptive field on the contralateral face, shoulder, arm, or torso. About half of the neurons are also visually responsive. The visual receptive field is typically adjacent to the tactile receptive field and extends 5–30 cm outward from the body surface. Cells that have a tactile receptive field on the side or back of the head also often respond to auditory stimuli in the space near the tactile receptive field, within about 30 cm of the head (5). Thus, the multimodal neurons in PZ represent the space immediately surrounding the body through touch, vision, and sometimes audition.
In a recent experiment (6), electrical stimulation of sites within PZ evoked coordinated movement patterns consistent with defending the body from an impending threat. For example, if the sensory receptive fields were on and near the left side of the head, stimulation caused a squint and facial grimace on the left side, a head turn to the right, and a thrusting of the left hand into the space near the left of the head. If the sensory receptive fields were on and near the arm, stimulation caused a fast withdrawal of the arm to a guarding-like posture behind the back. These results suggest that PZ may be part of a sensory-motor pathway that detects and localizes potentially threatening objects near the body and organizes defensive movements.
In our previous work studying the response properties of neurons in PZ, we observed a puzzling phenomenon. While testing tactile receptive fields, we found that the neurons responded to some tactile stimuli and not others. A touch with a cotton swab evoked a robust response and enabled us to plot the borders of the receptive field. In contrast, when the tactile receptive field was touched by a part of the primate chair to which the monkey was habituated, the neurons did not respond. Here, we describe these qualitative observations. One interpretation is that nearby familiar objects that pose no potential threat to the monkey are not encoded by neurons in PZ.
Materials and Methods
All husbandry, surgical, and behavioral procedures were approved by the Princeton University Institutional Animal Care and Use Committee and the consultant veterinarian and were in accordance with National Institutes of Health and U.S. Department of Agriculture guidelines. Responses of single neurons in the central part of the precentral gyrus were studied in two adult male Macaca fascicularis (6–7 kg). For each monkey, an initial surgical operation was performed under deep pentobarbitol anesthesia and strict aseptic conditions, during which an acrylic skull cap was affixed to the skull with bone screws. A stainless steel recording chamber, 2.5 cm in diameter, was embedded in the acrylic over the frontal lobe for a vertical approach to the precentral gyrus. A steel bolt for holding the head was also imbedded in the acrylic. Each animal recovered from the effects of the surgery within several days and was given 3 additional weeks to allow the skull to grow tightly around the skull screws. (For details of surgical procedures, see ref. 3.) In a subsequent procedure, also under deep anesthesia and aseptic conditions, the recording chamber was opened, and a hole ≈2 mm in diameter was drilled through the layer of acrylic and the bone, exposing the dura. As the experiment progressed, new holes were added to allow access to different portions of the precentral gyrus.
During the daily recording sessions, the monkey sat in a primate chair (see Fig. 1A). The animal was restrained by a rigid Plexiglas collar bolted to the sides of the chair. The Plexiglas walls of the chair formed a box around the monkey about 40 cm across. One arm extended through a hole in the front of the chair (5 × 5 cm) and was strapped down with Velcro strips to a metal arm holder. The hole was cut into a part of the chair that could slide laterally, such that the monkey's arm could be adjusted to a different position to the right or left. The head was held in place by the head bolt. A hydraulic microdrive was mounted to the top of the recording chamber. A steel guide cannula (an 18-gauge syringe needle) was lowered through the hole in the skull and into the dura. Then, a varnish-coated tungsten microelectrode (Frederick Haer, Bowdoinham, ME, impedance 0.5–5 MΩ) was advanced from the guide cannula into the brain to record from neurons in the cortex immediately below the dura.
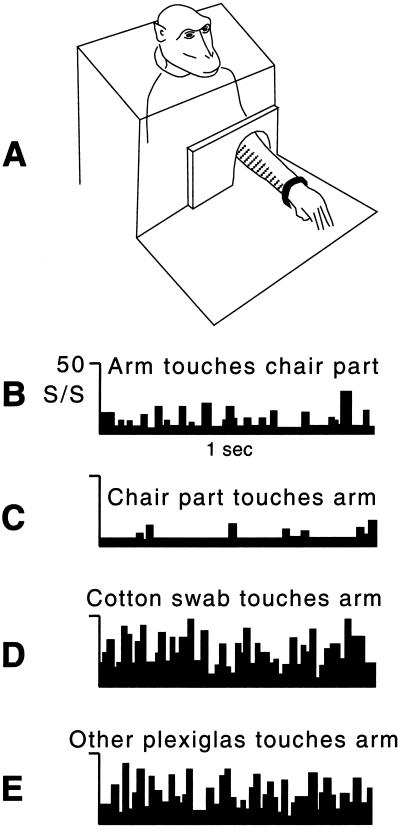
Figure 1.
Tactile responses of a neuron from the PZ. (A) The monkey sat in a chair with the arm extended through a hole and strapped in an arm holder. The Plexiglas plate surrounding the arm could be moved laterally, thereby touching the arm. The striped area on the medial surface of the arm indicates the tactile receptive field. This tactile receptive field extended up the arm and ended about halfway between the elbow and arm pit. (B) The firing rate (mean of 10 trials) during tactile stimulation caused by the experimenter pushing the arm such that the tactile receptive field came into contact with the adjacent Plexiglas chair part. (C) The firing rate (mean of 10 trials) during tactile stimulation caused by the experimenter moving the chair part such that it touched the tactile receptive field on the arm. (D) The firing rate (mean of 10 trials) during tactile stimulation with a cotton swab on the same part of the tactile receptive field that touched the chair part in B and C. (E) The firing rate (mean of 10 trials) during tactile stimulation with a disconnected piece of Plexiglas on the same part of the tactile receptive field that touched the chair part in B and C.
Once a cell was isolated, as indicated by the repeatability of its wave form on the oscilloscope, it was studied by presenting a standard battery of stimuli. Somatosensory responsiveness was studied by using manual palpation, manipulation of joints, gentle pressure, and stroking with cotton swabs. Somatosensory receptive fields were plotted by repeated presentation of the most effective of these stimuli. Responses on the face were tested while the eyes were covered. Most PZ neurons do not respond to visual stimuli projected onto a tangent screen, even when the screen is placed close to the face (3). Instead, they respond best to objects near the animal. Therefore, we used real objects, such as a ping-pong ball mounted on the end of a rod, to plot visual receptive fields. To ensure that the responses to stimuli close to the body were not caused by inadvertent tactile stimulation, for example by static electricity or air movement, the visual stimuli were also presented while the eyes were covered, while the animal was shielded with a piece of clear Plexiglas, or under both conditions.
In a few cases, we recorded the level of neuronal activity during presentation of hand-held tactile stimuli in the following fashion. A light-emitting diode placed out of view of the monkey cued the experimenter to apply a tactile stimulus to the monkey. The diode turned on at the start of each 2-s trial and turned off at the end of the trial. Single neuron spike data were collected during the period that the LED was on.
At the completion of the experiment, each monkey was given an overdose of sodium pentobarbitol (100 mg/kg) and was perfused transcardially with saline and then 10% formalin. The head was put in a stereotaxic apparatus, the skull was opened, and the brain was exposed. The positions of the arcuate and central sulci were measured stereotaxically. The recording sites were just posterior to the bend in the arcuate sulcus, in the expected location of the polysensory zone (4). The brain was sectioned in the coronal plane on a freezing microtome. Sections were cut at 50 mm and stained with cresyl violet. Damage from the microelectrode was clearly visible as streaks of gliosis in the tissue, confirming the locations of recording sites.
Results
Here, we describe qualitative observations of three example neurons from PZ and then summarize the findings for all 24 neurons tested. Fig. 1 illustrates the results for the first example cell, a typical bimodal, visual-tactile neuron. The tactile response was tested by touching the monkey with a cotton swab while the arm was held in the arm holder and the monkey's vision was blocked. The tactile receptive field was located on the medial surface of the contralateral arm and extended from the wrist up the arm to about half way between the elbow and shoulder (see Fig. 1A). When the monkey's vision of the arm was not blocked, the cell also responded to the sight of objects placed just medial to the forearm, within about 5 cm of the arm. This cell therefore exhibited the well established properties of multimodal neurons in PZ.
While studying this cell, we were surprised to find no neuronal response when the tactile receptive field touched the Plexiglas sides of the monkey chair. As shown in Fig. 1A, the arm extended through a hole in a Plexiglas plate. Movement of the arm therefore sometimes caused the edge of the hole to touch the forearm, just in front of the elbow. When the tactile receptive field was stimulated in this fashion, the neuron did not respond. Fig. 1B shows the low level of activity when the monkey's arm was pushed gently by the experimenter, causing the tactile receptive field to touch the edge of the Plexiglas. Fig. 1D shows a similar low level of activity when the monkey's arm was stationary and the Plexiglas plate was moved laterally, causing the edge of the plastic to touch the tactile receptive field. The cell also did not respond when the monkey actively moved its arm, causing the tactile receptive field to touch the edge of the Plexiglas. Thus, regardless of how the chair part came into contact with the tactile receptive field, whether because the arm was passively moved against it, it was moved against the arm, or the monkey actively moved the arm and touched it, the cell did not respond.
In contrast, Fig. 1D shows the high level of neuronal activity when the same location on the forearm was lightly touched with a cotton swab. Other tactile stimuli, such as the experimenter's gloved hand, a wooden dowel, and a puff of air from a bulb syringe, also evoked a response. Even touching a single hair evoked a robust response. As shown in Fig. 1E, touching with a hand-held piece of Plexiglas of the same type used in the chair construction evoked a high level of activity. Thus, ineffective tactile stimuli included any part of the chair with which the monkey was familiar through many months of training; effective stimuli included everything else that we tried. The neuron appeared to exhibit a “clothing effect,” in that the familiar feel of the chair surrounding the body did not elicit responses.
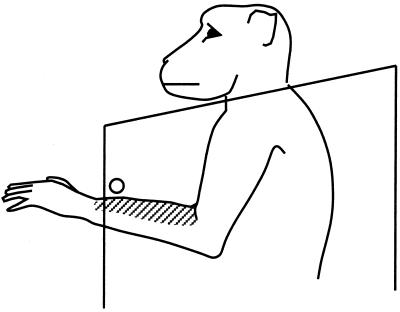
A second example neuron had a tactile receptive field on the dorsal surface of the contralateral forearm (see Fig. 2). Like the previous neuron, this one responded when the tactile receptive field was touched by a cotton swab, the gloved hand of the experimenter, or a disconnected piece of Plexiglas. When it came into contact with the edge of the chair, however, the neuron did not respond. We then tested the neuron by releasing the monkey's arm from the arm holder, removing the restrictive Plexiglas plate covering the front of the chair, and allowing the monkey to move its arm freely. Under this condition, the neuron still did not respond when the tactile receptive field came into contact with the edges of the chair. We then fixed a 1-cm diameter metal rod to the chair 10 cm in front of the monkey's chest, extending from the left to the right side of the chair (see Fig. 2). Because the monkey's head was held in a rigid posture by the head bolt, the monkey could not look down far enough to see the rod. Under these conditions, the neuron responded when the dorsal surface of the forearm came into contact with the metal rod, but not when the ventral surface of the forearm touched the rod. Over a period of several minutes as the monkey moved its arm, the neuron responded each time the tactile receptive field touched the rod. We then removed the rod from the chair. To ensure that the monkey did not feel the rod being removed, we held the monkey's hands down at its feet. After the rod was removed, we released the arms. The monkey lifted its arm, and as the dorsal surface of the arm approached the location where the rod had been, the neuron began to respond. However, because the rod was now absent, the arm passed through that region of space without obstruction, and the firing of the neuron subsided to baseline. Continuing its upward trajectory, the dorsal surface of the forearm came into contact with the neck plate of the chair, and the neuron did not respond. The monkey then lowered its arm and raised it again, and the neuron responded once again as the tactile receptive field approached the location where the rod had been. This response to a “phantom” rod occurred three times, and then was no longer observed; the neuron no longer responded as the arm moved, whether the tactile receptive field crossed the location where the rod had been or touched any part of the chair. Similar responses of PZ neurons to the remembered location of a stimulus have been described in the visual modality (7).
Figure 2.
A neuron from PZ with a tactile receptive field (striped) on the arm. As the monkey moved its arm spontaneously, the tactile receptive field touched parts of the chair, but the cell did not respond. When a metal bar (shown by the circle) was inserted into the chair, the cell responded each time the tactile receptive field touched the metal bar.
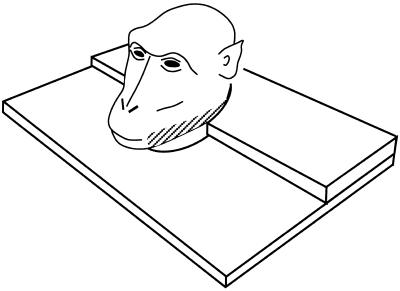
A third example neuron had a tactile receptive field on the contralateral jaw (see Fig. 3). We loosened the monkey's head bolt, thus allowing the head to turn to the right or left, causing the tactile receptive field to touch the plastic neck plate. Whether the head was turned passively by the experimenter or actively by the monkey, the neuron did not respond when the tactile receptive field came into contact with the plastic neck plate. In contrast, when a screwdriver was fixed to the neck plate out of the monkey's view, such that head turning resulted in the monkey's jaw coming into contact with the unseen novel object not normally part of the chair, the neuron responded. Touching the tactile receptive field with a cotton swab also evoked a response, whether the swab was advanced from behind the monkey and was thus out of view, or was advanced from in front of the monkey and was thus in view.
Figure 3.
A neuron from PZ with a tactile receptive field (striped) on the jaw and a visual response to objects presented in the space near the tactile receptive field. When the monkey turned its head, thus causing the tactile receptive field to rub against the adjacent chair part, the cell did not respond.
For all 24 cells tested, 20 in monkey no. 1 and 4 in monkey no. 2, the tactile response was present when the receptive field was touched by a cotton swab, the experimenter's hand, or a variety of other stimuli, but absent when the tactile receptive field was touched by a part of the monkey's chair that had come into contact with the monkey over many months of training.
Discussion
Recent findings suggest that PZ may participate in monitoring objects near the body and organizing a defensive reaction (6). One way to interpret the present observations is that familiar objects posing no possible threat to the monkey did not activate this defensive mechanism. In this view, any object held by the experimenter or any unfamiliar object, once it has entered a margin of safety around the body, is potentially threatening. Familiar parts of the primate chair are not. The following observations are consistent with this interpretation.
Bimodal neurons in PZ respond vigorously to the sight of real, three-dimensional objects moving in the space near the body, but respond poorly or not at all to visual stimuli moving on a screen, even when the screen is fixed close to the body (3).
Bimodal neurons in PZ respond better to faster visual stimuli (2). If the visual stimulus is advanced toward the tactile receptive field from a distance, the neuron will begin to respond once the stimulus has entered the space near the body. The faster the stimulus approaches, the sooner the neuron responds, effectively increasing the outer distance of its visual receptive field.
Bimodal neurons in PZ often respond better to visual stimuli that evoke an avoidance reaction than to stimuli that evoke an approach reaction (M.S.G., unpublished observations). A rubber snake, eliciting an alarm response from the monkey, was an especially effective stimulus when placed in the visual receptive field of a bimodal neuron; an apple placed in the visual receptive field evoked a smaller response. We observed 12 such “biblical” cells capable of distinguishing an apple from a snake.
Bimodal neurons respond to the sight of objects near the tactile receptive field regardless of the location of the visual stimulus on the retina. For 70% of the bimodal cells with a tactile response on the arm, the visual receptive field is anchored to the arm, moving when the arm is moved (3, 8). For 95% of the bimodal cells with a tactile response on the face, the visual receptive field is anchored to the head, moving when the head is rotated (3). For almost all bimodal neurons (94%), when the eyes fixate different locations, the visual receptive field does not move (2, 3, 8–11). The bimodal neurons in PZ thus encode the location of nearby visual stimuli with respect to the body surface.
In summary, the properties of neurons in PZ, including some previously puzzling observations, are consistent with a mechanism for maintaining a margin of safety around the body. It is not yet clear if PZ functions exclusively for this defensive purpose, or if it serves other functions as well. Future experiments involving the deactivation of PZ may further clarify its role in behavior.
Acknowledgments
This work was supported by National Institutes of Health Grant EY-11347 and Burroughs Wellcome Grant 992817.
Abbreviation
- PZ
polysensory zone
References
- 1.Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Behav Brain Res. 1981;2:147–163. doi: 10.1016/0166-4328(81)90053-x. [DOI] [PubMed] [Google Scholar]
- 2.Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. J Neurophysiol. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Graziano M S A, Hu X, Gross C G. J Neurophysiol. 1997;77:2268–2292. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- 4.Graziano M S A, Gandhi S. Exp Brain Res. 2000;135:259–266. doi: 10.1007/s002210000518. [DOI] [PubMed] [Google Scholar]
- 5.Graziano M S A, Reiss L A J, Gross C G. Nature (London) 1999;397:428–430. doi: 10.1038/17115. [DOI] [PubMed] [Google Scholar]
- 6.Graziano M S A, Taylor C S R, Moore T. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 7.Graziano M S A, Hu X, Gross C G. Science. 1997;277:239–241. doi: 10.1126/science.277.5323.239. [DOI] [PubMed] [Google Scholar]
- 8.Graziano M S A, Yap G S, Gross C G. Science. 1994;266:1054–1057. doi: 10.1126/science.7973661. [DOI] [PubMed] [Google Scholar]
- 9.Fogassi L, Gallese V, di Pellegrino G, Fadiga L, Gentilucci M, Luppino M, Pedotti A, Rizzolatti G. Exp Brain Res. 1992;89:686–690. doi: 10.1007/BF00229894. [DOI] [PubMed] [Google Scholar]
- 10.Gentilucci M, Scandolara C, Pigarev I N, Rizzolatti G. Exp Brain Res. 1983;50:464–468. doi: 10.1007/BF00239214. [DOI] [PubMed] [Google Scholar]
- 11.Graziano M S A, Gross C G. Exp Brain Res. 1998;118:373–380. doi: 10.1007/s002210050291. [DOI] [PubMed] [Google Scholar]