Abstract
Salvia divinorum, whose main active ingredient is the neoclerodane diterpene Salvinorin A, is a hallucinogenic plant in the mint family that has been used in traditional spiritual practices for its psychoactive properties by the Mazatecs of Oaxaca, Mexico. More recently, S. divinorum extracts and Salvinorin A have become more widely used in the U.S. as legal hallucinogens. We discovered that Salvinorin A potently and selectively inhibited 3H-bremazocine binding to cloned κ opioid receptors. Salvinorin A had no significant activity against a battery of 50 receptors, transporters, and ion channels and showed a distinctive profile compared with the prototypic hallucinogen lysergic acid diethylamide. Functional studies demonstrated that Salvinorin A is a potent κ opioid agonist at cloned κ opioid receptors expressed in human embryonic kidney-293 cells and at native κ opioid receptors expressed in guinea pig brain. Importantly, Salvinorin A had no actions at the 5-HT2A serotonin receptor, the principal molecular target responsible for the actions of classical hallucinogens. Salvinorin A thus represents, to our knowledge, the first naturally occurring nonnitrogenous opioid-receptor subtype-selective agonist. Because Salvinorin A is a psychotomimetic selective for κ opioid receptors, κ opioid-selective antagonists may represent novel psychotherapeutic compounds for diseases manifested by perceptual distortions (e.g., schizophrenia, dementia, and bipolar disorders). Additionally, these results suggest that κ opioid receptors play a prominent role in the modulation of human perception.
Salvia divinorum, a member of the mint family, is a psychoactive plant that has been used in traditional spiritual practices by the Mazatec people of Oaxaca, Mexico for many centuries (1). S. divinorum also grows in California and has been used as a legal hallucinogen for several years (2). Traditionally, S. divinorum is ingested as a quid or smoked for its psychoactive properties (1) and has been reported to have potent hallucinatory actions (1, 3). The main active ingredient of S. divinorum is Salvinorin A (Fig. 1), a novel neoclerodane diterpene of known absolute configuration (4) whose structure was determined by single-crystal x-ray analysis in two independent studies (5, 6). Salvinorin A is structurally distinct from the naturally occurring hallucinogens N,N-dimethyltryptamine, psilocybin, and mescaline and synthetic hallucinogens such as lysergic acid diethylamide (LSD), 4-bromo-2,5-dimethoxyphenylisopropylamine (DOB), and ketamine. Salvinorin A has been reported to be the most potent naturally occurring hallucinogen, with an effective dose in humans in the 200- to 1,000-μg range when smoked (1, 3). Salvinorin A thus rivals the synthetic hallucinogens LSD and DOB in potency. Salvinorin A has been reported to induce an intense hallucinatory experience in humans, with a typical duration of action being several minutes to an hour or so (1).
Figure 1.
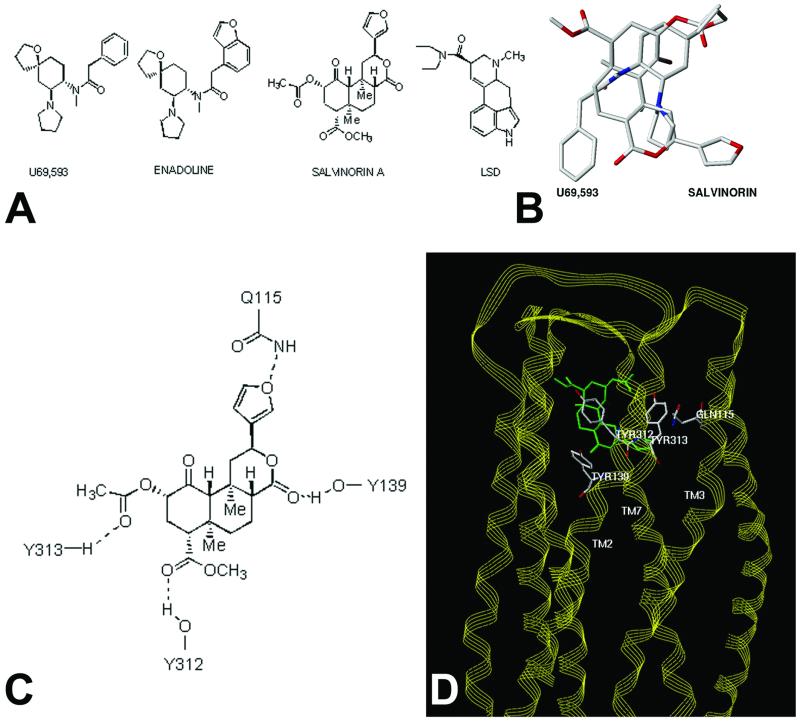
Molecular modeling predicts Salvinorin A is a structurally novel κ opioid ligand. A shows the structure of Salvinorin A, enadoline, U69593 and LSD whereas B shows a superimposition of the structures of Salvinorin A and U69593. C shows potential residues on the KOR identified by molecular modeling, which might interact with Salvinorin A, and D shows a model of Salvinorin A's interactions with the KOR (see supporting information for further details).
Several prior investigations attempted unsuccessfully to identify the molecular and cellular targets responsible for the actions of Salvinorin A (1, 3) by using mainly nonhuman molecular targets. Since then, it has become widely recognized that the pharmacological properties of rodent and human molecular targets are frequently distinct (7), and that tissue-based radioligand binding assays frequently yield inaccurate estimates of drug potency and selectivity. Accordingly, we reexamined the molecular pharmacological profile of the novel diterpene Salvinorin A at a large number of cloned human G protein-coupled receptors (GPCRs), channels, and transporters. We report here that Salvinorin A is a potent and selective κ opioid receptor (KOR) agonist and represents, to our knowledge, the first nonalkaloid opioid subtype-selective drug. We suggest that because the KOR has long been recognized as a target for psychotomimetic agents, KOR antagonists may represent a novel class of psychotherapeutic compounds. Our results also suggest that the KOR/dynorphin peptide system functions to modulate human perception.
Materials and Methods
Materials.
Two sources of Salvinorin A were used for the studies described here: Biosearch and the Salvia divinorum Research and Information Center, Malibu, CA; both samples were identical by thin-layer chromatography and mass spectroscopy and showed the expected molecular ion in the mass spectrum. In addition, the Biosearch sample showed the reported melting point (6), and the Varian 300 MHz NMR spectrum was identical with that reported. The coding region of the KOR was cloned via PCR-amplification of “Quick-Clone” cDNA (CLONTECH) and subcloned into the eukaryotic expression vector pIRESNEO via NotI adaptors to yield pIRESNEO-KOR. The entire insert was verified by automated double-stranded DNA sequencing (Cleveland Genomics, Cleveland). A stable human embryonic kidney-293 cell line expressing the KOR was also constructed (KOR-293) and was used for radioligand-binding and functional assays. GF-62 cells, a stable cell line expressing the 5-HT2A receptor (8), was used for functional studies of 5-HT2A receptors. All other receptors were obtained as previously described (9, 10) as part of the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP) resource.
Frozen guinea pig brains and rat brains were purchased from Harlan Bioproducts for Science (Indianapolis). [d-Ala-2-MePhe4,Gly-ol5]enkephalin (DAMGO), d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), and H-Tyr-Tic-Phe-Phe-OH (TIPP) were obtained from Multiple Peptide Systems (San Diego) through arrangement with Paul Hillery of the Research Technology Branch, National Institute on Drug Abuse. SNC-80 was obtained from K.C.R. (−)-Nor-binaltorphine 2HCl (Nor-BNI) and (+)-U69593 were obtained from Research Biochemicals (Natick, MA). [35S]Guanosine 5′-(γ-thio)-triphosphate ([35S]-GTP[γS], 45 TBq/mmol) was purchased from DuPont/NEN. BSA, naloxone, GDP, and GTP[γS] were purchased from Sigma.
Radioligand-Binding Assays.
Radioligand-binding assays at human cloned GPCRs, ion channels, and transporters were performed as previously detailed (9, 10) by using the resources of the NIMH-PDSP. Detailed on-line protocols are available for all assays at the NIMH-PDSP web site (http://pdsp.cwru.edu). κ opioid radioligand-binding assays in situ in guinea pig brain and μ opioid receptor (MOR)- and δ opioid receptor (DOR)-binding assays in rat brain were performed as previously detailed (11). Initial screening assays were performed by using 10 μM Salvinorin A or 10 μM LSD by using quadruplicate determinations and the percent inhibition of specific binding determined. Where 10 μM test compound inhibited >50% of specific binding, Ki determinations were performed by using six concentrations of unlabeled ligand spanning a 10,000-fold dose range. Kis were calculated by using graphpad prizm and represent the mean ± SEM of quadruplicate determinations.
Functional Assays.
Phosphoinositide hydrolysis assays at 5-HT2A receptors were performed as previously described (8, 12). κ opioid agonist-dependent inhibition of adenylate cyclase was performed by using the KOR-cell line. Briefly, cells were split into polylysine-coated 24-well plates and then incubated overnight in serum-free medium. The next day, medium was replaced with Hanks' F12 medium containing 100 μM isobutylmethylxanthine and 100 μM forskolin together with various concentrations of test agent. After incubation at 37°C for 15 min, the reaction was terminated and cAMP content determined as described previously (13). All data reported here represent the results of at least four separate experiments with EC50 and Emax values calculated by using graphpad prizm (Graph PAD, San Diego).
The 35S-GTP[γS]-binding assay proceeded with modifications of the methods described previously (14, 15). Guinea pig caudate membranes, prepared as described previously (16) (10–20 μg of protein in 300 μl of 50 mM Tris⋅HCl, pH 7.4, with 1.67 mM DTT and 0.15% BSA) were added to polystyrene 96-well plates filled with 200 μl of a reaction buffer containing 50 mM Tris⋅HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 100 μM GDP, 0.1% BSA, 0.05–0.01 nM 35S-GTP[γS], and varying concentrations of drugs. The reaction mixture was incubated for 3 h at 22°C (equilibrium). The reaction was terminated by the addition of 0.5 ml of ice-cold Tris⋅HCl, pH 7.4 (4°C) followed by rapid vacuum filtration through Whatman GF/B filters previously soaked in ice-cold Tris⋅HCl, pH 7.4 (4°C). The filters were washed twice with 0.5 ml of ice-cold distilled H2O (4°C). Bound radioactivity was counted at an efficiency of 98% by liquid scintillation spectroscopy. Nonspecific binding was determined in the presence of 10 μM GTP[γS].
Molecular Modeling.
Molecular modeling investigations were conducted by using the sybl molecular modeling package (Ver. 6.7, 2001, Tripos Associates, St. Louis). Molecular mechanics minimizations of receptor models and complexes were performed after the addition of hydrogen atoms by using the Tripos force field with Gasteiger–Hückel charges (distance-dependent dielectric constant, nonbonded cutoff = 8 Å) without constraints and were terminated at an energy gradient of 0.05 kcal/mol/Å, essentially as previously described (17–19) The unity program within sybl was used to perform the three-dimensional database searches. Full details of the modeling methods and results are published as supporting information (Tables 3–5) on the PNAS web site, www.pnas.org.
Results
Salvinorin A Selectively Inhibits KOR Binding.
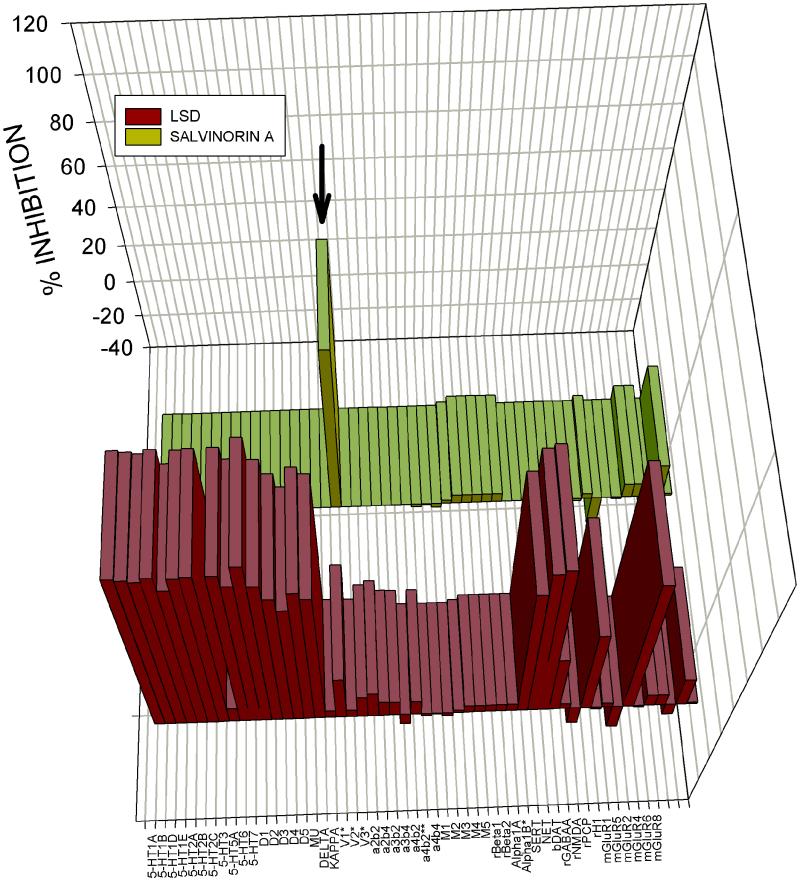
To identify Salvinorin A's molecular target, we screened Salvinorin A (10 μM) at a large panel of mainly cloned human GPCRs, transporters, and ligand-gated ion channels by using the resources of the NIMH-PDSP. For comparison, we screened the same molecular targets with the prototypic hallucinogen LSD, also at 10 μM. As shown in Fig. 1 and in supporting information, Salvinorin A inhibited only [3H]-bremazocine-labeled KORs and did not significantly inhibit binding to cloned human μ (MOR) or δ opioid (DOR) receptors or any of the 48 other molecular targets screened. Ki determinations (Table 1) showed that Salvinorin A was a potent agonist of KOR and guinea pig (gp)KOR. Additionally, Salvinorin A had Ki values >5,000 nM at the gpMORs and gpDORs (Table 1). These results indicate that Salvinorin A is, to our knowledge, the first naturally occurring κ opioid selective ligand. By comparison, LSD potently inhibits the binding of a large number of biogenic amine receptors (Fig. 1) with Kis <50 nM for several GPCRs (data not shown). Interestingly, Salvinorin A had no detectable affinity for the 5-HT2A serotonin receptor and did not activate 5-HT2A receptors (not shown), which represent the main molecular target responsible for the classical hallucinogens such as LSD, N,N′-dimethyltryptamine, psilocybin, mescaline, and 4-bromo-2,5-dimethoxyphenylisopropylamine (20, 21).
Table 1.
Salvinorin A is a potent and selective κ opioid ligand
| μ Ki | δ Ki | κ Ki (pKi ± SEM) | |
|---|---|---|---|
| Cloned receptors | >10,000 nM | >10,000 nM | 16 (7.2 +/− 0.06) |
| Brain receptors | >5,000 nM | >5,000 nM | 4.3 (8.6 +/− 0.05) |
Shown are mean Ki values for Salvinorin A at cloned receptors expressed in human embryonic kidney-293 cells or opioid receptors expressed in situ in rat (μ, δ) or guinea pig brain (κ). Data represent mean ± SEM of computer-derived estimates of Ki and pKi values for n > 3 separate experiments.
Salvinorin A Represents a Structurally Novel KOR Ligand.
Because Salvinorin A represents a structurally novel hallucinogen, we next performed molecular modeling studies to provide insights into how this compound might interact with KORs. A previously reported model of the KOR complexed with the KOR-selective agonist U69593 was used as a starting point (22). This model has the advantage that it was derived from a set of distance constraints between potential hydrogen bond-forming pairs unique to the opioid receptor sequences themselves. The result thus does not depend directly on any direct experimental structural data for rhodopsins. Although this model was constructed before the publication of the crystal structure of rhodopsin (23), it is remarkable that the overall configurations are quite similar (rms deviation = 4 Δ by fitting the helix C-α atoms of identical residues in both sequences). The U69593 KOR complex places the arylacetamide portion of the ligand in a position analogous to the tyramine moiety with the carbonyl hydrogen bonded to Y139 (22). The only structural similarity between U69593 and Salvinorin A (Fig. 2A) is the presence of an aromatic ring and the amide and ester carbonyl groups separated by a short linkage. Because of this similarity, and the nearly complete lack of similarity of salvinorin and any known KOR ligand, the salvinorin crystal structure (5) was initially docked by superimposition of aromatic centroids and the carbonyl atoms of salvinorin with those of bound U69593. The role of the carbonyl functionality for arylacetamide ligands as a hydrogen bond acceptor has been demonstrated experimentally (24) and indirectly supports the proposed role of Y139 and its interaction with the lactone carbonyl of salvinorin. Multiple sterically allowed complexes were generated by using a systematic conformational search about all rotatable Y139 bonds, a dummy bond between a Y139 OH hydrogen atom, and salvinorin carbonyl, following a previously described method (18). Candidate complexes were evaluated interactively for steric fit and hydrogen bond donating properties of the receptor cavity visualized as a Connolly channel plot color coded for hydrogen-bonding potential.
Figure 2.
Large-scale screening of human cloned GPCRs reveals Salvinorin A is selective for KOR. Shown is the mean percent inhibition of radioligand binding or functional activity (metabotropic glutamate receptors only) to 50 receptors and transporters for LSD (yellow bars) and Salvinorin A (red bars) tested at 10 μM. With the exception of the rat β1 and β2 adrenergic and bovine dopamine transporter (DAT) all of the assays were performed with cloned human receptors heterologously expressed (see Materials and Methods and supporting information on the PNAS web site for details). As can be seen (arrow), Salvinorin A inhibited only KOR binding at 10 μM. See Table 5 for details. SERT, serotonin transporter; NET, norepinephrine transporter; DAT, dopamine transporter; rGABAA, rat GABA-A receptor.
Only one family of complexes allowed simultaneous hydrogen bond formation between the receptor side chains and ligand features shown in Fig. 2C (see Table 3 for additional modeling results and details of modeling procedures). In this orientation, the furan substituent of Salvinorin A pointed toward TM1 and TM2, the 4-methoxycarbonyl toward TM5 and TM6, with the A and C rings toward the extra- and intracellular sides, respectively (Fig. 2D and supporting information). Not unexpectedly, there is very little atom-by-atom correspondence between bound U69593 and Salvinorin A, although both occupy a similar space (Fig. 2B). Docking of salvinorin into hydrogen bond potential-coded Connolly channels defining the binding sites of the MOR and DOR models (22) indicates that salvinorin is sterically compatible with each in slightly different binding modes but could not as readily accommodate the four-point hydrogen bond donor/acceptor scheme (Fig. 2D) seen with the KOR receptor (e.g., the KOR models could accommodate the furan oxygen and 4-methoxycarbonyl functionality but not the 2-acetoxy group). Residues potentially forming the salvinorin-binding site of the KOR receptor model are listed in Table 4. The identities of 11 of these are conserved in both the MOR and DOR, whereas the remaining seven are variable. The variable residues cause significant alterations in the steric and electronic characteristics of MOR and DOR in the regions analogous to the salvinorin-binding site of the KOR. The substantial differences in the region of the salvinorin-binding site between the KOR and MOR/DOR receptors are consistent with the observed KOR selectivity of salvinorin.
The proposed KOR salvinorin-binding site model is also consistent with what little is known about the structural features of salvinorin required for psychotropic activity. For example, the one-position carbonyl of salvinorin is not able to form specific donor/acceptor contacts with residues in the receptor model, partially because of its sterically hindered environment, and is not essential for psychotropic activity (25). The 2-acetoxy group of salvinorin does make specific donor/acceptor contacts in the model and is required for activity (5). Interestingly, a three-dimensional search of the National Cancer Society Database using the pharmacophore features and geometries derived from salvinorin docked with the KOR model produced splendidin (26) and deoxydeoxygedunin (27) (not shown). Splendidin was originally isolated from Salvia splendens, a species distinct from S. divinorum and from which salvinorin is derived. S. splendens has been reported to have psychotropic activity.
Salvinorin A Is a Potent KOR Agonist at Recombinant KORs and KORs Expressed in Situ.
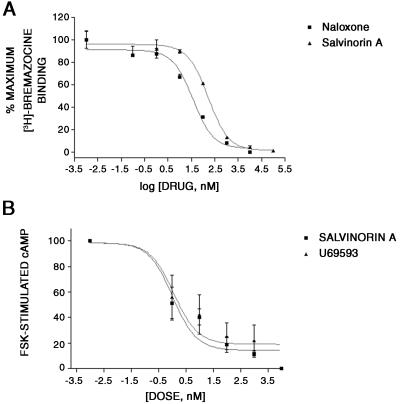
We next examined the agonist/antagonist properties of Salvinorin A by using two model systems: KOR stably expressed in human embryonic kidney-293 cells and gpKOR expressed in situ in guinea pig brain. As shown in Fig. 3, Salvinorin A was a potent KOR agonist with an EC50 for inhibition of adenylate cyclase of 1.05 nM as compared with an EC50 for the KOR agonist U69593 of 1.2 nM (Table 2). Salvinorin A was also a potent agonist at gpKOR expressed in situ with an EC50 for [35S]GTP[γS] binding of 235 nM with U69593 having an EC50 of 377 nM (Table 2). Taken together, these results indicate that Salvinorin A represents, to our knowledge, the first nonnitrogenous KOR-selective agonist.
Figure 3.
Salvinorin A is a potent KOR agonist. A shows that Salvinorin A potently inhibits 3H-bremazocine binding to cloned KORs, whereas B shows the ability of Salvinorin A to inhibit forskolin-stimulated adenylate cyclase in KOR-393 cells. Data represent the mean ± SD of triplicate determinations from a representative experiment that has been replicated three times. For the inhibition of forskolin-stimulated cyclase activity, an EC50 value of 1 ± 0.5 nM was calculated for Salvinorin A, compared with an EC50 value of 1.2 ± 0.6 nM for U69593.
Table 2.
Salvinorin A is a potent κ-opioid agonist: [35S]GTP-γ-S studies using guinea pig brain caudate membranes
| Drug | Unblocked | CTAP, 200 nM | TIPP, 20 nM | Nor-BNI, 0.2 nM |
|---|---|---|---|---|
| Percent stimulation | ||||
| DAMGO | 414 ± 47 | 11,124 ± 2,126 | 494 ± 56 | 355 ± 61 |
| 0.96 ± 0.02 | 0.99 ± 0.09 | 0.97 ± 0.02 | 0.92 ± 0.03 | |
| SNC80 | 758 ± 131 | 987 ± 120 | 9,565 ± 4,115 | 855 ± 119 |
| 0.91 ± 0.04 | 0.94 ± 0.03 | 0.90 ± 0.19 | 0.86 ± 0.3 | |
| U69,593 | 377 ± 39 | 540 ± 57 | 442 ± 76 | 1,554 ± 168 |
| 1.70 ± 0.04 | 1.70 ± 0.04 | 1.60 ± 0.06 | 1.60 ± 0.05 | |
| Percent of maximal stimulation produced by 10 μM U69,593 | ||||
| Salvinorin A | 235 ± 26 | 204 ± 20 | 259 ± 40 | 643 ± 128 |
| 0.79 ± 0.04 | 0.83 ± 0.03 | 0.81 ± 0.06 | 0.89 ± 0.10 | |
[35S]-GTP-γ-S-binding assays were conducted as described in Materials and Methods. Agonist dose–response curves were generated by using 8–10 drug concentrations in the absence and presence of fixed concentrations of selective antagonists: CTAP to block μ receptors, TIPP to block δ receptors, and nor-BNI to block κ receptors. The concentrations were chosen on the basis of previous studies to selectively block the targeted receptor. Values in parentheses are the maximal percent stimulation. For Salvinorin-A, the value is reported as a percent of the stimulation produced by 10 μM U69,593. Each value is ± SD (n = 3). DAMGO, [d-Ala-2-MePhe4,Gly-ol5]enkephalin; CTAP, d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2; TIPP, H-Tyr-Tic-Phe-Phe-OH.
Discussion
The main finding of this paper is that Salvinorin A, the active ingredient of the hallucinogenic plant S. divinorum, is a potent and selective KOR agonist. Salvinorin A is a novel nonalkaloid diterpene that has no structural resemblance to any known hallucinogens but does have modest structural homology to enadoline, a selective KOR agonist (Fig. 1 A and C). Salvinorin A thus represents a class of hallucinogens with potent actions at KORs. Because KOR agonists have long been known to have psychotomimetic actions (28), these results imply that the actions of Salvinorin A in humans are mediated, at least in part, via activation of KORs. Additionally, these results imply that KOR-selective antagonists could conceivably represent treatments for diseases in which hallucinations are prominent, including schizophrenia, depression with psychotic features, and the hallucinosis associated with certain dementias (Alzheimer's, Huntington's, and Pick diseases) and certain types of drug abuse (e.g., amphetamine and cocaine psychosis) (29, 30). Previous studies evaluating naltrexone, which is a nonselective opioid antagonist, for the treatment of schizophrenia have yielded equivocal results (31, 32).
Dynorphin was discovered in 1979 by Goldstein et al. (33) and was demonstrated to be an extraordinarily potent endogenous peptide with selectivity for the KOR (34), a GPCR in the opioid receptor family (35). Before the cloning of the KOR, a large amount of behavioral (36, 37), developmental (38, 39), and biochemical (40, 41) data suggested the existence of distinct KORs. Although dynorphin and related peptides represent, in some cases, potent and relatively selective endogenous ligands for the KOR, other types of naturally occurring ligands have heretofore not been identified. The discovery that Salvinorin A is a potent naturally occurring nonalkaloid agonist for the KOR is thus unexpected.
It is now well established that the activation of KORs induces a large number of behavioral effects that include analgesia, sedation, and perceptual distortions. In the past, studies on the precise role of KORs in humans were hampered by the lack of selective agonists, although studies with compounds such as cyclazocine and ketocyclazocine suggested that KOR agonists were psychotomimetic (28). More recently, human studies with the highly selective KOR agonist enadoline (42) indicated that KOR activation induced visual distortions, feelings of unreality, and depersonalization. These effects of enadoline are reminiscent of those previously reported for Salvinorin A (2, 3). Taken together, these results suggest that the KOR/dynorphinergic system functions to modulate human perception and cognition, as might be inferred from detailed anatomical studies of dynorphin peptide distribution studies (43–45).
One of the implications of these results is that KORs or KOR signaling may also be important in the pathogenesis of diseases characterized by perceptual distortions. The most obvious diseases implicated are schizophrenia, dementia, and bipolar disorders, because all are characterized by hallucinations and delusions. Prior studies evaluating KORs in schizophrenia have yielded conflicting results (46–48), whereas one study examining affective disorder was negative (47). On the other hand, two well-controlled studies have demonstrated an up-regulation of KORs in Alzheimer's disease (49, 50), whereas MORs and DORs were down-regulated (50) or unchanged (49).
In conclusion, we report the discovery that Salvinorin A is a potent selective KOR agonist. Salvinorin A thus represents a unique structural class of nonnitrogenous opioid subtype-selective agonists. Additionally, these results suggest that KORs play a prominent role in the regulation of human perception and suggest that KOR antagonists could represent a novel drug class with specific activity in diseases in which alterations in perception are predominant. Finally, these results imply that the KOR/dynorphinergic system functions to modulate human perception and cognition.
Supplementary Material
Acknowledgments
We gratefully acknowledge Christina M. Dersch, Beth Popadok, and Sandra Hufesein for superb technical assistance. This work was supported in part by a Research Scientist Development Award KO2MH01366 (B.L.R.) and by the NIMH-PDSP NO2MH80004 (B.L.R.).
Abbreviations
- KOR
κ opioid receptor
- MOR
μ opioid receptor
- DOR
δ opioid receptor
- LSD
lysergic acid diethylamide
- NIMH-PDSP
National Institute of Mental Health Psychoactive Drug Screening Program
- GPCR
G protein-coupled receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Valdes L J., III J Psychoactive Drugs. 1994;26:277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- 2.Giroud C, Felber F, Augsburger M, Horisberger B, Rivier L, Mangin P. Forensic Sci Int. 2000;112:143–150. doi: 10.1016/s0379-0738(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 3.Siebert D J. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 4. Koreeda, M., Brown, L. & Valdes, I. L. (1990) Chem. Lett. 2015–2018.
- 5.Valdes L J, Butler W M, Hatfield G M, Paul A G, Koreeda M. J Org Chem. 1984;49:4716–7720. [Google Scholar]
- 6. Ortega, A., Blount, J. F. & Manchand, P. S. (1982) J. Chem. Soc. Perkins Trans. 1, 2505–2508.
- 7.Adham N, Tamm J A, Salon J A, Vaysse P J, Weinshank R L, Branchek T A. Neuropharmacology. 1994;33:387–391. doi: 10.1016/0028-3908(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Roth B L, Palvimaki E, Berry S, Khan N, Sachs N, Uluer A, Choudhary M. J Pharmacol Exp Ther. 1995;275:1638–1646. [PubMed] [Google Scholar]
- 9.Glennon R A, Lee M, Rangisetty J B, Dukat M, Roth B L, Savage J E, McBride A, Rauser L, Hufeisen S, Lee D K. J Med Chem. 2000;43:1011–1018. doi: 10.1021/jm990550b. [DOI] [PubMed] [Google Scholar]
- 10.Rothman R B, Baumann M H, Savage J E, Rauser L, McBride A, Hufeisen S J, Roth B L. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 11.Ananthan S, Kezar H S, III, Carter R L, Saini S K, Rice K C, Wells J L, Davis P, Xu H, Dersch C M, Bilsky E J, et al. J Med Chem. 1999;42:3527–3538. doi: 10.1021/jm990039i. [DOI] [PubMed] [Google Scholar]
- 12.Roth B L, Shoham M, Choudhary M, Khan N. Mol Pharmacol. 1997;52:259–266. doi: 10.1124/mol.52.2.259. [DOI] [PubMed] [Google Scholar]
- 13.Gray J A, Sheffler D J, Bhatnagar A, Woods J A, Hufeisen S J, Benovic J L, Roth B L. Mol Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- 14.Sim L J, Selley D E, Xiao R, Childers S R. Eur J Pharmacol. 1996;307:97–105. doi: 10.1016/0014-2999(96)00211-7. [DOI] [PubMed] [Google Scholar]
- 15.Partilla J S, Carroll F I, Thomas J B, Rice K C, Zimmerman D M, Rothman R B. Analgesia. 1999;4:27–32. [Google Scholar]
- 16.Thomas J B, Mascarella S W, Burgess J P, Xu H, McCullough K B, Rothman R B, Flippen-Anderson J L, George C F, Cantrell B E, Zimmerman D M, Carroll F I. Bioorg Med Chem Lett. 1998;8:3149–3152. doi: 10.1016/s0960-894x(98)00576-9. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro D A, Kristiansen K, Weiner D M, Kroeze W K, Roth B L. J Biol Chem. 2002;277:11441–11449. doi: 10.1074/jbc.M111675200. [DOI] [PubMed] [Google Scholar]
- 18.Westkaemper R B, Runyon S P, Savage J E, Roth B L, Glennon R A. Bioorg Med Chem Lett. 2001;11:563–566. doi: 10.1016/s0960-894x(01)00010-5. [DOI] [PubMed] [Google Scholar]
- 19.Choudhary M S, Sachs N, Uluer A, Glennon R A, Westkaemper R B, Roth B L. Mol Pharmacol. 1995;47:450–457. [PubMed] [Google Scholar]
- 20.Glennon R A, Titler M, McKenney J D. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 21.Roth B L, Willins D L, Kristiansen K, Kroeze W K. Pharmacol Ther. 1998;79:231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 22.Pogozheva I D, Lomize A L, Mosberg H I. Biophys J. 1998;75:612–634. doi: 10.1016/S0006-3495(98)77552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palczewski K, Kumasaka T, Hori T, Behnke C A, Motoshima H, Fox B A, Le Trong I, Teller D C, Okada T, Stenkamp R E, et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 24.Lavecchia A, Greco G, Novellino E, Vittorio F, Ronsisvalle G. J Med Chem. 2000;43:2124–2134. doi: 10.1021/jm991161k. [DOI] [PubMed] [Google Scholar]
- 25.Valdes I L, Chang H M, Visger D C, Koreeda M. Org Lett. 2001;3:3935–3937. doi: 10.1021/ol016820d. [DOI] [PubMed] [Google Scholar]
- 26. Savona, G., Paternostro, M. P. & Piozzi, F. (1979) J. Chem. Soc. Perkins Trans. 1, 533–534.
- 27. Bevan, C. W. L., Halsall, T. G., Nwaji, M. N. & Taylor, D. A. H. (1962) J. Chem. Soc. Perkins Trans. 1, 768–771.
- 28.Pfeiffer A, Brantl V, Herz A, Emrich H M. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 29.Rothman R B, Gorelick D A, Heishman S J, Eichmiller P R, Hill B H, Norbeck J, Liberto J G. J Subst Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 30.Rothman R B. Analgesia. 1994;1:27–49. [Google Scholar]
- 31.Sernyak M J, Glazer W M, Heninger G R, Charney D S, Woods S W, Petrakis I L, Krystal J H, Price L H. J Clin Psychopharmacol. 1998;18:248–251. doi: 10.1097/00004714-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Marchesi G F, Santone G, Cotani P, Giordano A, Chelli F. Prog Neuropsychopharmacol Biol Psychiatr. 1995;19:1239–1249. doi: 10.1016/0278-5846(95)00263-4. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein A, Tachibana S, Lowney L I, Hunkapiller M, Hood L. Proc Natl Acad Sci USA. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavkin C, James I F, Goldstein A. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 35.Xie G X, Meng F, Mansour A, Thompson R C, Hoversten M T, Goldstein A, Watson S J, Akil H. Proc Natl Acad Sci USA. 1994;91:3779–3783. doi: 10.1073/pnas.91.9.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin W R, Eades C G, Thompson J A, Huppler R E, Gilbert P E. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 37.Martin W R. Br J Clin Pharmacol. 1979;7:273S–279S. doi: 10.1111/j.1365-2125.1979.tb04700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spain J W, Bennett D B, Roth B L, Coscia C J. Life Sci. 1983;33:235–239. doi: 10.1016/0024-3205(83)90486-1. [DOI] [PubMed] [Google Scholar]
- 39.Spain J W, Roth B L, Coscia C J. J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang K J, Hazum E, Cuatrecasas P. Proc Natl Acad Sci USA. 1981;78:4141–4145. doi: 10.1073/pnas.78.7.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosterlitz H W, Paterson S J, Robson L E. Br J Pharmacol. 1981;73:939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh S L, Strain E C, Abreu M E, Bigelow G E. Psychopharmacology (Berlin) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- 43.Chavkin C, Bakhit C, Weber E, Bloom F E. Proc Natl Acad Sci USA. 1983;80:7669–7673. doi: 10.1073/pnas.80.24.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGinty J F, van der Kooy D, Bloom F E. J Neurosci. 1984;4:1104–1117. doi: 10.1523/JNEUROSCI.04-04-01104.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGinty J F, Henriksen S J, Goldstein A, Terenius L, Bloom F E. Proc Natl Acad Sci USA. 1983;80:589–593. doi: 10.1073/pnas.80.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owen F, Bourne R C, Poulter M, Crow T J, Paterson S J, Kosterlitz H W. Br J Psychiatr. 1985;146:507–509. doi: 10.1192/bjp.146.5.507. [DOI] [PubMed] [Google Scholar]
- 47.Peckys D, Hurd Y L. Brain Res Bull. 2001;55:619–624. doi: 10.1016/s0361-9230(01)00525-1. [DOI] [PubMed] [Google Scholar]
- 48.Izenwasser S, Staley J K, Cohn S, Mash D C. Life Sci. 1999;65:857–862. doi: 10.1016/s0024-3205(99)00315-x. [DOI] [PubMed] [Google Scholar]
- 49.Barg J, Belcheva M, Rowinski J, Ho A, Burke W J, Chung H D, Schmidt C A, Coscia C J. Brain Res. 1993;632:209–215. doi: 10.1016/0006-8993(93)91155-l. [DOI] [PubMed] [Google Scholar]
- 50.Mathieu-Kia A M, Fan L Q, Kreek M J, Simon E J, Hiller J M. Brain Res. 2001;893:121–134. doi: 10.1016/s0006-8993(00)03302-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.