Abstract
The physiological meaning of the coexpression of adenosine A2A receptors and group I metabotropic glutamate receptors in γ- aminobutyric acid (GABA)ergic striatal neurons is intriguing. Here we provide in vitro and in vivo evidence for a synergism between adenosine and glutamate based on subtype 5 metabotropic glutamate (mGluR5) and adenosine A2A (A2AR) receptor/receptor interactions. Colocalization of A2AR and mGluR5 at the membrane level was demonstrated in nonpermeabilized human embryonic kidney (HEK)-293 cells transiently cotransfected with both receptors by confocal laser microscopy. Complexes containing A2AR and mGluR5 were demonstrated by Western blotting of immunoprecipitates of either Flag-A2AR or hemagglutinin-mGluR5 in membrane preparations from cotransfected HEK-293 cells and of native A2AR and mGluR5 in rat striatal membrane preparations. In cotransfected HEK-293 cells a synergistic effect on extracellular signal-regulated kinase 1/2 phosphorylation and c-fos expression was demonstrated upon A2AR/mGluR5 costimulation. No synergistic effect was observed at the second messenger level (cAMP accumulation and intracellular calcium mobilization). Accordingly, a synergistic effect on c-fos expression in striatal sections and on counteracting phencyclidine-induced motor activation was also demonstrated after the central coadministration of A2AR and mGluR5 agonists to rats with intact dopaminergic innervation. The results suggest that a functional mGluR5/A2AR interaction is required to overcome the well-known strong tonic inhibitory effect of dopamine on striatal adenosine A2AR function.
Adenosine is a neuromodulator that plays a very important role in basal ganglia function (1). Its actions are mediated by specific G protein-coupled receptors, which are currently classified in A1, A2A, A2B, and A3 subtypes (2). Compared with the other adenosine receptor subtypes, A2A receptors (A2ARs) are concentrated in the striatum (1, 3), where they are expressed mostly by γ-aminobutyric acid (GABA)ergic striatopallidal neurons (4). The recent ultrastructural analysis performed by Hettinger et al. (5) has demonstrated that, in the rat, A2ARs are localized mostly postsynaptically in the dendrites and dendritic spines of striatal GABAergic neurons. A2AR immunoreactivity was observed primarily at glutamatergic (asymmetric) synapses (5). Therefore, it was suggested that A2AR plays a prominent role in modulating glutamatergic input to striatal GABAergic neurons (5).
Glutamate acts on both ionotropic and metabotropic G protein-coupled receptors (mGluRs). Molecular and pharmacological characterization studies have currently divided the mGluR family into three groups (I–III) (6). Group I mGluR includes mGluR1 and mGluR5, with the latter being highly expressed in the striatum, particularly in the striatal GABAergic efferent neurons (7). In the striatopallidal complex in primates, mGluR5 showed a localization very similar to that described for A2AR in rats. Thus, mGluR5 immunoreactivity was commonly found postsynaptically and perisynaptically to asymmetric synapses (8). These studies provide a morphological basis for the possible existence of functional interactions between striatal A2AR and mGluR5. In fact, in recent in vivo microdialysis experiments we found functional evidence for the possible existence of synergistic A2AR/mGlluR5 interactions modulating the function of the GABAergic striatopallidal neurons originating in the nucleus accumbens (9). In the present study we provide evidence for the existence of A2AR/mGluR5 heteromeric complexes in membrane preparations from human embryonic kidney (HEK)-293 cells transiently cotransfected with both receptors and from rat striatum. Furthermore, the same kind of functional A2AR/mGluR5 synergistic interaction (induction of the immediate-early gene c-fos) could be demonstrated both in cotransfected cells and the rat striatum. These results suggest that A2AR/mGluR5 synergistic interactions can have important implications for striatal neuronal function and dysfunction.
Methods
Antibodies and cDNA Constructs for Experiments with HEK-293 Cells.
Primary antibodies used were: mouse anti-Flag antibody (Clone M2, Sigma); mouse anti-hemagglutinin (HA) antibody (Clone 12CA5, Roche Diagnostics); goat anti-c-Fos antibody (sc-52, Santa Cruz Biotechnology); rabbit anti-c-Fos antibody (sc-7202, Santa Cruz Biotechnology); mouse anti-β-tubulin (Clone TUB 2.1, Sigma); rabbit affinity-purified anti-mGluR1α antibody F2-Ab and rabbit affinity-purified anti-mGluR1 antibody F1-Ab (10), both antibodies showing reactivity toward mGluR5 (data not shown); rabbit anti-mGluR5 (AB5232, Chemicon); rabbit affinity-purified anti-A2AR polyclonal antibody VC21-Ab (11); rabbit anti-extracellular signal-regulated kinase (ERK) 1/2 (M-5670, Sigma); and mouse anti-phosphorylated ERK1/2 (M-8159, Sigma). Secondary antibodies used were: horseradish peroxidase-conjugate swine anti-rabbit IgG and horseradish peroxidase-conjugate swine anti-mouse IgG (Dako); fluorescein (FITC)-conjugate affinity-purified donkey anti-mouse IgG, Texas red-conjugate affinity-purified donkey anti-rabbit IgG, Cy3-conjugate affinity-purified donkey anti-mouse IgG, Cy5-conjugate affinity-purified donkey anti-rabbit IgG and Cy2-conjugate affinity-purified donkey anti-goat IgG (Jackson ImmunoResearch). The cDNAs encoding human A2AR and the rat N-terminal HA-tagged mGluR5 (mGluR5a isoform) were gifts from S. Rivkees (Yale Pediatrics, New Haven, CT) (12) and J. P. Pin (Centre National de la Recherche Scientifique-Unité Propre de Recherche 9023, Montpellier, France) (13), respectively. The Flag epitope (DYKDDDDK) was introduced into the N terminal of the A2AR, between amino acids 6 and 7, by using a PCR-based mutagenesis approach (10). The sequence of the obtained Flagged A2AR cDNA was confirmed by DNA sequencing.
Cell Cultures and Transfection.
HEK-293 cells were grown as described (10). For the transient expression of HA-mGluR5 and Flag-A2AR or Flag-mGluR1β, cells were transfected with 10 μg of cDNA encoding the human adenosine receptor and/or rat mGluR (ratio 1:1; pcDNA containing LacZ reporter was used to equilibrate the amount of total DNA) by calcium phosphate precipitation (10). Cells at either 24 or 48 h after transfection were grown in glutamate-free medium (ICN) in the absence of both glutamine and glutamate for 3 h before their use.
Immunolabeling Experiments in Transfected HEK-293 Cells.
HEK-293 cells were fixed in 4% paraformaldehyde for 15 min and washed with PBS containing 20 mM glycine (buffer A) to quench the aldehyde groups. Where indicated, cells were permeabilized with buffer A containing 0.2% Triton X-100 for 5 min. Cells were treated with PBS containing 1% BSA (buffer B). After 1 h at room temperature, cells were labeled with the indicated primary antibody for 1 h, washed, and stained with the indicated secondary antibody. Coverslips were rinsed for 30 min in buffer B and mounted with Immuno Fluoro mounting medium (ICN). Confocal microscope observations were made with a Leica TCS-SP (Leica Lasertechnik, Heidelberg) confocal scanning laser microscope adapted to an inverted Leitz DMIRBE microscope.
Coimmunoprecipitation Experiments.
Transfected HEK-293 cells or rat striatal membranes were solubilized in ice-cold lysis buffer [PBS, pH 7.4, containing 1% (vol/vol) Nonidet P-40] (Calbiochem) for 1 h on ice and centrifuged at 80,000 × g for 90 min. The supernatant (1 mg protein/ml) was processed for immunoprecipitation as described (10) with the mouse anti-Flag, anti-HA antibodies (2 μg/ml) or VC21-Ab (2 μg/ml). Immune complexes were dissociated in SDS-polyacrylamide gel sample buffer by heating to 37°C for 1 h, and resolved by SDS/PAGE in 7% gels (10, 14, 15). Proteins were transferred to poly(vinylidene difluoride) membranes (Immobilon-P, Millipore) by using a semidry transfer system and immunoblotted by using the primary antibodies indicated and horseradish peroxidase-conjugated swine anti-rabbit IgG as a secondary antibody. The immunoreactive bands were developed with the enhanced chemiluminiscence detection kit (Pierce SuperSignal), as described (14, 15).
Intracellular Calcium and cAMP Measurements.
For calcium determination, transiently transfected HEK-293 cells (106 cells per ml) were loaded with 5 μM Fura-2/AM for 30 min at 37°C. Cells were washed and subsequently incubated in Hanks's balanced salt solution (GIBCO) containing 0.2 units/ml adenosine deaminase. Calcium peak induction was achieved by the addition of the group I mGluR agonist quisqualic acid (Tocris Neuramin, Bristol, U.K.) and/or the selective A2AR agonist CGS 21680 (Sigma). Intracellular calcium was determined at 37°C in a dual-wavelength Shimadzu RF-5000 spectrofluorophotometer as described (10). The accumulation of cAMP was measured by a cAMP[3H] assay system (Amersham Pharmacia) as described in the manufacturer's manual, with transfected HEK-293 cells (2 × 106 cells/sample) in serum-free DMEM (GIBCO) preincubated with 50 μM Ro 20–1724 (Calbiochem) for 10 min and then stimulated with the indicated concentrations of agonists for 15 min.
ERK Assay.
Transfected HEK-293 cells were serum-starved for 24 h before stimulation with CGS 21680 (200 nM) and/or quisqualic acid (100 μM) for 5 min in the presence or absence of the ERK 1/2 kinase inhibitor PD98059 (75 nM; Calbiochem). After treatment, cells were washed with ice-cold PBS and scraped into 1 ml of lysis buffer containing 1% Triton X-100, 50 mM Tris⋅HCl, pH 7.6, 45 mM B-glycerophosphate, 50 mM NaF, 1 mM NaVO4, and protease inhibitors. Lysed cells were centrifugated for 1 min at 14,000 rpm at 4°C and equal protein concentrations were resolved on SDS-10% polyacrylamide gel, blotted onto inmobilon-P membrane, and incubated with rabbit anti-ERK1/2 (1/40,000) or mouse anti-phospho-ERK1/2 (1/10,000). Quantitative analysis of detected bands was performed by densitometric scanning.
Nuclear c-Fos Protein Levels.
Transiently transfected HEK-293 cells treated or not with CGS 21680 and quisqualic acid were chilled down and washed with ice-cold PBS and once with hypotonic buffer A (10 mM Hepes, pH 7.9/10 mM KCl/0.1 mM EDTA/0.1 mM EGTA/1 mM DTT). Cells were allowed to swell in 1 ml of buffer A for 20 min and 60 μl of a 10% solution of Nonidet P-40 (Calbiochem) was added and then vortexed for 10 s. The crude extract was centrifuged for 5 min at 4,000 rpm in a microfuge and the pellet (nuclei) was dissolved in SDS/PAGE sample buffer. Nuclei and crude extracts were analyzed by SDS/PAGE and immunoblotted by using rabbit anti-c-Fos (1/500) and mouse anti-β-tubulin (1/500), respectively. The intensities of the immunoreactive bands on x-ray film corresponding to c-Fos protein were measured by densitometric scanning. All values were normalized by using tubulin as a control protein.
Phencyclidine (PCP)-Induced Motor Activity in Rats.
Male Sprague–Dawley rats weighing 300–350 g were used in all experiments. The animals were stereotaxically implanted with stainless steel guide cannulae (22 gauge, Pastic ONE) in the right ventricle (ICV administration) under Equithesin (sodium pentobarbital, National Institute on Drug Abuse Pharmacy) anesthesia (coordinates with respect to bregma: A −0.8, L −1.3, V −4.5). Guide cannulae were fixed with dental acrylic and stainless steel screws to the skull surface. Stainless steel stylets were inserted into the cannulae to prevent occlusion. A recovery period of 6–7 days was allowed before testing. For ICV administration injection needles (28 gauge) extending 0.5 mm below the guide were inserted into the cannulae. CGS 21680 (3 μg), (RS)-chloro-5-hydroxyphenylglycine (CHPG, Tocris; 9 μg), or saline were administered ICV at a rate of 1 μl/min by means of a microdrive pump (final injection volume, 10 μl). The doses of the A2AR and mGluR5 agonists were chosen according to published studies (16, 17). The needle was then left in place for an additional 5 min. Ten minutes after ICV administration, PCP (5 mg/kg i.p.) was administered to habituated rats (30 min of habituation to the actimeters) and motor counts were automatically recorded (Automex II, Columbus Instruments, Columbus, OH) for 60 min. Correct cannula placement was ascertained immediately after the animal's death (overdose of Equithesin) by injecting ink to the ventricular system.
c-Fos Immunohistochemistry in Rat Brain Sections.
Two hours after the administration of drugs, rats were anesthetized with Equithesin (sodium pentobarbital, National Institute on Drug Abuse Pharmacy) and perfused intracardially with 0.1 M PBS followed by 4% paraformaldehyde in 0.1 M sodium phosphate monobasic (pH = 7.4). Brains were postfixed in the same fixative for 2 h and immersed in 20% sucrose/0.01 M sodium phosphate (pH = 7.4) solution for 48 h at 4°C. The brains were frozen rapidly in dry ice and stored at −80°C until sectioning. Thirty-micrometer sections were cut on the cryostat, collected in PBS buffer, and incubated in blocking buffer (PBS/0.3% Triton/3% BSA) for 60 min before incubation in rabbit anti-c-Fos-antibody (1:1,000 dilution; sc-7202; Santa Cruz Biotechnology) in blocking buffer for 48 h at 4°C. Sections were washed and incubated in 1:200 goat anti-rabbit antibody (Vector Laboratories) for 1 h, then washed and incubated for 1 h in ABC reagent (Vector Laboratories; PK-4000), washed again, treated with 0.33 mg/ml 3,3′-diaminobenzidine and 0.01% peroxide for 6 min, transferred to PBS, and mounted onto chromalum gelatin-coated slides, air-dried, dehydrated through graded ethanols, cleared in Citrasolv (Fisher Scientific), and coverslipped with Permount (Sigma). Bright-field images of the caudate putamen, nucleus accumbens, and the cingulate cortex were digitally captured by using a Photometrics Coolsnap charge-coupled device camera (Photometrics, Tucson, AZ) attached to a Zeiss Axioskop two-light microscope with a ×5 objective. The images were digitized and the number of c-Fos-immunoreactive nuclei were counted by using IPLAB 3.5 software (Scanalytics, Billerica, MA).
Results
Complexes Containing A2AR and mGluR5 in Cotransfected HEK-293 Cells.
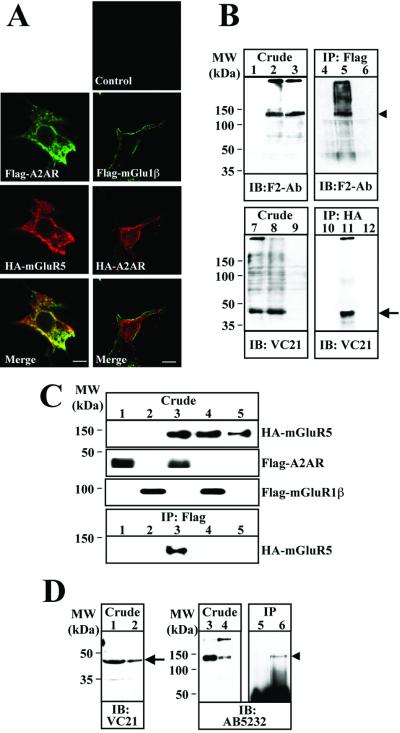
By confocal microscopy analysis of nonpermeabilized HEK-293 cells transiently transfected with the cDNAs for Flag-A2AR and HA-mGluR5 some overlapping in the distribution of the two proteins was found (Fig. 1A). This colocalization was specific because it was not found in cells cotransfected with HA-A2AR and Flag-mGluR1β, a related G protein-coupled receptor (Fig. 1A). The existence of A2AR/mGluR5 heteromeric complexes was subsequently assayed by coimmunoprecipitation experiments. From cotransfected HEK-293 cell extracts the mouse anti-Flag antibody against Flag-A2AR coimmunoprecipitated a band of ≈120 kDa, which corresponds to the HA-mGlu5 receptor (Fig. 1B, lane 5). This band did not appear in immunoprecipitates from cells transfected with only one cDNA (Fig. 1B, lanes 4 and 6) or when an irrelevant antibody was used (data not shown). Similarly, in cotransfected cells the analysis by SDS/PAGE and immunoblotting with anti-A2AR antibodies of immunoprecipitates obtained with mouse anti-HA showed a band of ≈45 kDa, which corresponds to the A2AR (Fig. 1B, lane 11). As a control no immunoprecipitation of HA-mGlu5 receptor was obtained from cells cotransfected with HA-mGluR5 and Flag-mGluR1β, when Flag antibody was used (Fig. 1C, lane 4). The physiological significance of coimmunoprecipitation is strengthened by the fact that it also occurred with native receptors from rat striatum. Immunoprecipitates obtained with an antibody anti-A2AR coimmunoprecipitated a ≈120-kDa band corresponding to mGluR5 (Fig. 1D, lane 6).
Figure 1.
Heteromerization of A2AR and mGluR5 in A2AR/mGluR5-transfected HEK-293 cells. (A) Immunofluorescence localization of Flag-A2AR and HA-mGluR5 in HEK-293 cells. Cells were immunostained with mouse anti-Flag mAb (10 μg/ml), rabbit F2-Ab (5 μg/ml), or VC21 (5 μg/ml). The bound primary antibodies were detected by using either fluorescein (green)-conjugated donkey anti-mouse IgG antibody (1/100) or Texas red-conjugated donkey anti-rabbit (1/100). Cells were analyzed by double immunofluorescence with confocal microscopy. Superimposition of images reveals A2AR/mGluR5 colocalization in yellow (Merge). (Scale bar: 10 μm.) (B) Coimmunoprecipitation of A2AR and mGluR5 in HEK-293 cells. Cells transiently expressing Flag-A2AR alone (lanes 1 and 7), Flag-A2AR plus HA-mGluR5 (lanes 2 and 8), or HA-mGluR5 (lanes 3 and 9) were washed, solubilized, and processed for immunoprecipitation by using anti-Flag mAb (2 μg/ml; lanes 4–6) and anti-HA mAb (2 μg/ml; lanes 10–12). Solubilized membranes (Crude) and immunoprecipitates (IP) were analyzed by SDS/PAGE and immunoblotted by using rabbit F2-Ab (5 μg/ml) (similar results were obtained by using AB5232 or anti-HA antibodies, data not shown) or rabbit anti-A2AR antibody (VC21-Ab; 10 μg/ml) and horseradish peroxidase-conjugated swine anti-rabbit IgG as a secondary antibody. (C) Lack of coimmunoprecipitation of mGluR5 and mGluR1β in HEK-293 cells. Cells transiently expressing Flag-A2AR (lane 1), Flag-mGluR1β (lane 2), Flag-A2AR plus HA-mGluR5 (lane 3), Flag-mGluR1β plus HA-mGluR5 (lane 4), and HA-mGluR5 alone (lane 5) were processed for immunoprecipitation by using anti-Flag antibody (2 μg/ml). Solubilized membranes (Crude) were analyzed by SDS/PAGE and immunoblotted by using F1-Ab to detect HA-mGluR5 and Flag-mGlu1β, and VC21-Ab to detect Flag-A2AR. Immunoprecipitates were immunoblotted by using F2-Ab to detect HA-mGluR5. (D) Coimmunoprecipitation of A2AR and mGluR5 in rat striatum. Rat striatum membranes were solubilized and processed for immunoprecipi- tation by using anti-A2A antibody (VC21-Ab, 2 μg/ml) (lanes 5 and 6). Solubilized membranes (Crude) from HEK transfected with Flag-A2AR (lane 1), HA-mGluR5 (lane 3), and rat striatum (lanes 2 and 4) and immunoprecipitates (IP) (lanes 5 and 6) were analyzed by SDS/PAGE and immunoblotted with VC21-Ab or AB5232. In B and D the positions of A2AR and mGluR5 are indicated by an arrow and arrow head, respectively.
Synergism on Signaling in Cotransfected HEK-293 Cells.
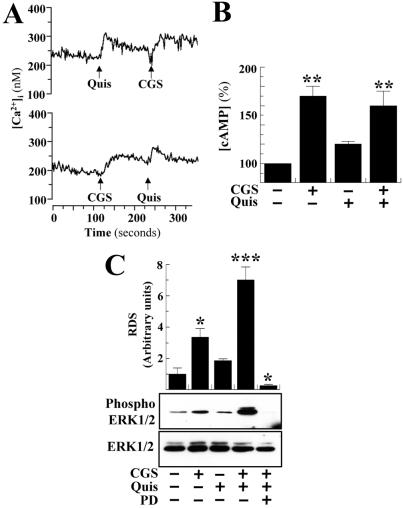
In HEK cells transiently expressing Flag-A2AR plus HA-mGluR5 (receptor densities were controlled by means of Western blotting) both quisqualic acid (100 μM) and CGS 21680 (200 nM) mobilized intracellular calcium (Fig. 2A). Preincubation with quisqualic acid or CGS 21680 did not change the calcium peak obtained in response to A2AR or mGluR5 activation, respectively (Fig. 2A). The cotransfected cells showed a nonsignificant increase in cAMP accumulation when treated with quisqualic acid (100 μM) (Fig. 2B) and a significant stimulation of adenylyl cyclase activity when treated with CGS 21680 (200 nM) (Fig. 2B). The effect induced by the A2AR agonist was not significantly modified by concomitant treatment with quisqualic acid (Fig. 2B). Although a lack of A2AR/mGluR5 synergism was observed at the second messenger level, a synergism on mitogen-activated protein kinase (MAPK) pathway was found. Treatment with quisqualic acid (100 μM) produced a nonsignificant increase in ERK1/2 phosphorylation (Fig. 2C). On the other hand, CGS 21680 (200 nM) induced a significant ERK1/2 phosphorylation, which was synergistically potentiated by concomitant mGluR5 activation (Fig. 2C). The effect of quisqualic acid plus CGS 21680 was completely reverted by PD98059, an inhibitor of the ERK1/2 kinase (Fig. 2C). Quisqualic acid or CGS 21680 failed to provide any signal in nontransfected HEK-293 cells (data not shown).
Figure 2.
(A) Ca2+ mobilization, (B) cAMP accumulation, and (C) ERK phosphorylation in A2AR/mGluR5 transiently cotransfected HEK-293 cells. (A) Cells transiently expressing Flag-A2AR plus HA-mGluR5 were preincubated with 0.2 unit/ml adenosine deaminase for 30 min and loaded with FURA-2/AM, and the intracellular Ca2+ mobilization was determined after stimulation with quisqualic acid (100 μM, Quis) or CGS21680 (200 nM, CGS). (B) Cells were preincubated with 0.2 units/ml adenosine deaminase for 30 min and stimulated during 15 min with 100 μM quisqualic acid (Quis) and/or 200 nM CGS21680 (CGS) and the cAMP levels were determined. All values are normalized by using the nontreated transfected cells. The results are expressed as means + SEM (in percent of the values obtained in nontreated transfected cells) of three independent experiments performed in duplicate. Raw data from the different groups analyzed with repeated measures ANOVA followed by Newman–Keuls post hoc comparisons. **, P < 0.01 vs. nontreated transfected cells. (C) Serum-starved cells were preincubated with adenosine deaminase and stimulated with CGS 2160 (200 nM) and/or quisqualic acid (100 μM) for 5 min in the presence or absence of PD98059 (75 nM). The phosphorylation of ERK1/2 was determined by immunoblotting (see Methods). (Lower) Representative ERK assay; (Upper) the intensities of the immunoreactive bands on x-ray film corresponding to ERK1/2 and phospho ERK1/2 protein were measured by densitometric scanning. All values of phosphorylated ERK1/2 were normalized by using ERK1/2 and expressed as means + SEM (in relative densitometric scanning obtained in nontreated transfected cells) of three independent experiments. ANOVA followed by Newman–Keuls post hoc comparisons. * and ***, P < 0.05 and P < 0.001, respectively, versus nontreated transfected cells.
Synergism on c-Fos Expression in Cotransfected HEK-293 Cells.
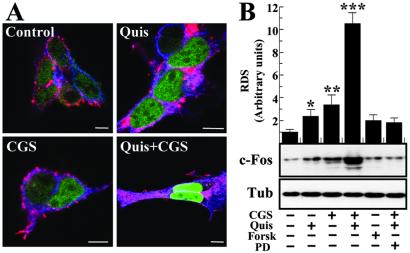
The expression of the immediate-early gene c-fos has been shown to be strongly regulated by the MAPK (ERK1/2) pathway (see ref. 18). An overlap in the distribution of the two proteins was found by confocal microscopy analysis of HEK-293 cells transiently expressing Flag-A2AR and HA-mGluR5 (Fig. 3A; purple staining in all four panels). In nontreated cells a low expression of c-Fos (green) in the nuclei was observed. In cells incubated with either quisqualic acid (100 μM) or CGS 21680 (200 nM) a small increase of c-Fos levels in the nuclei was observed. In cells treated simultaneously with both agonists a large increase in the expression of nuclear c-Fos was obtained (Fig. 3A). Quantitation of c-Fos immunoreactivity was performed by nuclear extraction, immunoblotting, and densitometric scanning of the immunoreactive band on the film. As observed in Fig. 3B, quisqualic acid (100 μM) or CGS 21680 (200 nM) induced a small but significant increase (2- and 3-fold with respect to untreated cells) of c-Fos in the nuclei. The increase in the amount of c-Fos in cells treated with quisqualic acid plus CGS 21680 was more pronounced, about 10-fold higher than in nontreated cells. This increase was not reproduced by forskolin and, more interesting, it was completely reverted by the ERK 1/2 kinase inhibitor PD98059, corroborating that the synergism on c-Fos is mediated by MAPK activation (Fig. 3B).
Figure 3.
c-Fos expression in A2AR/mGluR5-transfected HEK-293 cells. (A) Immunofluorescence localization of A2AR, mGluR5, and c-Fos protein. Cells were preincubated with 0.2 units/ml adenosine deaminase for 30 min and treated 1 h with vehicle, quisqualic acid (100 μM) (Quis), CGS21680 (200 nM) (CGS), and both agonists. Cells were washed, fixed, permeabilized, and processed for immunostaining with mouse anti-HA mAb (10 μg/ml), rabbit F2-Ab (5 μg/ml), and goat anti-c-Fos (1/50). The bound primary antibodies were detected by using Cy3-conjugated donkey anti-mouse (1/200), Cy5-conjugated donkey anti-rabbit (1/200), and Cy2-conjugated donkey anti-goat (1/200) IgG antibodies. Cells were analyzed by triple immunofluorescence with a confocal microscope. Images show mGluR5 in blue, A2AR in red, c-Fos protein in green, and A2AR/mGluR5 colocalization in purple. (Scale bar: 10 μm.) (B) c-Fos protein levels in the nuclei of HEK-293 cells. Cells were treated with vehicle, quisqualic acid (100 μM) (Quis), CGS21680 (200 nM) (CGS), or forskolin (1 μM) in the presence or absence of PD98059 (75 nM). After 1 h nuclear fractionation was performed. The nuclear fraction (Nuclei) and crude extract (Total Extract) were analyzed by SDS/PAGE and immunoblotted by using rabbit anti-c-Fos (1/500) and mouse anti-β-tubulin (1/500), respectively. (C) The intensities of the immunoreactive bands on x-ray film corresponding to c-Fos protein were measured by densitometric scanning. All values are normalized by using tubulin as a control protein. The results are presented as means + SEM (in % of the relative densitometric scanning values obtained in nontreated transfected cells) of three independent experiments. ANOVA followed by Newman–Keuls post hoc comparisons. ***, P < 0.001 vs. nontreated transfected cells and vs. cells treated with quisqualate or CGS 21680. * and **, P < 0.05 and P < 0.01, respectively, vs. nontreated transfected cells.
Synergism on Phencyclidine-Induced Motor Activity in Rats and on the Rat Striatal Expression of c-fos.
In previous studies we found that the systemic administration of CGS 21680 counteracts the motor-activating effects of psychostimulants, like amphetamine and PCP (19). In the present work, to avoid peripheral side effects, we studied the effect of the central administration of CGS 21680 (3 μg/10 μl, ICV) and/or CHPG (9 μg/10 μl, ICV) on the motor activity induced by a relatively high dose of PCP (0.5 mg/kg, i.p.). PCP produced a significant increase (ANOVA with Newman–Keuls post hoc test, P < 0.01) in motor activity (3,441 ± 386 counts per 60 min; n = 12) compared with the control group (1,201 ± 203 counts per 60 min; n = 8). Neither CGS 21680 nor CHPG alone significantly influenced PCP-induced increase in motor activity (2,630 ± 473 and 3,135 ± 389 counts per 60 min, respectively; n = 9 in both groups). However, a very significant counteracting effect (ANOVA, P < 0.001) was observed after their coadministration (1,788 ± 272 counts per 60 min; n = 11). The same protocol of CGS 21680 and CHPG administration that allowed the finding of a synergism in the experiments on PCP-induced motor activity was applied to investigate the existence of an A2A/mGluR5 synergism at the level of striatal c-fos expression. Initial microscopic examination showed some c-Fos immunoreactivity immediately adjacent to the lateral ventricle ipsilateral to the implanted cannula in all groups, probably as a nonspecific reaction. Therefore, quantification of c-Fos-positive nuclei was performed in the contralateral hemisphere (Fig. 4A). Neither CGS 21680 (3 μg/10 μl, ICV), nor CHPG (9 μg/10 μl, ICV) produced any significant increase in the number of c-Fos-positive nuclei in the cingulate cortex, the caudate putamen, or the nucleus accumbens (Fig. 4C). However, coadministration of the A2AR and the mGluR5 agonists induced an increase in the number of c-Fos-positive nuclei in the striatum, which was highly significant in the nucleus accumbens (Fig. 4 B and C).
Figure 4.
Rat striatal expression of c-fos. (A) Scheme of the rat brain sections showing the level of cannula implantation for ICV administration of CGS 21680 and CHPG and the level of the brain areas analyzed for c-Fos immunohistochemistry (cingulate cortex, caudate putamen, and nucleus accumbens). (B) (Left) Photomicrograph of the nucleus accumbens of a rat treated with CGS 2160 (3 μg, ICV) plus CHPG (9 μg, ICV); the encircled area corresponds to the area analyzed for quantification of c-Fos nuclei. (Right) Magnification of the rectangular area shown Left, showing c-Fos-positive nuclei (black dots). (C) Quantification of c-Fos immunoreactivity from the cingulate cortex (CgC) (triangular area in A), the caudate putamen (CPu) (rectangular area in A), and the nucleus accumbens (NAc) (elliptic area in A) of rats treated as indicated above. The results are expressed as means + SEM (n = 5–6 per group) of the total number of c-Fos-positive nuclei. ANOVA followed by Newman–Keuls post hoc comparisons were used for statistical comparisons. ***, P < 0.001 vs. control group.
Discussion
Evidence for the existence of G protein-coupled receptor homomerization and heteromerization is growing continuously (20). We recently reported the formation of functional heteromeric complexes between adenosine A1 receptors and dopamine D1 receptors (14), adenosine A1 and mGluR1α receptors (10), and A2AR and dopamine D2 receptors (D2R) (15). In the present study, results obtained from confocal laser microscopy experiments in nonpermeabilized cotransfected HEK-293 cells showed that A2AR and mGluR5 can be colocalized in some membrane domains. This colocalization is a prerequisite for the formation of A2AR/mGluR5 heteromeric complexes at the membrane level. The results obtained from coimmunoprecipitation experiments from membrane preparations of cotransfected HEK-293 cells or rat striatum strongly suggest that A2AR and mGluR5 can also form heteromeric complexes. It remains to be resolved whether A2AR and mGluR5 are physically associated by means of direct protein–protein interactions (heterodimers) or they are linked by additional cytosolic proteins, such as Homer proteins and the Shank family of scaffold proteins, which have been recently shown to be involved in the coupling between mGluR5 and N-methyl-D-aspartate receptors (21).
The existence of A2AR/mGluR5 heteromers provides the possibility for close cross-talk between A2AR and mGluR5, which would synchronize A2AR and mGluR5 activation to produce synergistic effects at the cellular level. The most studied signal transduction pathways used by A2AR and mGluR5 depend on the activation of adenylyl cyclase (by means of Gs/Golf coupling) and phospholipase C (by means of Gq proteins), respectively (1, 6). As described for other cell lines (22), in A2A/mGluR5-cotransfected cells, the A2AR agonist CGS 21680 produced a significant increase in intracellular cAMP accumulation, which was not modified by the stimulation of mGluR5 with quisqualate. As expected, stimulation of mGluR5 induced an increase in intracellular Ca2+ concentration ([Ca2+]i) in A2AR/mGluR5 cotransfected cells. In addition, the A2AR agonist CGS 21680 produced a significant increase in [Ca2+]i, which agrees with recent studies in rat striatal slices that suggest that A2AR also uses phospholipase C/inositol triphosphate signaling (23). However, no synergistic A2AR/mGluR5 interaction was observed at the level of [Ca2+]i.
The lack of synergistic interaction between A2AR/mGluR5 at the second messenger level (cAMP and Ca2+) contrasted with the strong synergism found at the MAPK pathway level and at the level of the expression of the immediate-early gene c-fos. This synergism could be caused by cross-talk between independent signaling pathways converging at the level of MAPK cascade (24). Furthermore, other levels of interaction upstream to MAPK could be the nonreceptor tyrosine kinase src or other enzymes recently demonstrated to be activated by G protein-coupled receptors through G protein-independent signaling (25).
The results obtained after the administration of a selective mGluR5 agonist plus a selective A2AR agonist in rats suggest that a synergistic A2AR/mGluR5 interaction similar to that observed in cotransfected cells also occurs in the striatum. The preferential effect in the ventral striatum (nucleus accumbens) compared with the dorsal striatum (caudate putamen) agrees with studies showing a more powerful effect of CGS 21680 in the ventral striatum (see ref. 19). Furthermore, these results also agree with results obtained in recent microdialysis experiments on the ventral GABAergic striatopallidal neurons (9). Striatal A2AR seems to be involved in the increased striatal c-fos expression induced by the systemic administration of antipsychotics and D2R antagonists (26, 27). This effect occurs in the GABAergic striopallidal neurons, which are tonically inhibited by dopamine acting on D2R (28). It is well known that D2R stimulation strongly counteracts the effects induced by A2AR stimulation, which includes induction of c-fos expression (1, 22). In fact, A2AR-mediated striatal c-fos expression has only been shown to be clearly induced when interrupting D2R signaling (27, 29). The results presented here show that the c-fos induction by A2AR activation is very poor but markedly increases when mGluR5 is also activated, which strongly suggest that concomitant stimulation of A2AR and mGluR5 receptors is one of the mechanisms by which A2AR stimulation can overcome the tonic inhibitory effect of dopamine and induce striatal c-fos expression. We favor the idea that this mechanism can take place under conditions of intense glutamatergic neurotransmission, which is known to induce adenosine release, most probably, because of the neuronal metabolic demand imposed by the increased excitatory input (see ref. 30). The results obtained from the experiments with PCP agree with this hypothesis and suggest that costimulation of A2AR and mGluR5 can even counteract the effects induced by a strong stimulation of dopaminergic neurotransmission. The motor hyperactivity induced by PCP depends on postsynaptic D2R, and a high degree of D2R blockade is required to significantly counteract the stimulatory action of high doses of PCP (31).
Given the key role of the MAPK cascade and immediate-early genes in the coupling of early neuronal responses to long-term adaptive changes (18, 24, 32), the present results suggest that A2AR/mGluR5 interactions are involved in striatal neuronal plasticity. More specifically, this mechanism may underlie the recently described dopamine-independent increased c-fos expression in the striopallidal neurons associated with sensitization to psychostimulants (33). In fact, both striatal A2AR and mGluR5 have recently been shown to be involved in certain forms of striatal plasticity, including corticostriatal long-term potentiation and long-term depression (34, 35).
Acknowledgments
We acknowledge the technical help received from Susana Castel (confocal microscopy section). F.C. currently holds a research contract with the Catalan Commission for Research and Technological Innovation (CIRIT).
Abbreviations
- A2AR
adenosine A2A receptor
- mGluR
metabotropic glutamate receptor
- HA
hemagglutinin
- PCP
phencyclidine
- D2R
dopamine D2 receptor
- ERK
extracellular signal-regulated kinase
- CHPG
(RS)-chloro-5-hydroxyphenylglycine
- MAPK
mitogen-activated protein kinase
- GABA
γ-aminobutyric acid
- HEK
human embryonic kidney
References
- 1.Ferré S, Fredholm B B, Morelli M, Popoli P, Fuxe K. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm B B, Ijzerman A P, Jacobson K A, Klotz K-N, Linden J. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 3.Rosin D L, Robeva A, Woodard R L, Guyenet P G, Linden J. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- 4.Schiffmann S N, Jacobs O, Vanderhaeghen J-J. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- 5.Hettinger B D, Lee A, Linden J, Rosin D L. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Hermans E, Challis R A. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Testa C M, Standaert D G, Landwehrmeyer G B, Penney J B, Young A B. J Comp Neurol. 1995;354:241–252. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- 8.Smith Y, Charara A, Hanson J E, Paquet M, Levey A I. J Anat. 2000;196:555–576. doi: 10.1046/j.1469-7580.2000.19640555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Díaz-Cabiale Z, Vivó M, Del Arco A, O'Connor W T, Harte M K, Müller C E, Martínez E, Popoli P, Fuxe K, Ferré S. Neurosci Lett. 2002;324:154–158. doi: 10.1016/s0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 10.Ciruela F, Escriche M, Burgueno J, Angulo E, Casado V, Soloviev M M, Canela E I, Mallol J, Chan W Y, Lluis C, et al. J Biol Chem. 2001;276:18345–18351. doi: 10.1074/jbc.M006960200. [DOI] [PubMed] [Google Scholar]
- 11.Sarrio S, Casado V, Escriche M, Ciruela F, Mallol J, Canela E I, Lluis C, Franco R. Mol Cell Biol. 2000;20:5164–5174. doi: 10.1128/mcb.20.14.5164-5174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivkees S A, Lasbury M E, Barbhaiya H. J Biol Chem. 1995;35:20485–20490. doi: 10.1074/jbc.270.35.20485. [DOI] [PubMed] [Google Scholar]
- 13.Ango F, Albani-Torregrossa S, Joly C, Robbe D, Michel J-M, Pin J-P, Bockaert J, Fagni L. Neuropharmacology. 1999;38:793–803. doi: 10.1016/s0028-3908(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 14.Ginés S, Hillion J, Torvinen M, Le Crom S, Casado V, Canela E I, Rondin S, Lew J Y, Watson S, Zoli M, et al. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah M E, Mallol J, et al. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 16.Ferré S, Rubio A, Fuxe K. Neurosci Lett. 1991;130:162–164. doi: 10.1016/0304-3940(91)90387-9. [DOI] [PubMed] [Google Scholar]
- 17.Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, Fuxe K, Ferré S. Neuropsychopharmacology. 2001;25:505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 18.Valjent E, Caboche J, Vanhoutte P. Mol Neurobiol. 2001;23:85–101. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- 19.Ferré S. Psychopharmacology. 1997;17:82–91. [Google Scholar]
- 20.Bouvier M. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- 21.Sheng M, Kim E. J Cell Sci. 2000;113:1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 22.Kull B, Ferré S, Arslan G, Svenningsson P, Fuxe K, Owman C, Fredholm B B. Biochem Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 23.Wirkner K, Assmann H, Koles L, Gerevich Z, Franke H, Norenberg W, Boehm R, Illes P. Br J Pharmacol. 2000;130:259–269. doi: 10.1038/sj.bjp.0703234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweatt J D. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 25.Heuss C, Gerber U. Trends Neurol Sci. 2000;23:469–475. doi: 10.1016/s0166-2236(00)01643-x. [DOI] [PubMed] [Google Scholar]
- 26.Boegman R J, Vincent S R. Synapse. 1996;22:70–77. doi: 10.1002/(SICI)1098-2396(199601)22:1<70::AID-SYN8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 27.Pinna A, Wardas J, Cozzolino A, Morelli M. Neuropsychopharmacology. 1999;20:44–51. doi: 10.1016/S0893-133X(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 28.Robertson G S, Vincent S R, Fibiger H C. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 29.Morelli M, Pinna A, Wardas J, Di Chiara G. Neuroscience. 1995;67:49–55. doi: 10.1016/0306-4522(94)00602-2. [DOI] [PubMed] [Google Scholar]
- 30.Ferré S, Fuxe K. Prog Brain Res. 2000;125:353–361. doi: 10.1016/S0079-6123(00)25024-3. [DOI] [PubMed] [Google Scholar]
- 31.Ogren S O, Goldstein M. Neuropsychopharmacology. 1994;11:167–177. doi: 10.1038/sj.npp.1380103. [DOI] [PubMed] [Google Scholar]
- 32.Berke J D, Hyman S E. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 33.Uslaner J, Badiani A, Norton C S, Day H E, Watson S J, Akil H, Robinson T E. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 34.D'Alcantara P, Ledent C, Swillens S, Schiffmann S N. Neuroscience. 2001;107:455–464. doi: 10.1016/s0306-4522(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 35.Sung K-W, Choi S, Lovinger D M. J Neurophysiol. 2001;86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]