Abstract
Vascular endothelial growth factor (VEGF) is an angiogenic protein with neurotrophic and neuroprotective effects. Because VEGF promotes the proliferation of vascular endothelial cells, we examined the possibility that it also stimulates the proliferation of neuronal precursors in murine cerebral cortical cultures and in adult rat brain in vivo. VEGF (>10 ng/ml) stimulated 5-bromo-2′-deoxyuridine (BrdUrd) incorporation into cells that expressed immature neuronal marker proteins and increased cell number in cultures by 20–30%. Cultured cells labeled by BrdUrd expressed VEGFR2/Flk-1, but not VEGFR1/Flt-1 receptors, and the effect of VEGF was blocked by the VEGFR2/Flk-1 receptor tyrosine kinase inhibitor SU1498. Intracerebroventricular administration of VEGF into rat brain increased BrdUrd labeling of cells in the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), where VEGFR2/Flk-1 was colocalized with the immature neuronal marker, doublecortin (Dcx). The increase in BrdUrd labeling after the administration of VEGF was caused by an increase in cell proliferation, rather than a decrease in cell death, because VEGF did not reduce caspase-3 cleavage in SVZ or SGZ. Cells labeled with BrdUrd after VEGF treatment in vivo include immature and mature neurons, astroglia, and endothelial cells. These findings implicate the angiogenesis factor VEGF in neurogenesis as well.
Vascular endothelial growth factor (VEGF) is a hypoxia-inducible secreted protein that interacts with receptor tyrosine kinases on endothelial cells to promote angiogenesis. Recent evidence indicates that VEGF can also act directly on neurons to produce neurotrophic and neuroprotective effects. For example, VEGF stimulates axonal outgrowth and improves the survival of cultured superior cervical and dorsal root ganglion neurons (1, 2), enhances the survival of mesencephalic neurons in organotypic explant cultures (3), protects HN33 (mouse hippocampal neuron × neuroblastoma) cells from death induced by serum withdrawal (4), reduces hypoxic death of HN33 cells and cultured cerebral cortical neurons (5), and protects cultured hippocampal neurons from glutamate toxicity (6). Conversely, inhibition of VEGF signaling leads to apoptosis in cortical neuron cultures (7), and deletion of the hypoxia-response element from the VEGF promoter causes motor-neuron degeneration in mice (8), perhaps because of loss of a direct neurotrophic effect of VEGF.
Neurogenesis, the process through which precursor cells differentiate toward a mature neuronal phenotype, persists in discrete regions of the adult brain, including the rostral subventricular zone (SVZ) (9, 10) and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) (11). Comparatively little is known regarding the possible role of VEGF in adult neurogenesis, although other growth factors, including epidermal growth factor (EGF) (12), fibroblast growth factor-2 (FGF-2) (13), brain-derived neurotrophic factor (BDNF) (14), and erythropoietin (15), have been implicated. In addition, the VEGF receptor Flk-1 is expressed in neural progenitor cells of the mouse retina (16), and its activation stimulates their differentiation into amacrine neurons and photoreceptor cells (17). Finally, neurogenesis in the adult SGZ appears to occur in intimate association with angiogenesis, suggesting that common factors, such as VEGF, might be involved in both processes (18).
To investigate the possibility that VEGF is a neurogenic as well as an angiogenic factor, we examined its effects in in vitro and in vivo models of neurogenesis, by using the cell-proliferation marker 5-bromodeoxyuridine (BrdUrd). The in vitro model employs primary cultures of embryonic rat cortical neurons, and has been used to identify growth factors involved in hypoxia-induced neurogenesis, including heparin-binding EGF (19). The in vivo model involves intracerebroventricular (i.c.v.) administration of growth factors in the adult rat, and has been used to study the induction of neurogenesis in the brain in vivo by factors such as EGF, FGF-2, and BDNF (20–22).
Materials and Methods
Materials.
VEGF, BrdUrd 5′-monophosphate sodium salt, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were from Sigma, and SU1498 was from Calbiochem–Novabiochem.
Cell Culture.
Animal studies were approved by the local Institutional Animal Care and Use Committee. Cortical cultures enriched in cells of neuronal lineage were prepared from 16-day Charles River CD1 mouse embryos as described (5), except that Neurobasal medium containing 1× B27 supplement, 2 mM glutamate, and 1% penicillin and streptomycin was used (23). After 4 days, one-half of the medium was replaced with Neurobasal medium containing 2% B27, and experiments were conducted at 5 days in vitro.
BrdUrd Labeling in Vitro.
BrdUrd 5′-monophosphate (50 μg/ml) was added to cultures for 24 h. For immunocytochemistry, cultures were fixed in 4% paraformaldehyde in PBS (pH 7.5) for 40 min, washed with PBS, incubated for 2 h with blocking buffer (2% horse serum/1% BSA/0.1% Triton X-100 in PBS, pH 7.5), incubated overnight at 4°C with mouse monoclonal anti-BrdUrd (Roche Applied Science; 2 μg/ml), and then incubated for 1 h at room temperature with rhodamine-conjugated goat anti-mouse IgG (Jackson ImmunoResearch; 1:200). BrdUrd-immunoreactive cells were counted in five 200× microscope fields per well (at the12-, 3-, 6-, and 9-o'clock positions and in the center). In some experiments, BrdUrd incorporation was measured with a commercially available enzyme-linked immunosorbent assay kit (Roche Applied Science).
Cell Viability.
Cell viability was assayed by MTT absorbance and by counting cells on photomicrographs of 4′,6-diamidino-2-phenylindole (DAPI)- stained cultures (Vector Laboratories, Burlingame, CA). For MTT assays, cultures were incubated with 5 mg/ml MTT at 37°C for 2 h, and absorbance at 570 nm was measured in solubilized cells by using a Cytofluor series 4000 multiwell plate reader (Applied Biosystems, Foster City, CA). For cell counting, the number of intact DAPI-stained nuclei in five 200× microscope fields per well (at the 3-, 6-, 9-, and 12-o'clock positions and in the center) was recorded. In both cases, results were expressed as a percentage of values obtained in control cultures not treated with VEGF.
Immunocytochemistry.
Cell cultures (24) and brain sections (25) were processed for immunocytochemistry and double-label immunocytochemistry as described. For double-label immunocytochemistry, sections were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature, washed twice with PBS, and incubated in 2 M HCl at 37°C for 1 h. Sections were incubated with blocking solution, with primary antibodies at 4°C overnight, and with secondary antibodies in blocking solution at room temperature for 2 h. Primary antibodies were as follows: mouse monoclonal anti-BrdUrd (2 μg/ml; Roche Applied Science); sheep polyclonal anti-BrdUrd (25 μg/ml; Biodesign International, Saco, ME); rat monoclonal anti-embryonic nerve cell adhesion molecule (ENCAM, 1:100) and mouse monoclonal anti-nestin (1:400) (BD Biosciences Pharmingen, San Diego); affinity-purified rabbit polyclonal anti-VEGFR1/Flt-1 (1:500), mouse monoclonal anti-VEGFR-2Flk-1 (1:500) and affinity-purified goat polyclonal anti-doublecortin (Dcx, 1:100) (Santa Cruz Biotechnology); rabbit polyclonal anti-cleaved (17–20 kDa) caspase-3 (1:500; New England Biolabs); mouse monoclonal anti-neuronal nuclear antigen (NeuN) (1:200; Chemicon); and mouse monoclonal anti-glial fibrillary acidic protein (GFAP, 1:500) and rabbit polyclonal anti-von Willebrand factor (vWF; 1:500; Sigma). Biotinylated goat anti-mouse IgG and FITC-(Vector Laboratories) or rhodamine-conjugated secondary antibodies (Jackson ImmunoResearch) were used (all 1:200). DAPI was used to counterstain nuclei in some cases and fluorescence signals were detected with a Nikon E300 or E800 microscope at excitation/emission wavelengths of 535/565 nm (rhodamine, red), 470/505 (FITC, green), and 360/400 (DAPI, blue). Biotin signals were detected with 3,3′-diaminobenzidine (DAB; brown) and Vector VIP (purple). Results were recorded with a Magnifire digital camera (ChipCoolers, Warwick, RI). Controls included omitting or preabsorbing primary antibody and omitting secondary antibody. For confocal microscopy, a Nikon PCM-2000 laser-scanning confocal microscope and Simple PCI imaging software (Compix, Cranberry Township, PA) were used.
VEGF Administration and BrdUrd Labeling in Vivo.
VEGF was administered i.c.v. in artificial cerebrospinal fluid (aCSF) consisting of 128 mM NaCl, 2.5 mM KCl, 0.95 mM CaCl2, and 1.99 mM MgCl2. VEGF (10 μg/ml) or aCSF was infused at 1 μl/h for 3 days with an osmotic minipump (Alzet 1003D; Alza Scientific Products, Mountain View, CA), and BrdUrd 5′-monophosphate (50 mg/kg in saline i.p.) or saline was given twice daily for the same number of days. Brains (four per condition) were removed after perfusion with saline and 4% paraformaldehyde in PBS. Fifty-micrometer coronal sections were cut with a cryostat and stored at −80°C. Sections were treated with 50% formamide/280 mM NaCl/30 mM sodium citrate at 65°C for 2 h, incubated in 2 M HCl at 37°C for 30 min, and rinsed in 0.1 M boric acid (pH 8.5) at room temperature for 10 min. Sections were then incubated in 1% H2O2 in PBS for 15 min, in blocking solution (2% goat serum/0.3% Triton X-100/0.1% BSA in PBS) for 2 h at room temperature, and with 2 μg/ml mouse monoclonal anti-BrdUrd antibody (Roche Applied Science) at 4°C overnight. Sections were washed with PBS, incubated with biotinylated goat-anti-mouse secondary antibody (Vector Laboratories, 1:200) for 2 h at 25°C, washed, and placed in avidin-peroxidase conjugate (Vector Laboratories) solution for 1 h. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine and 0.03% H2O2. Processing was stopped with H2O and sections were dehydrated through graded alcohols, cleared in xylene, and coverslipped in permanent mounting medium (Vector Laboratories). Sections were examined with a Nikon E300 epifluorescence microscope.
Statistics.
All experiments were repeated at least three times. The statistical significance of differences between means was evaluated by Student's t test for single, and by ANOVA followed by post hoc t tests for multiple, comparisons, with P < 0.05 considered significant.
Results
VEGF Stimulates Neurogenesis in Cortical Cultures in Vitro.
Cortical cultures primarily contained cells of neuronal lineage, as demonstrated by the expression of phenotypic markers in the following percentages of cells: calbindin, ≈15%; Hu, ≈80%; ENCAM, ≈80%; βIII tubulin, ≈75%; nestin, ≈25%; Neuro D, ≈5%; and GFAP, ≈2% (19). When these cultures were treated with VEGF for 24 h in vitro, they showed increased incorporation of BrdUrd into cells that expressed the immature neuronal marker ENCAM or the neuroepithelial precursor cell marker nestin (Fig. 1). This effect was concentration-dependent and was associated with an increase in cell number (DAPI-stained nuclei). The concentration–response relationships for stimulation of BrdUrd labeling and for elevation of cell number by VEGF were similar, with maximal effects at 10 ng/ml.
Figure 1.
VEGF stimulates BrdUrd incorporation in murine cortical cultures in vitro. (A) Immunodetection of BrdUrd labeling (red) in control (Left), and 10 ng/ml VEGF-treated (Right) cortical cultures at 24 h in vitro. (Original magnification, 20×; Insets, 40×.) (B) Quantification of VEGF-stimulated BrdUrd incorporation by ELISA, expressed as a percentage of BrdUrd incorporation in control cultures. (C) Cell counts (number of DAPI-stained nuclei) in VEGF-treated cultures, expressed as a percentage of cell counts in control cultures. (D) Colocalization of BrdUrd labeling (red) with immunoreactivity for the immature neuronal marker ENCAM (green, Left) and the neuroepithelial precursor cell marker nestin (green, Right). Data are representative fields (A and D) or mean values ± SEM (B and C) from at least three experiments per panel.
VEGF Stimulates Neurogenesis in Vitro Through VEGFR2/Flk-1 Receptors.
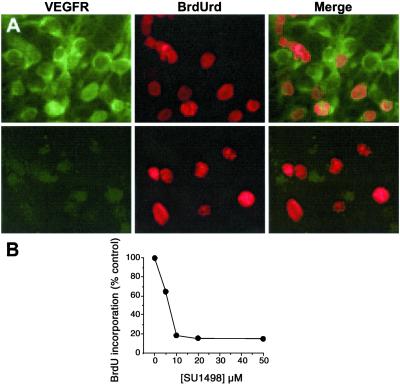
To identify the receptor mechanism involved in the stimulation of BrdUrd labeling by VEGF, cultures were immunostained with antibodies against VEGFR1/Flt-1 and VEGFR2/Flk-1. VEGFR2/Flk-1 was expressed in almost all cells, whereas VEGFR1/Flt-1 was barely detectable by immunocytochemistry (Fig. 2), despite the fact that the antibody used is capable of staining neuronal VEGFR1/Flt-1 receptors (5). In addition, the VEGFR2/Flk-1 receptor tyrosine kinase inhibitor SU1498 blocked the ability of 10 ng/ml VEGF to increase BrdUrd labeling, at concentrations (1–100 μM) associated with previously reported effects of SU1498, such as inhibition of VEGF-induced up-regulation of endothelial nitric oxide synthase (26).
Figure 2.
Association of VEGF-stimulated BrdUrd incorporation with VEGFR2/Flk-1 receptors in murine cortical cultures in vitro. (A) Immunocolocalization of VEGFR2/Flk-1 (green, Top) but not VEGFR1/Flt-1 (green, Bottom) receptors with BrdUrd labeling (red). (Original magnification, 40×.) (B) Inhibition of VEGF (10 ng/ml for 24 h)-stimulated BrdUrd incorporation into cortical cultures by the VEGFR2/Flk-1 receptor tyrosine kinase inhibitor SU1498. Data are representative fields (A) or mean values ±SEM (B) from at least three experiments per panel.
VEGF Stimulates Neurogenesis in Rat Brain in Vivo.
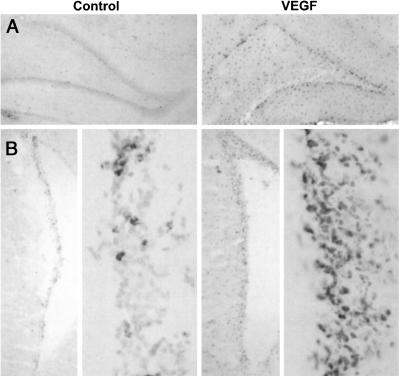
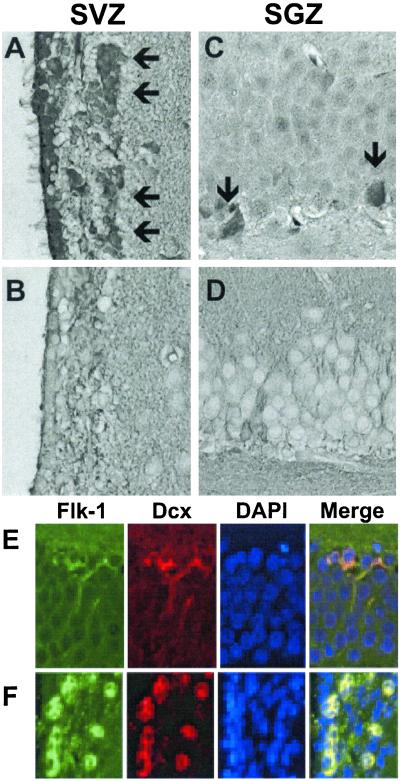
To determine whether VEGF also stimulates BrdUrd incorporation in the rat brain in vivo, VEGF (10 μg/ml) was continuously infused i.c.v. at 1 μl/h for 3 days by means of a minipump and BrdUrd 5′-monophosphate (50 mg/kg i.p.) was given twice daily over the same period, and brains were examined 1 wk later. Sections through the two principal neuroproliferative regions of the adult brain showed that VEGF increased BrdUrd labeling in both SGZ and SVZ (Fig. 3). As was also true of cells that incorporated BrdUrd in response to VEGF in our cell cultures, these regions expressed abundant VEGFR2/Flk-1 but not VEGFR1/Flt-1 receptors (Fig. 4). Moreover, VEGFR2/Flk-1 receptor expression colocalized with the immature neuronal marker protein Dcx (27, 28).
Figure 3.
VEGF stimulates BrdUrd incorporation into neuroproliferative zones of rat brain in vivo. aCSF (Left) or 10 μg/ml of VEGF (Right) was infused i.c.v. at 1 μl/h and BrdUrd 5′-monophosphate (50 mg/kg) was given i.p. twice daily for 3 days. Four days later, BrdUrd was localized by immunohistochemistry in hippocampus (A) and rostral SVZ (B) (Original magnification, 4×; high-power views of SVZ, 20×). Note that BrdUrd incorporation is not confined to neuroproliferative zones, but extends, for example, into the dentate hilus in (A) consistent with the labeling of both neuronal and non-neuronal (e.g., astroglial) cells (see Fig. 6). Data are representative fields from at least three experiments per panel.
Figure 4.
VEGF receptor expression in neuroproliferative zones of rat brain in vivo. Brain sections through SVZ (A and B) and SGZ (C and D) were stained with antibodies against VEGFR2/Flk-1 (A and C) and VEGFR1/Flt-1 (B and D). (Original magnification, 4×.) VEGFR2/Flk-1, but not VEGFR1/Flt-1, immunostaining was observed in SVZ and SGZ (arrows), as well as in ependymal cells (ciliated cells at far left in A). VEGFR2/Flk-1 expression (green) was colocalized with Dcx (red) in SGZ (E) and SVZ (F). Data are representative fields from at least three experiments per panel.
VEGF Does Not Modify Caspase-3-Dependent Cell Death in SGZ of Rat Brain.
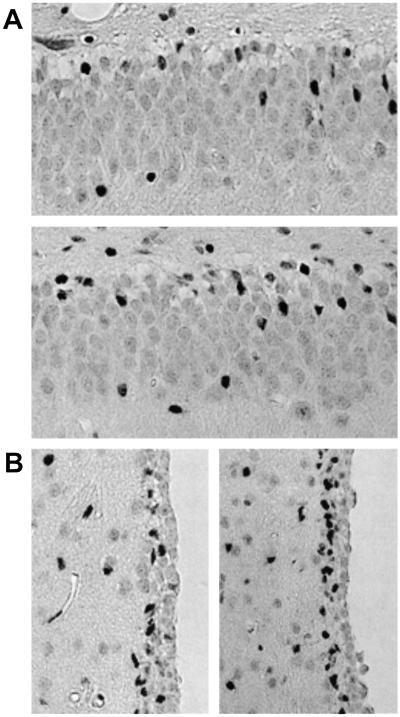
A significant proportion of newly generated cells in neuroproliferative zones of the rodent brain normally die soon after mitosis (29), which appears to be because of caspase-3-dependent programmed cell death (30). Therefore, VEGF could increase the number of BrdUrd-labeled cells by inhibiting cell death rather than stimulating cell birth. To distinguish between these possibilities, SGZ and SVZ from VEGF- and aCSF-vehicle-treated mouse brains were stained with an antibody against activated caspase-3. The number of activated caspase-3-immunopositive cells in SGZ and SVZ was modestly increased in VEGF-treated compared with control rats (Fig. 5), indicating that the increase in BrdUrd labeling after VEGF treatment is not because of a reduction in caspase-3-mediated programmed cell death. (That VEGF might reduce caspase-3-independent cell death remains a possibility.) The increase in the number of cells expressing activated caspase-3 (Fig. 5) is considerably less than the corresponding increase in cells that show BrdUrd labeling (Fig. 3), consistent with a net increase in cell numbers after VEGF treatment.
Figure 5.
Caspase-3 cleavage in SGZ (A) and SVZ (B) from control (A Top; B Left) and VEGF-treated (A Bottom; B Right) rat brains in vivo. Brain sections through SGZ were stained with an antibody against the 17 to 20-kDa cleavage product of caspase-3. (Original magnification, 4×; high-power views, 20×.) Data are representative fields from at least three experiments per panel.
VEGF Stimulates Incorporation of BrdUrd into Both Neuronal and Non-Neuronal Cells of Rat Brain in Vivo.
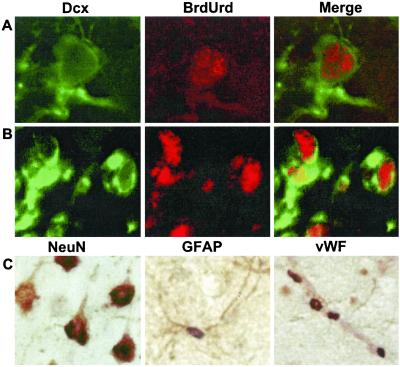
To ascertain whether VEGF induced the incorporation of BrdUrd into neurons in vivo, as demonstrated above in vitro, brain sections from VEGF- and aCSF-vehicle-treated rats were processed for double-label immunohistochemistry with antibodies against BrdUrd and against cell-type-specific markers. Cells that incorporated BrdUrd by 7 days after the onset of VEGF treatment included Dcx-expressing immature neurons of SGZ and SVZ, as well as NeuN-immunopositive mature neurons, GFAP-staining astrocytes, and endothelial cells that expressed vWF (Fig. 6). Therefore, VEGF stimulated BrdUrd incorporation into brain cells of both neuronal and nonneuronal lineages in vivo.
Figure 6.
VEGF stimulates incorporation of BrdUrd into neurons, astrocytes, and endothelial cells in vivo. Rats were given VEGF and BrdUrd as described in the legend to Fig. 3. Brain sections through SGZ (A) and SVZ (B) were stained with antibodies against the immature neuronal marker Dcx (green) and against BrdUrd (red). (C) Sections were stained with antibodies against BrdUrd (purple) and either the mature neuronal marker NeuN (Left), the astroglial marker GFAP (Center), or the endothelial cell marker vWF (Right; brown). Note that BrdUrd and NeuN are nuclear markers, whereas Dcx, GFAP, and vWF are extranuclear. In C Left, all cells shown stain for NeuN, and all except the cell at far left also stain for BrdUrd. In C Center, a single cell is seen, with GFAP-positive processes and BrdUrd-positive nucleus. In C Right, the vessel running diagonally from top left to bottom right stains for vWF, and the four associated nuclei stain for BrdUrd. Data are representative fields from at least three experiments per panel.
Discussion
The major finding of this study is that VEGF, identified originally as an angiogenic and vascular permeability factor, and more recently as a neurotrophic and neuroprotective factor, can also stimulate neurogenesis, identified by increased BrdUrd labeling of neuronal precursor cells in vitro and in vivo. The in vitro effect appears to be mediated through VEGFR2/Flk-1 receptors because these were the VEGF receptors expressed in our cultures, and because the VEGFR2/Flk-1 receptor tyrosine kinase inhibitor SU1598 blocked VEGF-stimulated BrdUrd labeling. The in vivo effect was observed in both of the major neuroproliferative zones of the adult rat brain—SGZ and SVZ, where VEGFR2/Flk-1 receptors also predominated—and extended to astroglial and endothelial as well as neuronal cell lineages.
VEGF exerts multiple effects on endothelial cells, acting as a mitogen (31, 32), promoting cell survival (33, 34), and protecting cells from injury (35, 36). Taken together with prior evidence for survival-promoting and protective effects of VEGF on neurons, our findings suggest that VEGF regulates a similarly broad range of functions in neurons. This is not necessarily surprising, since neurons express some of the same VEGF receptors and VEGF receptor-coupled signal transduction pathways that are found in the endothelium (1–8).
Palmer et al. (18) reported that in the adult rat SGZ, neurogenesis occurs in close proximity to blood vessels, where VEGF expression is high and angiogenesis is ongoing. Based on this and other evidence, they argued that neurogenesis and angiogenesis might be mechanistically linked, citing VEGF as a factor that might provide such a linkage. The results presented here are consistent with that hypothesis.
The ability of VEGF to stimulate the proliferation of neuronal precursors may be of interest in the context of lesion-induced neurogenesis, which occurs after excitotoxicity (38), seizures (39), oxidative stress (40), and global (41) and focal (25) ischemia. Particularly in cerebral ischemia, where VEGF expression is known to be increased (42) and to promote angiogenesis (43), coordinate induction of angiogenesis and neurogenesis by a common factor could have adaptive value, by efficiently promoting brain recovery and repair. The induction of neurogenesis and angiogenesis in tandem could also be important in development, and a role for VEGF in this process has been proposed (44).
Acknowledgments
This work was supported by National Institutes of Health Grant NS37695 (to D.A.G.).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- BrdUrd
5-bromo-2′-deoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- Dcx
doublecortin
- EGF
epidermal growth factor
- ENCAM
embryonic nerve cell adhesion molecule
- GFAP
glial fibrillary acidic protein
- i.c.v.
intracerebroventricular
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NeuN
neuronal nuclear antigen
- SGZ
subgranular zone
- SVZ
subventricular zone
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sondell M, Lundborg G, Kanje M. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sondell M, Sundler F, Kanje M. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Silverman W F, Krum J M, Mani N, Rosenstein J M. Neuroscience. 1999;90:1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 4.Jin K L, Mao X O, Greenberg D A. J Mol Neurosci. 2000;14:197–203. doi: 10.1385/JMN:14:3:197. [DOI] [PubMed] [Google Scholar]
- 5.Jin K L, Mao X O, Greenberg D A. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek M P, Mitsuda N, Tohyama M. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- 7.Ogunshola O O, Antic A, Donoghue M J, Fan S-Y, Kim H, Stewart W B, Madri J A, Ment L R. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 8.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, et al. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 9.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 11.Altman J. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds B A, Tetzlaff W, Weiss S. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray J, Peterson D A, Schinstine M, Gage F H. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschenbaum B, Goldman S A. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shingo T, Sorokan S T, Shimazaki T, Weiss S. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K, Cepko C L. J Neurosci. 1996;16:6089–6099. doi: 10.1523/JNEUROSCI.16-19-06089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yourey P A, Gohari S, Su J L, Alderson R F. J Neurosci. 2000;20:6781–6788. doi: 10.1523/JNEUROSCI.20-18-06781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer T D, Willhoite A R, Gage F H. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Mao X O, Sun Y, Xie L, Jin L, Nishi E, Klagsbrun M, Greenberg D A. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn H G, Winkler J, Kempermann G, Thal L J, Gage F H. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigova T, Pencea V, Wiegand S J, Luskin M B. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- 22.Pencea V, Bingaman K D, Wiegand S J, Luskin M B. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brewer G J, Torricelli J R, Evege E K, Price P J. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Jin K, Mao X O, Zhu Y, Greenberg D A. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin K, Minami M, Lan J Q, Mao X O, Batteur S, Simon R P, Greenberg D A. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen B Q, Lee D Y, Zioncheck T F. J Biol Chem. 1999;274:33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 27.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet M C, Friocourt G, McDonnell N, Reiner O, Kahn A, et al. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 28.Gleeson J G, Lin P T, Flanagan L A, Walsh C A. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 29.Morshead C M, van der Kooy D. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pompeiano M, Blaschke A J, Flavell R A, Srinivasan A, Chun J. J Comp Neurol. 2000;423:1–12. [PubMed] [Google Scholar]
- 31.Leung D W, Cachianes G, Kuang W-J, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 32.Keck P J, Hauser S D, Krivi G, Sanzo K, Warren T, Feder J, Connolly D T. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 33.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 34.Gerber H P, McMurtrey A, Kowalski J, Yan M, Keyt B A, Dixit V, Ferrara N. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 35.Yang W, de Bono D P. FEBS Lett. 1997;403:139–142. doi: 10.1016/s0014-5793(96)01486-x. [DOI] [PubMed] [Google Scholar]
- 36.Castilla M A, Caramelo C, Gazapo R M, Martin O, Gonzalez-Pacheco F R, Tejedor A, Bragado R, Arroyo M V. Life Sci. 2000;67:1003–1013. doi: 10.1016/s0024-3205(00)00693-7. [DOI] [PubMed] [Google Scholar]
- 37.Jin K L, Mao X O, Nagayama T, Goldsmith P C, Greenberg D A. Neuroscience. 2000;100:713–717. doi: 10.1016/s0306-4522(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 38.Gould E, Tanapat P. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 39.Parent J M, Yu T W, Leibowitz R T, Geschwind D H, Sloviter R S, Lowenstein D H. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magavi S S, Leavitt B R, Macklis J D. Nature (London) 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Solway K, Messing R O, Sharp F R. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovács Z, Ikezaki K, Samoto K, Inamura T, Fukui M. Stroke. 1996;27:1865–1873. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z G, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soker S, Takashima S, Miao H Q, Neufeld G, Klagsbrun M. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]