Abstract
Recent evidence suggests that bone marrow-derived angioblasts or endothelial progenitor cells circulate in peripheral blood and can incorporate at sites of pathologic neovascularization or during the ovarian cycle. However, the incorporation of endothelial progenitor cells into vessels of nonischemic tissues in adult animals has not been observed. We hypothesized that the vascular microenvironment differs between newborn and adult animals, and that donor endothelial cell progenitors would engraft in rapidly growing normal tissues during the neonatal period. After nonablative administration of bone marrow cells either at birth or at 4 weeks of age, donor-derived endothelial cells were found only in the neovasculature of the newborn recipients. Both the incorporation of donor endothelial cells into the newborn neovasculature as well as tissue vascularity were significantly increased by coadministering vascular endothelial growth factor with bone marrow cells. These findings suggest that bone marrow-derived endothelial progenitor cells can contribute to neovascularization during the newborn period and are responsive to vascular endothelial growth factor.
Keywords: angiogenesis‖development‖plasticity‖vasculogenesis‖transplantation
Over the last 30 years, the study of new blood vessel formation has focused largely on pathologic conditions such as tumor growth, metastasis, ischemia, and proliferative retinopathies (1–3). Neovascularization and vascular repair and maintenance during early postnatal development remains poorly understood. Blood vessel formation is divided into vasculogenesis and angiogenesis (3). Vasculogenesis occurs during early embryonic development and involves the de novo differentiation of vascular endothelial cells from precursor cells, known as endothelial progenitor cells (EPCs) or angioblasts (3). Thereafter, neovascularization is believed to occur exclusively by a distinct process denoted as angiogenesis, a remodeling process characterized by the sprouting of new blood vessels from preexisting ones (3). Although the rate and extent of new blood vessel formation are known to vary during development, there are few studies thus far addressing neovascularization in the context of different developmental stages.
It was recently demonstrated that specific subsets of both bone marrow (BM) and circulating cells seem to be enriched in EPCs and could incorporate into the vasculature of experimentally induced wounds or ischemic areas (4–7). The only nonpathologic site of incorporation was tissue involved in the ovarian cycle (5). To date, there is no evidence that vasculogenesis contributes to postnatal neovasculature formed during normal tissue growth and maintenance (4–7).
We hypothesized that early neonatal development, which represents a period of accelerated tissue growth and active neovascularization, may provide a distinct milieu for vessel growth and formation. By using a newborn mouse transplant model, we determined whether donor BM-derived EPCs can engraft when administered to the neonate in the absence of any marrow-ablative preconditioning. We also assessed the engraftment, survival, and long-term fate of EPCs after transplantation. Finally, we studied how the endothelial cell mitogen, vascular endothelial growth factor (VEGF), modulates EPC engraftment in the newborn recipient. The demonstration of donor BM-derived EPC engraftment in the absence of radioablation (8–9) may allow us to study the use of BM-EPCs as a therapeutic vehicle for cellular and gene therapy for various congenital disorders.
Methods
Animals and Administration of BM and rVEGF.
β-Glucuronidase (GUSB)-deficient homozygous mutant mice were obtained from a B6.C-H-2bm1/ByBir-gusmps/+ colony maintained by M.S.S. at Washington University (St. Louis, MO). Homozygous transgenic donor mice were obtained from a separately bred group of syngeneic animals carrying the human GUSB cDNA as a transgene to increase cellular GUSB expression. Homozygous GUSB-deficient mice were identified at birth by the absence of GUSB activity as described (10). Unfractionated BM from syngeneic donor mice was obtained as described (10). We injected 5 × 106 or 1 × 108 nucleated cells (100 μl per mouse) i.v. from same-sex donors into newborn mice through the superficial temporal vein (11) or into 4-week-old GUSB-deficient mice through the lateral tail vein, respectively. Thirty-three newborns and 10 4-week-old GUSB-deficient mice were used for this study. At least three animals were studied at each time point. A portion of the GUSB-deficient newborn mice received either 1.25 ng or 0.4 ng of rVEGF164 (R & D Systems) either with or without BM cells at day 1 of birth and a repeat administration of an equivalent dose of rVEGF164 alone i.v. on day 3 of birth. VEGF-injected wild-type and saline-injected GUSB-deficient mice were used as controls.
Analysis of Hematopoietic Engraftment and GUSB Quantitative Biochemistry.
Hematopoietic engraftment was assessed 6 months after administration of BM cells by flow cytometry using FACScan (Becton Dickinson). Marrow isolated from transplanted recipients was resuspended at 1 × 106 cells/100 μl in Iscove's modified Dulbecco's medium/2% (vol/vol) FCS and analyzed as previously described by using a fluorogenic GUSB substrate (Molecular Probes), and CD45/PE (12). For the purpose of comparison to nonablated BM recipients, three newborn GUSB-deficient recipients received a sublethal dose of 200 rads of γ-irradiation from a 137Cs irradiator just before injection. Biochemical analysis for GUSB activity of murine tissues was performed as described (13, 14).
Histochemistry, Immunofluorescence, Immunohistochemistry, and Morphometry.
Tissue sections from organs were harvested at 2, 4, and 8 weeks after BM administration, processed, and histochemical analysis for GUSB was performed as described (10, 14). For immunofluorescence, sections were fixed for 20 min at 4°C in 100% acetone and then incubated with MOM block [5% (wt/wt) papain/2 μM EDTA, pH 7.4/20 mM l-cysteine] at dilution of 1:20, a PBS-blocking buffer (0.01 g/ml BSA/2 μg powdered milk/ml/3 μl/ml Triton X-100) and 10% (vol/vol) goat serum for 1–2 h. The slides then were washed with PBS and incubated with rabbit polyclonal antibody against human von Willebrand Factor (vWF; DAKO; 1:250) and mouse monoclonal antibody against mouse GUSB (1:100; gift of W. Sly) in PBS-BB/10% (vol/vol) goat serum overnight at 4°C. Slides were washed and then incubated with goat anti-mouse and goat anti-rabbit IgG FITC- or rhodamine-conjugated fluorochromes for 1–2 h (Calbiochem). For immunohistochemistry, tissues were fixed as above and processed as described (10). Anti-vWF or BS-1 lectin were used at 1:100 or 1:50 dilutions, respectively.
Morphometric quantitation of changes in vascularity in the liver in response to rVEGF was done on 7–14 randomly chosen fields (×20) from tissues obtained from at least two experimental animals by fluorescence microscopy using an antibody to vWF, as described above. The total number of vWF-positive cells was counted for each image. Analysis of the heart was performed by counting the number of times vWF-positive vessels crossed the lines of a grid superimposed on a digitally captured image. Quantitation of numbers of GUSB-positive cells that colocalized with endothelial cells expressing vWF was done by counting the numbers of colocalized vessels or cells in 18–30 random sections of tissue on at least two different animals. The area of each section was measured and data was expressed as numbers of colocalization of GUSB and vWF per square centimeter.
Histopathology.
Tissue samples were removed, fixed in 10% (vol/vol) neutral-buffered formalin, embedded in paraffin, and then sectioned for staining. Immunohistochemistry was performed on deparaffinized sections after antigen retrieval. Slides then were incubated with either anti-vWF (1:100) or BS-1 lectin (1:160) overnight, washed, and secondary antibody was applied and visualized by using the LSAB peroxidase kit (DAKO). Slides were counterstained with hematoxylin.
Statistical Analysis.
Student's t tests were performed to compare different data sets. All data are presented as mean ± SEM; a P < 0.05 was interpreted to denote statistical significance.
Results
To study the fate of putative BM-derived EPCs in vivo and their role in new blood vessel formation, we used the GUSB-deficient mouse as a recipient to track wild-type (GUSB-positive) donor BM cells from a syngeneic mouse. This mouse is a model of the human disease mucopolysaccharidosis type VII (MPSVII) and is completely deficient in GUSB activity (15). The GUSB-deficient mouse also has been used extensively by our laboratory and others to track in vivo GUSB-positive neuronal progenitor cells (16), macrophages (10), highly purified BM stem cells (12), and lymphocytes (P.P.Y., C. Vogler, A.A.H., and M.S.S., unpublished data). More recently a monoclonal antibody to mouse GUSB has been developed that allows detection of GUSB-positive donor cells by immunofluorescence or immunohistochemistry. To enhance the sensitivity for detecting donor-derived GUSB-positive cells, we used a syngeneic transgenic mouse that expresses supraphysiologic levels of human GUSB in all tissues, including BM cells (P.P.Y., C. Vogler, A.A.H., and M.S.S., unpublished data). No abnormalities in the morphology or organization of the vascular system have been reported in either human patients with MPSVII or in the GUSB-deficient mouse (17–18).
Newborn GUSB-deficient mice were injected i.v. within 3 days of birth with 5 × 106 nucleated BM cells isolated from the syngeneic transgenic adult mice expressing both human and mouse GUSB. In parallel, 4-week-old GUSB-deficient mice were injected i.v. with an approximately equal weight-adjusted dose (1 × 108 cells) of unfractionated BM cells isolated from the same donor mice as used for the newborn recipients. The cells were administered without any radioablative or chemical preconditioning to quantify the extent of BM-derived EPC engraftment into vasculature while minimizing hematopoietic engraftment. Although some hematopoietic engraftment can occur in the absence of myeloablative regimens, the extent of engraftment is relatively low and usually requires multiple injections of high numbers of cells (19). To evaluate the appearance and distribution of donor-derived cells in the recipient mice, animals were killed 2, 4, and 8 weeks after administration of BM.
A histochemical survey showed that donor-derived cells were found in varying amounts in newborn recipients 2 weeks after administration in all tissues examined, including brain, heart, lung, muscle, fat, liver, spleen, kidney, and bone (data not shown). Most organs, with the exception of the brain, contained numerous clusters of donor cells located throughout the tissue. Parallel analysis of the tissues from the 4-week-old recipients 2 weeks after administration showed similar distribution and numbers in all tissues with the exception of the brain, which contained no donor cells (data not shown).
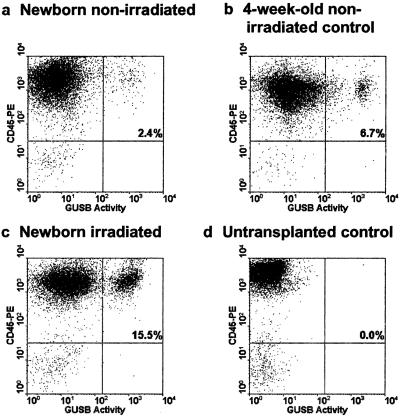
Many of these are likely to be nonendothelial cells of hematopoietic origin. Therefore, we assessed the degree of hematopoietic engraftment in spleen and BM from both newborn and 4-week-old recipients 6 months after administration of syngeneic BM by costaining cells with an anti-CD45 antibody and a fluorogenic GUSB substrate followed by FACS analysis (Fig. 1). Hematopoietic engraftment of donor cells in BM was very low in newborn recipients that did not receive radioablation (≈2.3 ± 0.35%) as compared with newborn recipients preconditioned with 200 rads that had 15 ± 4.8% engraftment (Fig. 1). Engraftment levels were somewhat higher (≈6.67 ± 1.1%) for 4-week-old nonablated recipients when compared with newborns and may reflect the large number of cells administered (1 × 108). Because of the low level hematopoietic engraftment in both newborn and 4-week-old recipients, it was necessary to use additional cell-specific markers to distinguish endothelial cell engraftment from hematopoietic engraftment.
Figure 1.
Low-level hematopoietic engraftment is observed after i.v. administration of bone marrow cells without prior radioablation. (a and b) Bone marrow from a representative mouse that received 5 × 106 cells per g of body weight syngeneic bone marrow (a) at birth or (b) at 4 weeks in the absence of any preconditioning radiation. The cells were incubated with the fluorogenic GUSB substrate (C12FdGlcU) followed by labeling with anti-mouse CD45-PE-conjugated antibody. (c) A newborn mouse given an equivalent number of GUSB-positive bone marrow cells after receiving 200 rads of radiation. (d) The CD45 and GUSB-positive quadrant was set to exclude greater than 99.9% of signal in similarly stained nontransplanted GUSB-deficient mice. The number in the upper right quadrant denotes the average engraftment of the animals analyzed. Three animals were analyzed for each nonablative transplantation condition.
Donor-Derived GUSB-Positive Cells Incorporated into Neovasculature of Newborn Recipients.
To determine whether donor BM-derived cells incorporated into vascular structures and exhibited the characteristics of endothelial cells, we performed immuno-localization experiments by using antibodies that recognize endothelial cells [anti-vWF (20, 21) or BS-1 lectin (22)], and an antibody identifying donor-derived cells, anti-mouse GUSB (gift from W. Sly). GUSB and vWF factor colocalized in cells of vascular structures throughout the heart and liver of newborn recipients analyzed 2 weeks after administration of BM cells without radioablation (Fig. 2). The number of donor-derived cells exhibiting the endothelial marker vWF declined dramatically after 8 weeks in newborn recipients in heart (Fig. 2 j–l) and liver (data not shown). Areas of continued colocalization were primarily small capillaries. Analysis of the heart and liver of recipients that received donor cells at 4 weeks of age failed to detect any colocalization of GUSB and vWF at any time point analyzed (data not shown).
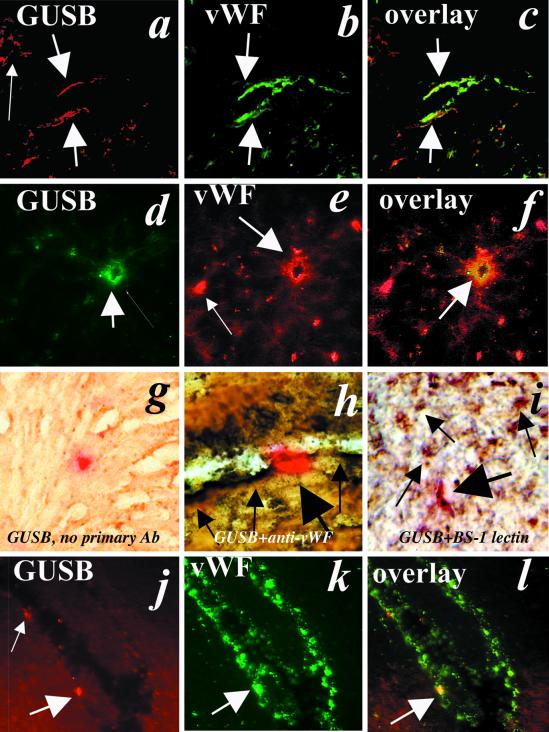
Figure 2.
Donor-derived cells colocalize with endothelial cell markers in association with vascular structures. Immunofluorescence with anti-GUSB and anti-vWF antibodies was used to detect donor-derived and endothelial cells, respectively. Colocalization is observed in a GUSB-deficient recipient mouse heart (a–c and g–l) and liver (d–f) obtained 2 weeks (a–i) or 8 weeks (j–l) after administration of syngeneic GUSB-positive bone marrow cells at birth (×40). (h) Immunohistochemistry using anti-vWF (brown) and histochemistry for GUSB enzyme activity (red) demonstrates colocalization in heart tissue obtained from newborn GUSB-deficient mice killed 2 weeks after bone marrow administration. (i) A similar dual-staining procedure was performed with BS-1 lectin and GUSB histochemical staining. Thick arrows denote GUSB-positive cells (red) that colocalize with vWF- or BS-1 lectin-positive cells (brown). Thin arrows denote vWF-expressing cells that do not express GUSB (×40).
To increase our confidence that endothelial cell engraftment occurred during the newborn period, we performed immunohistochemistry on frozen sections obtained from heart by using either an anti-vWF antibody or BS-1 lectin and subsequently stained the sections histochemically for GUSB activity (10). Overexpression of human GUSB in the transgenic donor cells aided in this analysis because the GUSB-specific staining was partially quenched during the initial immunohistochemical procedure. Numerous GUSB-positive (red) cells colocalized with either BS-1 or vWF-positive cells in association with vascular structures in sections obtained from newborn heart isolated 2 weeks after administration of BM cells (Fig. 2). Hearts obtained from recipients transplanted at 4 weeks of age did not show any colocalization (data not shown).
One potential weakness of our model may be that GUSB enzyme can be secreted in small amounts by nonendothelial donor cells and taken up by surrounding endothelial cells, which may then become immunopositive. That result seems unlikely for two reasons. First, GUSB-positive endothelial cells were observed juxtaposed to GUSB-negative cells in the vasculature. If cross-correction were a problem, we would predict that multiple cells in a region rather than single isolated cells would be GUSB-positive. Second, no GUSB-positive endothelial cells were identified in recipients transplanted at 4 weeks of age, which had higher levels of hematopoietic engraftment, suggesting that such cross-correction either did not occur or that it was below the level of detection by our assays.
Together, these data suggest that BM-derived EPCs contribute to new blood vessel formation occurring in the newborn period. However, no evidence of a role of BM-derived EPCs in normal tissue growth was observed when the cells were administered to 4-week-old recipients.
VEGF Increased Vascularity and Donor-Derived EPC Engraftment in Newborn Heart and Liver.
Syngeneic BM cells were coadministered with either a low dose (0.4 ng per newborn mouse) or high dose (1.25 ng per newborn mouse) of recombinant VEGF to 1-day-old GUSB-deficient mice in the absence of radioablation. A second identical dose of VEGF was administered on day 3. Control GUSB-deficient mice were injected with BM cells alone and received an injection of saline on day 3. In parallel, wild-type newborn mice were injected with either saline or low or high doses of VEGF on days 1 and 3 after birth. No acute toxicity was observed in recipients receiving BM cells, saline, or low dose (0.4 ng per mouse × 2) VEGF alone or in combination with BM cells. Both GUSB-deficient and wild-type newborns receiving a high dose (1.25 ng per mouse × 2) of VEGF with or without BM cells had transient hypoxemia and exhibited mildly prolonged bleeding at the injection site. Systemic administration of VEGF has been shown to cause hypotension in other animal models (23). Importantly, no angiomas were observed either grossly or on histologic sections in control or VEGF-treated animals (data not shown).
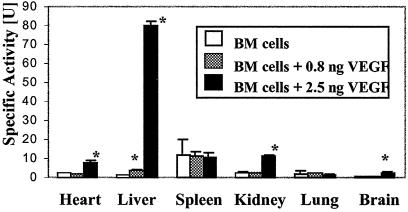
We inferred the presence of donor-derived, GUSB-positive cells in various organs by a quantitative biochemical assay for GUSB activity. Enzyme activity was increased in several tissues when BM cells were administered with high dose VEGF as compared with the same dose of BM cells without VEGF (Fig. 3). Enzyme levels in recipient spleens were not significantly different with VEGF administration at 2 weeks (Fig. 3) or 8 weeks (data not shown) after transplantation, suggesting that there was no increase in donor hematopoietic engraftment in the presence of VEGF when compared with recipients of BM cells alone (Fig. 3).
Figure 3.
GUSB-specific activities were increased 2 weeks after administration in several tissues when GUSB-positive bone marrow cells were coadministered i.v. with VEGF to newborn GUSB recipients. GUSB-specific activity is presented as nmol of substrate cleaved per h per mg of protein. *, significant (P < 0.05) increase in specific activity over recipients not receiving VEGF.
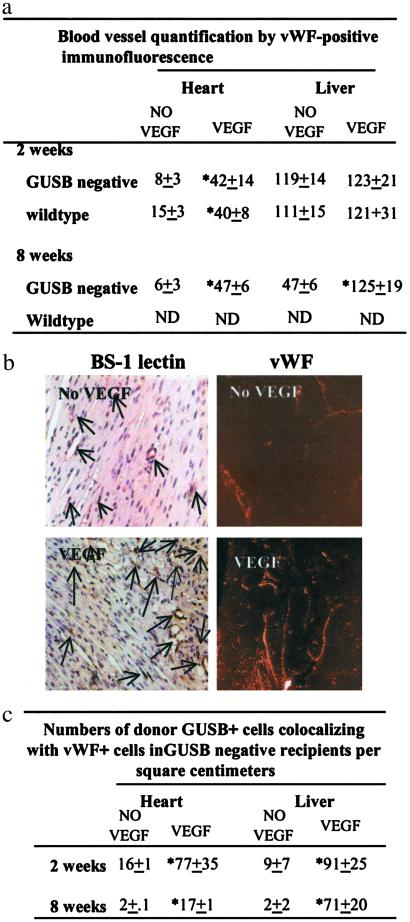
The effect of recombinant VEGF on vascularity was assessed by morphometric measurements of endothelial cells in various tissues by using an anti-vWF antibody. Because the levels of EPC incorporation in the absence of VEGF were best characterized in the heart and liver, we focused our morphometric analysis on those two organs. Vascular density in the heart was increased significantly 2 weeks after i.v. administration of high-dose VEGF in both wild-type and GUSB-deficient recipients (Fig. 4a). The increase persisted at 8 weeks after administration. Analysis of the liver showed no significant difference in the numbers of vWF-positive endothelial cells between VEGF-treated and untreated animals at 2 weeks in either GUSB-deficient or wild-type animals. In contrast, 8 weeks after administration, the VEGF-treated liver had greater numbers of vWF-positive cells as compared with untreated animals (Fig. 4a). The increase in vascular density in the heart was confirmed through immunohistochemistry by using BS-1 lectin on paraffin-embedded sections (Fig. 4b) and through morphologic examination by hematoxylin/eosin staining of these sections (data not shown).
Figure 4.
VEGF increases both newborn recipient vascularity and donor EPC engraftment. (a) Morphometric analysis of both GUSB-deficient and wild-type newborn mouse recipient heart and liver in the absence (No VEGF) or presence (VEGF) of 2.5 ng of VEGF per newborn mouse by fluorescent immunostaining with anti-vWF. (b) Histochemistry was performed on formalin-fixed paraffin-embedded sections by using BS-1 lectin (Left), or immunofluorescence was performed on cryosections by using anti-vWF antibody (Right) to detect endothelial cells in heart tissue obtained 2 weeks after administration of BM cells in the absence or presence of VEGF. (c) Numbers of vWF-positive cells that colocalized with GUSB-positive cells in the absence or presence of VEGF. *, statistically significant change over recipients not receiving VEGF at P ≤ 0.05.
The VEGF-mediated increase in donor-derived cells in multiple tissues (Fig. 3) may have resulted from VEGF-related changes in vascular permeability of recipient tissue and resultant accumulation of nonendothelial donor BM cells. To investigate whether VEGF had an effect on the number of donor-derived endothelial cells engrafted in newborn recipients, we performed immunofluorescent colocalization by using anti-vWF and anti-GUSB antibodies. There was a significant increase in the number of donor-derived cells that coexpress GUSB and vWF found in association with vessels of various sizes throughout the heart and liver 2 weeks after high-dose (2.5 ng per newborn mouse) VEGF administration over recipients who received cells and saline (Fig. 4c). Donor-derived endothelial cells (expressing both GUSB and vWF) persisted after 8 weeks in both heart and liver, albeit reduced in number from the 2-week time point (Fig. 4c). Interestingly, analysis of liver 8 weeks after administration showed that the donor-derived endothelial cells were restricted exclusively to sinusoids (data not shown). In summary, the number of GUSB-positive cells (donor-derived) that also expressed the endothelial cell marker, vWF, in both heart and liver in newborn recipients was significantly higher when the cells were administered with 2.5 ng (2 administrations of 1.25 ng per mouse) of VEGF.
Discussion
Recent studies suggest that BM-derived cells can contribute to vascularization in pathological states and during ovarian cycling in adult animals. Those studies provide the first evidence that in certain situations vascular repair or growth is not supported exclusively by angiogenesis, a process whereby endothelial cells arise from preexisting ones (4–7). However, those studies have not demonstrated postnatal vasculogenesis in normal tissues in adults or during postnatal development, but only in pathologic situations such as malignancy, ischemia, or wound repair (4–7, 24–25). By demonstrating the presence of donor-derived endothelial cells in vascular structures of normal newborn recipient tissues, this study provides the first report of postnatal vasculogenesis occurring during normal tissue growth and maintenance. These data suggest that vascular formation and maintenance during early postnatal growth and development may occur by a distinct mechanism and highlights the importance of studying these processes in their developmental context. Definitive molecular characterization of the EPC that distinguishes it from either more primitive progenitors or from sloughed, mature endothelial cells is incomplete (7, 26). Therefore, we chose to transplant unfractionated mouse bone marrow to ensure that we delivered EPCs to recipient animals.
Newborn serum VEGF levels increase sharply after birth and are higher than adult levels (27). Because VEGF is known to increase circulating BM-EPCs (28), the relative numbers of circulating BM-EPCs may be higher in the newborn period. Our experiments, however, do not address to what extent endogenous BM-EPCs and vasculogenesis contribute to neovascularization and vascular remodeling during this developmental period in unmanipulated animals.
The majority of the studies regarding the development of the vascular system have focused on the early embryonic period. During this period, VEGF is required, and angioblasts or EPCs arising from the blood islands differentiate in situ and assemble into capillary plexi (3). In the late embryo, as well as in the adult, normal growth and tissue maintenance are believed to occur exclusively by angiogenic remodeling, a process that is largely VEGF-independent and involves sprouting of capillaries from preexisting vessels (1–3). In adult mice, this hypothesis is supported by the lack of direct evidence of vasculogenesis in normal organs and tissues from either exogenously administered or tagged endogenous circulating EPCs (4–7). Our data showing a lack of donor-cell endothelial engraftment in the 4-week-old recipient mice are consistent with this hypothesis. Not only did the 4-week-old recipients receive an equivalent dose per weight of nucleated BM cells, but they also had higher levels of hematopoietic engraftment and greater theoretical generation of donor-derived EPCs. Although the growth rate is obviously greatly reduced in mice at 4 weeks of life as compared with the immediate newborn period, it is not completely abrogated. Weight gain of 3–5 g is typical from age 4 to 12 weeks (29). Thus, there is a continued need for active blood vessel formation, albeit reduced in magnitude.
Thus far, no reports have specifically addressed the mechanism of neovascularization during early postnatal development. However, recent studies suggest that the mechanism of neovascularization in the early postnatal period may be different from that of neovascular growth occurring later in development. Gerber et al. (30) inactivated VEGF in newborn mice within the first week after birth by either an inducible gene-targeting strategy or by using a soluble VEGF receptor. Their data suggest that early neonatal vascular growth was highly responsive to VEGF. Inactivation of VEGF within the first week of life led to high rates of mortality and histopathologic abnormalities in a number of tissues including heart, liver, and kidney. In contrast, disruption of VEGF ≈4 weeks after birth had no effect on mortality and only minor effects on tissue vascularity, suggesting a developmental switch in VEGF-mediated new blood vessel formation. Another group reported that VEGF levels increased dramatically after birth in human neonates (27). These findings suggest that neovascularization in the immediate postnatal period, in contrast to the adult animal, may be sensitive to VEGF.
Our findings that donor BM-derived angioblasts contribute at least in part to vascular growth during the early postnatal period suggest that vasculogenesis is involved in neovascular growth in the neonate. The data presented here are further supported by a previous study where we observed donor-derived cells associated with vascular-like structures in normal tissue after sublethal radiation and BM transplantation performed in newborn mice (31). There were more vascular-like structures containing donor cells in the previous study than in the current one, and those vascular-associated donor cells persisted for at least 10 months (31). Because the experimental design in the current study involved a single administration of unfractionated BM cells without radioablation, the levels of EPC engraftment observed were expectedly low. We chose to perform the current study without radioablation to ensure that endothelial cell engraftment would occur in the absence of any radiation-induced damage.
The finding that VEGF dramatically increased donor EPC engraftment into foci of new blood vessels in newborn mice supports our hypothesis that there may be a postnatal window of vasculogenesis that is regulated by VEGF. Although the 4-week-old recipients received an equal number of weight-adjusted donor cells, the absence of any colocalization of donor cells with endothelial markers supports the proposed (30) notion that there may be a mechanistic switch with regard to VEGF-mediated neovascularization during development. We propose that this switch also may coincide with a vasculogenic-to-angiogenic switch.
An alternative explanation of our results is that EPCs will incorporate into any foci of active angiogenesis. Two lines of evidence argue against this explanation. First, administration of equivalent numbers of BM cells per gram weight to 4-week-old mice did not result in any detectable EPC engraftment despite the fact that the recipients continued to grow during this period. Second, although coadministration of recombinant VEGF with BM cells to neonates increased tissue vascularity in the liver ≈3-fold by 8 weeks after administration, the numbers of vWF-positive, donor-derived sinus lining cells increased greater than 35-fold over recipients that did not receive VEGF. If EPC engraftment depended solely upon the extent of new blood vessel formation, then the increase in the number of donor-derived endothelial cells would be expected to parallel the increase in vascularity. Together, these findings suggest that VEGF may modulate the efficiency and/or persistence of EPC engraftment. Thus far, several groups have considered the possibility of exploiting enhanced endothelialization in various ischemic models; i.e., wound healing, diabetes, ischemic limb salvage, and myocardial infarction by the addition of viable EPCs to supplement vascularization (4–7, 28). It will be interesting to test whether VEGF also can modulate EPC engraftment in these pathologic models of neovascularization.
Abbreviations
- EPC
endothelial progenitor cells
- BM
bone marrow
- VEGF
vascular endothelial growth factor
- GUSB
β-glucuronidase
- vWF
von Willebrand factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Yancopoulos G D, Klagsburn M, Folkman J. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain R K. Nature (London) 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner J M. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner J M. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Schatteman G C, Hanlon H D, Jiao C, Dodds S G, Christy B A. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalka C, Masuda H, Takahashi T, Kalka-Moll W M, Silver M, Kearney M, Li T, Isner J M, Asahara T. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohlemiller K, Vogler C A, Roberts M, Galvin N, Sands M S. Exp Eye Res. 2000;71:469–481. doi: 10.1006/exer.2000.0897. [DOI] [PubMed] [Google Scholar]
- 9.Bastedo L, Sands M S, Lambert D T, Pisa M A, Birkenmeier E, Chang P L. J Clin Invest. 1994;94:1180–1186. doi: 10.1172/JCI117434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman B J, Roberts M S, Vogler C A, Nicholes A, Hofling A A, Sands M S. Blood. 1999;94:2142–2150. [PubMed] [Google Scholar]
- 11.Sands M S, Barker J E. Lab Anim Sci. 1999;49:328–330. [PubMed] [Google Scholar]
- 12.Soper B W, Duffy T, Vogler C A, Barker J E. Exp Hematol. 1999;27:1691–1704. doi: 10.1016/s0301-472x(99)00098-3. [DOI] [PubMed] [Google Scholar]
- 13.Glaser J H, Sly W S. J Lab Clin Med. 1973;82:969–971. [PubMed] [Google Scholar]
- 14.Wolfe J H, Sands M S. In: Gene Transfer into Neurones: Towards Gene Therapy of Neurological Disorders. Lowenstein P, Enquest L, editors. Essex, U.K.: Wiley; 1996. p. 263. [Google Scholar]
- 15.Sands M S, Birkenmeier E H. Proc Natl Acad Sci USA. 1993;90:6567–6570. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder E Y, Taylor R M, Wolfe J H. Nature (London) 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 17.Sly W S, Quinton B A, McAlister W H, Rimoin D L. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 18.Vogler C A, Birkenmeier E H, Sly W S, Levy B, Pegors C, Kyle J W, Beamer W G. Am J Pathol. 1990;136:207–217. [PMC free article] [PubMed] [Google Scholar]
- 19.Rao S S, Peters S O, Crittenden R B, Stewart F M, Ramshaw H S, Quesenberry P J. Exp Hematol. 1997;25:114–121. [PubMed] [Google Scholar]
- 20.Little D, Said J W, Siegel R J, Fealy M, Fishbein M C. J Pathol. 1986;149:89–95. doi: 10.1002/path.1711490203. [DOI] [PubMed] [Google Scholar]
- 21.Marder V J, Mannucci P M, Firkin B G, Hoyer L W, Meyer D. Thromb Haemostasis. 1985;54:871–872. [PubMed] [Google Scholar]
- 22.Alroy J, Goyal V, Skutelsky E. Histochemistry. 1987;86:603–607. doi: 10.1007/BF00489554. [DOI] [PubMed] [Google Scholar]
- 23.Henry T D. Br Med J. 1999;318:1536–1539. doi: 10.1136/bmj.318.7197.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix M J, Seftor E A, Meltzer P S, Gardner L M, Hess A R, Kirschmann D A, Schatteman G C. Proc Natl Acad Sci USA. 2001;90:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrix M J, Seftor R E, Seftor E A, Gruman L M, Lee L M, Nickoloff B J, Miele L, Sheriff D D, Schatteman G C. Cancer Res. 2002;62:665–668. [PubMed] [Google Scholar]
- 26.Peichev M, Naiyer A J, Pereira D, Zhu Z, Lane W J, Williams M, Oz M C, Hicklin D J, Witte L, Moore M A, Raffi S. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 27.Malamitsi-Puchner A, Tziotis J, Protonotariou E, Xyni K, Sarandakou A, Creatsas G. Pediatr Res. 1999;45:877–880. doi: 10.1203/00006450-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczed A, Iwaguro H, Hayashi S I, Isner J M, Asahara T. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 29.Daly T M, Ohlemiller K K, Roberts M S, Vogler C A, Sands M S. Gene Ther. 2001;8:1291–1298. doi: 10.1038/sj.gt.3301420. [DOI] [PubMed] [Google Scholar]
- 30.Gerber H, Hillan K J, Ryan A M, Kowalski J, Keller G, Rangell L, Wright B D, Radtke F, Aguet M, Ferrara N. Development (Cambridge, UK) 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 31.Sands M S, Barker J E, Vogler C, Levy B, Gwynn B, Galvin N, Sly W S, Birkenmeier E. Lab Invest. 1993;68:676–686. [PubMed] [Google Scholar]