Abstract
Protein tyrosine phosphatases (PTPases) exist in plants, but their role in plant signaling processes is unknown. One of the most important signaling networks in plants concerns the regulation of stomatal aperture, by which closure of stomatal pores restricts water loss in dry conditions, a process essential for plant survival. Closure is achieved by reduction in guard cell volume as a consequence of net efflux of potassium salt at both plasmalemma and tonoplast. To test whether protein tyrosine phosphorylation has any role in guard cell signaling processes, the effects on stomatal aperture and on guard cell K(Rb) fluxes of a number of specific inhibitors of PTPases have been investigated. Stomatal closure induced by abscisic acid, high external Ca2+, hydrogen peroxide, and dark were all prevented by one such inhibitor, phenylarsine oxide, which added to closed stomata promoted reopening. Flux measurements with 86Rb+ identified the efflux across the tonoplast as the sensitive process, implying that protein tyrosine dephosphorylation must occur at or downstream of the Ca2+ signal responsible for triggering ion efflux from the vacuole. There was no inhibition of efflux at the plasmalemma. A second inhibitor of PTPases, 3,4 dephosphatin, gave very similar effects, inhibiting closure induced by abscisic acid, high external Ca2+, and dark, and promoting reopening if added to closed stomata. Again, the efflux of K(Rb) at the tonoplast was the sensitive process. These results provide clear evidence for the involvement of PTPases in a major signaling network in plants.
Although the existence of protein tyrosine phosphatases (PTPases) in plants has recently been recognized, their role in plant signaling processes is unknown. A tyrosine-specific PTPase, AtPTP1, has been characterized from Arabidopsis (1) and shown to be encoded by a stress-responsive gene (up-regulated by high salt, down-regulated by cold). A dual-specificity PTPase, AtDsPTP1, which is capable of inactivating mitogen-activated protein (MAP) kinase from Arabidopsis, also has been identified (2). Other PTPase-like genes also have been identified in the Arabidopsis genome, but their functions remain to be identified (for review, see ref. 3). Protein tyrosine kinases/phosphatases play a major role in signal transduction in animal cells (4), and it is important to establish their functions in plants.
The bending of the touch-sensitive petiole in Mimosa pudica has been shown to be associated with a reduction in the degree of tyrosine phosphorylation of actin in the petiole (5), and both the tyrosine dephosphorylation in actin and the bending in response to touch are inhibited by phenylarsine oxide (PAO), a specific inhibitor of PTPases. Petiole bending is produced by the loss of turgor in motor cells in the lower half of the pulvinus which involves the loss of KCl from the motor cells, changes which are akin to the changes in guard cell turgor, volume, and solute content responsible for stomatal closure. To test whether protein tyrosine phosphorylation has any role in guard cell signaling processes, the effects on stomatal aperture and on guard cell K(Rb) fluxes of a number of specific inhibitors of PTPases have been investigated.
The signaling networks concerned with the regulation of stomatal aperture are of critical importance for plants, given that stomatal closure to restrict water loss in dry conditions is essential for their survival. Such closure requires net efflux of potassium salt at both plasmalemma and tonoplast. The changes associated with stomatal closing induced by abscisic acid (ABA) have been most extensively studied and are understood best. We have some incomplete understanding of the signal transduction chains involved in the changes in ion channel activity at the plasmalemma but much more limited understanding of the mechanisms of control of ion fluxes at the tonoplast. Tracer flux measurements offer the only method for investigating signaling events associated with tonoplast transport processes in an intact cell, given that the tonoplast is not electrically accessible in conditions in which cytoplasmic proteins and all potential signaling intermediates are present. Previous flux studies in isolated guard cells, using 86Rb+ as an analogue for K+, have investigated the effects of ABA, with the aim of understanding how this closing signal induces efflux of K+ at both plasmalemma and tonoplast. The results establish that ABA induces a transient stimulation of tracer efflux across the tonoplast; it was suggested that an increase in cytoplasmic Ca2+ to a threshold level acts as trigger for the efflux transient, that both influx of Ca2+ from outside and its release from internal stores contribute to the increase, in different relative proportions in different conditions (6), and that ABA acts to change the set-point of some regulatory system (6, 7).
The work reported in this paper shows that stomatal closure induced by four different closing signals, namely ABA, high external Ca2+, hydrogen peroxide, and dark, were all prevented by PAO. Flux measurements with 86Rb+ identified the efflux across the tonoplast as the sensitive process, implying that protein tyrosine dephosphorylation is involved at or downstream of the Ca2+ signal responsible for triggering ion efflux from the vacuole. A second such inhibitor, 3,4 dephosphatin (3,4 DP), gave very similar results; results consistent with this hypothesis also were seen with two vanadate derivatives which also inhibit PTPases, bpV(phen) [potassium bisperoxo(1,10-phenanthroline)oxovanadate], and DMHV [bis(N,N-dimethylhydroxamido)hydroxovanadate]. These results provide clear evidence for involvement of PTPases in a major signaling network in plants.
Materials and Methods
Inhibitors.
PAO, 3,4 DP, and DMHV were dissolved in DMSO before dilution into the incubation medium; final concentrations of DMSO (0.05%, 0.25%, and 0.2% respectively) had no effect in solvent controls. Bpv(phen) was dissolved directly in the incubation medium.
Aperture Measurements.
Leaves of Commelina communis L. were submerged in water and incubated in light for at least 1 h to open stomata. Abaxial epidermal strips were removed and floated on 30 mM KCl/0.1 mM CaCl2/10 mM Pipes, pH 6 in light, in a thermostated cabinet at 25°C, lit by a bank of fluorescent tubes (PAR 140 μmol·m−2·s−1). Initial apertures were measured before transfer to closing conditions, with or without inhibitors present. At intervals, strips were transferred to a slide for aperture measurements with an eyepiece micrometer, before return to the incubation. In general, apertures were measured over 5–6 h, but in some experiments, apertures were measured again after overnight incubation in closing conditions, with or without inhibitor.
Efflux Measurements.
Flux measurements were as described (6–8). Isolated guard cells of C. communis L. were prepared by treatment of epidermal strips at low pH to kill all cells other than guard cells. Cells were loaded by incubation overnight (about 16 h) in solutions containing 2 mM RbCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 10 mM Pipes, pH 6, and labeled with 86Rb+ (Amersham Pharmacia). (Thus, Rb+ is used as an analogue for K+, not as a tracer.) Effluxes were measured by transferring individual strips to successive 0.75-ml portions of nonradioactive solutions of the same chemical composition in the wells of plastic culture chambers on a vibrating shaker and counting both of these washout solutions and the residue left in the tissue at the end of the efflux by using standard scintillation methods.
Tracer was expressed as pmol·mm−2 on the basis of the area of each individual strip, and the rate of loss was calculated for each time interval. During the washout, the total tracer content in the tissue (Q*) will be represented by the sum of two exponentials, reflecting exchange in the cytoplasm (fast component) and vacuole (slow component), respectively. To reduce the variability between different strips arising from difference in number and size of guard cells, the rate constant for exchange (h−1) was calculated as (rate of loss of tracer)/(tracer content) and plotted against time. In constant conditions, the rate constant falls with time as the cytoplasmic efflux proceeds to reach a steady value equal to the rate constant for vacuolar exchange when the slow phase is reached. The addition of an inhibitor during the slow phase of exchange allows its effect on the vacuolar flux to be assessed, whereas addition in the latter stages of loading and throughout the washout allows the determination of its effect on the plasmalemma efflux.
Results
Effects of PAO on Stomatal Aperture.
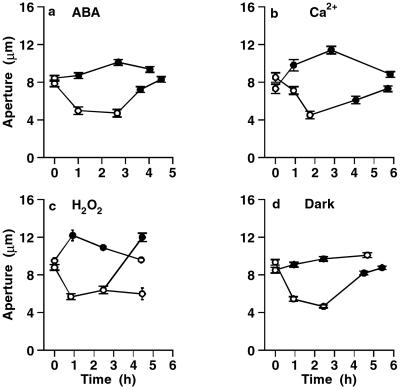
Because stomatal closure is the key process in guard cell function, the effect of 25 μM PAO was investigated on the response of stomatal aperture to four different treatments capable of closing stomata, namely ABA, high external Ca2+, hydrogen peroxide, and darkness. A set of representative results is shown in Fig. 1, with a very similar pattern of responses for each signal. Fig. 1a shows one of three experiments using 10 μM ABA as closing signal. In all, ABA-induced stomatal closure was prevented by PAO (n = 8/8), and stomata closed by ABA (in the absence of PAO) reopened when PAO was added after 1.5–2.5 h, with ABA still present (n = 7/7). The same pattern of responses was seen with the other three closing signals: addition of 10 mM Ca2+ externally, 0.2 mM H2O2, and transfer to the dark (Fig. 1 b–d, respectively). Thus, PAO prevented stomatal closure in response to all four closing signals, and indeed, if the starting aperture was relatively small, there was a degree of opening upon adding PAO plus the closing signal. It should be noted that the addition of PAO to open stomata had no effect on aperture. Furthermore, PAO added later was able to reopen stomata which had closed normally in response to any of the four signals. For each treatment, responses were measured on 4–12 strips.
Figure 1.
PAO (25 μM) prevents stomatal closure induced by four closing signals. The closing signal was applied immediately after the first aperture measurement, and changes in aperture followed with time. Each point shows the mean and SEM of 2–4 strips, from 24 to 88 pores. In each curve, white and black symbols show measurements in the absence and presence of 25 μM PAO, respectively, in 30 mM KCl. (a) 10 μM ABA. (b) Addition of 10 mM Ca2+ externally. (c) Addition of 0.2 mM H2O2. (d) Transfer to dark.
Effect of PAO on 86Rb+ Effluxes.
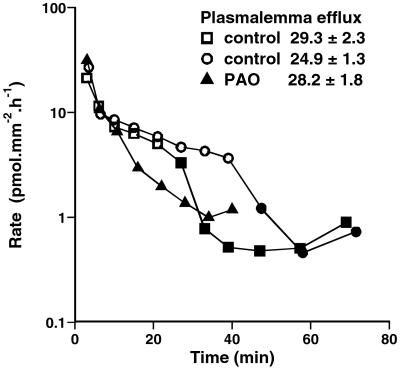
After loading isolated guard cells with 86Rb+ overnight, the rate of tracer loss was followed with time. Fig. 2 shows the effect of 25 μM PAO on the efflux when added at three different times: after 24 or 42 min of washout (at a stage of efflux when cytoplasmic exchange is effectively complete, and the remaining tracer is vacuolar), or when added to the loading solution for the last 30 min of loading, and to the washout solution from the start of efflux (allowing the effect on the efflux at the plasmalemma to be determined). PAO markedly inhibits the tonoplast efflux (by 85% and 88% in the two vacuolar curves shown) but does not affect the initial rate of loss, which is dominated by the flux at the plasma membrane. This distinction is most clearly seen in the fitting to two exponentials to distinguish cytoplasmic and vacuolar exchange, and the calculated values of initial efflux are shown in Fig. 2 Inset. In a replicate set (not shown), in which 5 μM PAO was added at 24 min of washout, the plasmalemma efflux was again unchanged, but the tonoplast efflux was severely inhibited (by 88%). The inhibition of vacuolar flux was confirmed in four further sets at 25 μM PAO (inhibition by 82–96%), one set at 10 μM (by 78%), and two further sets at 5 μM (by 72% and 87%); in four of these further sets, the effect on cytoplasmic exchange also was tested, and the lack of effect of PAO on the plasmalemma efflux was confirmed.
Figure 2.
Effect of PAO on the rate of loss of 86Rb+ from isolated guard cells; rate of loss of tracer against time of washout. White and black symbols refer to the absence and presence of 25 μM PAO. Each curve shows the means of four replicate strips (SEM of 10–20%). PAO was added at 24 or 42 min of washout in its absence (when tracer is predominantly vacuolar) or was added to the last 30 min of loading and from the start of the washout (when both cytoplasm and vacuole are labeled). Tracer content Q* (pmol·mm−2) for the first 24–47 min of efflux was fitted to two exponentials, and the efflux at the plasma membrane was calculated.
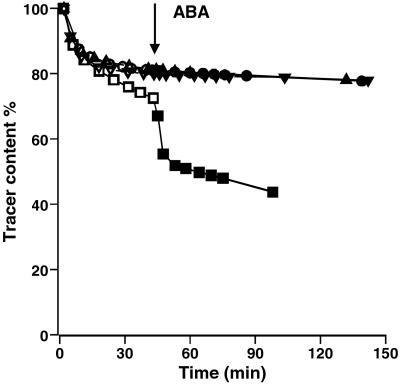
When ABA is added to guard cells, there is a marked transient stimulation of efflux across the tonoplast, with a much smaller stimulation of the efflux at the plasma membrane (8). The effect of PAO on the ability to generate the ABA-induced transient was therefore determined. Fig. 3 shows the plot of tracer content against time in replicate sets of tissue, with PAO added at the start of the washout and 10 μM ABA added after 40–44 min of efflux. There was a very large stimulation of efflux in the control, whereas in the presence of PAO (5, 10, or 25 μM), this transient was abolished. In a second experiment (not shown), cells were treated with 1, 5, or 25 μM PAO for 19–23 min (reducing the rate constant for vacuolar efflux to 60%, 28%, and 9% of the control value) before adding ABA at 42–59 min of washout; in this instance, the efflux transient was unaffected by 1 μM PAO but was abolished by 25 μM, and a much reduced transient was generated in the presence of 5 μM PAO, with the peak reduced to 30% of that in the absence of PAO. PAO added after the ABA transient (25 μM) reduced the rate constant for vacuolar efflux to 11% of the value in its absence.
Figure 3.
Effect of PAO on the ABA-activated release of vacuolar 86Rb+. Tracer content (percentage of initial value) against time. Initial washout in the absence of ABA (white symbols) in the presence of 0, 5, 10, or 25 μM PAO. ABA added to all four sets (black symbols) at 40–44 min of efflux. Each curve shows the mean of four replicate strips (SEM of 2–3% of each value). PAO concentration: □ and ■, control; ▵ and ▴, 25 μM; ○ and ●, 10 μM; ▿ and ▾, 5 μM.
Effect of Other Inhibitors of PTPases:Fluxes.
It is important to establish that the effects of PAO are the result of its ability to inhibit PTPases. Therefore, the specificity of action was tested by the use of three other inhibitors of PTPases, 3,4 DP and two vanadate derivatives, DMHV and bpV(phen).
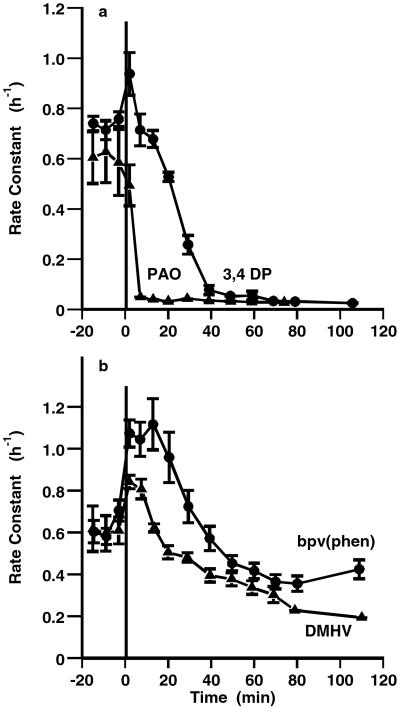
Fig. 4a shows an experiment in which 3,4 DP and PAO both inhibited the tonoplast efflux to the same extent (by about 95%), but the inhibition by 3,4 DP was slower to develop (over 40 min rather than 10 min). In the same experiment, the inhibition by the two vanadate derivatives was less effective and even slower to develop (Fig. 4b); DMHV (50 μM) inhibited by about 70% and bpv(phen) (100 μM) by about 50% over 80 min, but both showed a stimulation of efflux over the first 20 or 30 min, respectively. These results suggest that although all four inhibitors reduce tonoplast efflux, argued to be the result of inhibition of PTPase, the two vanadate derivatives have a second action initially, seen in the stimulation of tonoplast efflux. Vanadate itself, although commonly used to inhibit PTPases in animal cells, is not suitable for use in plants, given its ability to inhibit P-type ATPases (including Ca-ATPases). If the vanadate derivatives share this ability, then the initial transient stimulation of tonoplast efflux may reflect disturbance of calcium homeostasis in the initial period.
Figure 4.
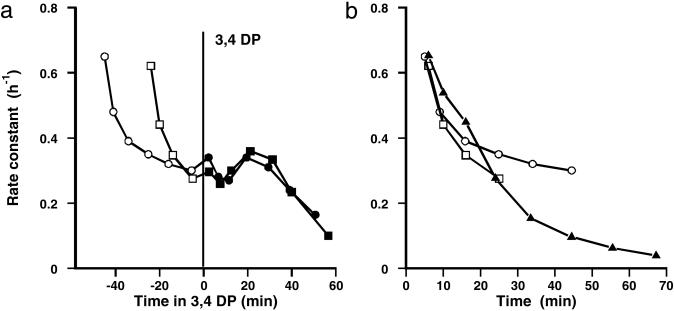
Comparative effects of different inhibitors of tyrosine phosphatases on vacuolar fluxes. Effects of (a) 25 μM PAO and 25 μM 3,4 DP and (b) 50 μM DMHV and 100 μM bpv(phen) on the rate of loss of 86Rb+ from isolated guard cells. Rate constant (h−1) plotted against time of washout. Inhibitor added at zero time on graph (36 min after start, during the phase of vacuolar efflux). Each curve shows the means of four replicate strips, with SEM.
In a second experiment, to distinguish effects at plasmalemma and tonoplast, 3,4 DP was added at different stages of washout. Fig. 5a shows the plot of rate constant against time in 3,4 DP, when the inhibitor was added after 30 and 50 min of washout in its absence (to assess the effect on the tonoplast efflux); it is shown in this form to highlight the close agreement between the two vacuolar time courses, with inhibition developing after 30 min but with a suggestion of an initial oscillation. The third set was treated with 3,4 DP for the last 40 min of labeling and throughout the washout (to assess the effect on the plasmalemma efflux). Fig. 5b shows the early stages of efflux in all three sets, and it is clear that 3,4 DP does not inhibit the initial efflux, the plasmalemma efflux, but only that at the tonoplast. Thus, the effects of 3,4 DP are very similar to those of PAO, but somewhat slower to develop (perhaps because of different penetration times).
Figure 5.
Effect of 3,4 DP on rate of loss of 86Rb+ from isolated guard cells. Rate constant (h−1) for loss of tracer against time of washout. White and black symbols refer to the absence and presence of 25 μM 3,4 DP. Each curve shows the means of four replicate strips (SEM of 10–20%). 3,4 DP was added at 24 or 42 min of washout in its absence (when tracer is predominantly vacuolar) or added to the last 30 min of loading and from the start of the washout (when both cytoplasm and vacuole are labeled) to measure the effect on efflux at the plasmalemma. (a) Effect of 3,4 DP on vacuolar efflux. (b) Comparison of initial stages of washout (dominated by the plasmalemma efflux) with and without 3,4 DP.
The Effect of 3,4 DP on Apertures.
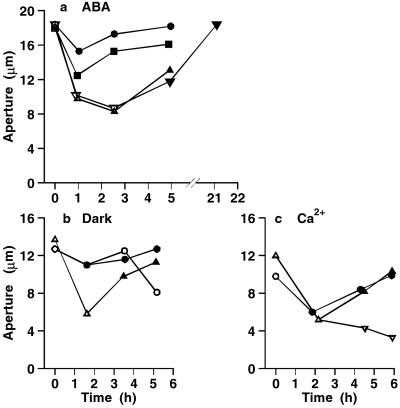
As might be predicted from the flux effects, the effects of 3,4 DP on stomatal closure induced by ABA, Ca2+, or transfer to the dark, were very similar to those of PAO, effectively preventing stomatal closure when applied at the start, and inducing reopening when added after normal closure in the absence of the inhibitor. In different experiments, the inhibitor was applied at concentrations in the range of 10 to 40 μM but with very similar results. Fig. 6 a–c shows representative results for the effects of ABA, dark, and Ca2+ respectively. In all, inhibition of closure was observed in 4/4 epidermal strips in ABA, 10/10 strips in high Ca2+, and 4/4 strips in the dark; reopening on adding the inhibitor to closed stomata was observed in 4/4 strips in ABA, 8/8 strips in high Ca2+, and 2/2 strips in the dark. Thus, two different inhibitors of PTPases, PAO and 3,4 DP, have very similar effects on both fluxes and apertures.
Figure 6.
3,4 DP prevents stomatal closure induced by ABA, dark, and Ca2+. The closing signal was applied immediately after the first aperture measurements, and changes in aperture followed with time. In each curve, white and black symbols show measurements in the absence and presence of 3,4 DP. (a) 10 μM ABA, 40 μM 3,4 DP. Each curve shows the mean of two replicate strips. (b) Dark, 25 μM 3,4 DP. Four strips were closed in the dark in the absence of inhibitor, and 3,4 DP was added after 1.6 h (▵, ▴). Four strips were treated in the dark in the presence of 3,4 DP; 3,4 DP was then removed from two strips (○), and two were left in 3,4 DP (●). (c) 10 mM external Ca2+, 25 μM 3,4 DP. Six replicate strips were transferred to 3,4 DP from zero time (○, ●). Of the six control strips closed in the absence of 3,4 DP (▵), four were treated with 3,4 DP after 2.2 h (▴), and two remained in the absence of inhibitor (▿). SEs (not shown) were in the range of 3% to 10%.
The Effect on Apertures of the Two Vanadate Derivatives, DMHV and bpv(phen).
The complex time course seen in the flux experiments in which DMHV and bpv(phen) were added during washout, with an initial stimulation of vacuolar efflux preceding the inhibition at longer times, would predict that the effects on aperture of adding these compounds also would be complex. This prediction was confirmed by the results, and for this reason, a full study of the effects of the two vanadate derivatives on aperture has not been attempted. In general, the two vanadate derivatives were much less effective than either PAO or 3,4 DP in preventing stomatal closure. Over the period of 5–6 h used for most aperture measurements, the vanadate derivative inhibited rather than prevented stomatal closure, and this was followed by slow reopening. The extent of both the initial closure and the subsequent recovery were variable in different batches of tissue. Both inhibition of dark-induced closure by 30–100 μM bpv(phen) and stimulation of some reopening when added after closure in the control were observed, and smaller effects on Ca2+-induced closure also were measured. In one of two experiments using ABA, 100 μM bpv(phen) inhibited, but did not prevent, ABA-induced closure over 4.6 h and promoted reopening after a further 17 h; in a second experiment, there was no significant inhibition of ABA-induced closure by bpv(phen) over 3–4 h (although there was a small inhibition of dark-induced closure in this tissue). In all, bpv(phen) inhibited dark-induced closure in 14/14 epidermal strips (although in two of these strips, the effect was small), ABA-induced closure in 4/8 strips, and Ca2+-induced closure (by a small amount) in 4/4 strips.
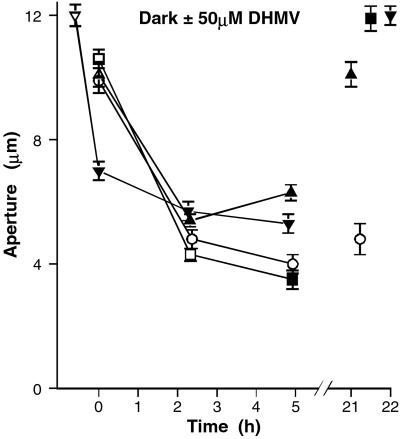
The complexity is best seen in the experiment shown in Fig. 7, showing the effect of DMHV on dark-induced closure. Stomata closed appreciably over 2 h in the dark in the presence of 50 μM DMHV but were nevertheless significantly more open than in the control (four strips in each set, P < 0.01); they reopened significantly over the next 3 h, and after a further 16 h, they had recovered the initial aperture, whereas the controls showed no reopening. If DMHV was added after closure in the dark, there was no reopening over the next 2.5 h; but, after a further 16 h, they were more open than at the start. Fig. 7 also shows that stomata pretreated with DMHV for 48 min in the light close significantly, with little further closure on transfer to the dark. However, in a second experiment, there was no inhibition of dark-induced closure by DMHV, measured over 5 h (four strips). Overall, the results are consistent with a dual effect of these two inhibitors, as suggested by the flux experiment involving both inhibition of PTPases, promoting opening, and a second effect, important in the initial stages, promoting stomatal closing.
Figure 7.
Effect of 50 μM DMHV on dark-induced closing. Aperture against time, after transfer to dark at zero time; changes in aperture in the presence and absence of inhibitor followed over 5 h and also at 21–22 h. One set of tissue was pretreated with DMHV in light for 48 min before transfer to dark. Each point shows the mean of two strips, from 32 to 40 pores. SEs (not shown) were in the range of 3% to 15%. In each curve, white and black symbols show measurements in the absence and presence of the inhibitor.
Discussion
The first key observation is that PAO, a specific inhibitor of PTPases, prevented stomatal closure in response to four different closing signals, namely ABA, high external Ca2+, transfer to the dark, and hydrogen peroxide, and furthermore, that PAO added later was able to reopen stomata which had closed normally in response to any of the four signals. Thus PAO also prevented the inhibition of opening by these closing agents.
High external Ca2+ produces an increase in cytoplasmic Ca2+ (9), and the inhibition of Ca2+-induced closure therefore shows that the process sensitive to PAO is either Ca2+ influx or downstream of the increase in cytoplasmic Ca2+ in the signaling chains. An ABA-induced efflux transient can be produced in the absence of external Ca2+ (6), and therefore a site downstream of Ca2+ is the more likely. The inhibition of H2O2-induced closure is important, because it rules out an alternative interpretation of the mode of action of PAO. Although it is widely used as a specific inhibitor of PTPases, it can also inhibit the activation of NADPH oxidase in human neutrophils (10). Application of ABA produces H2O2 in guard cells, and externally added H2O2 activates Ca2+ channels in the plasmalemma and increases cytoplasmic Ca2+, suggesting H2O2 lies on the signaling pathways downstream of ABA (11). However, if the effect of PAO were attributable to inhibition of the activation of NADPH oxidase in guard cells (thereby preventing the generation of H2O2), PAO would not be effective in blocking H2O2-induced stomatal closure. The results therefore suggest that the action of PAO in guard cells reflects its widespread effectiveness as an inhibitor of PTPases.
The ability of PAO to inhibit stomatal closure in response to different stimuli places the sensitive step central in the overall process of closing. Closure requires net efflux of ions at both plasmalemma and tonoplast, and the second key observation is that PAO strongly inhibited the efflux of 86Rb+ at the tonoplast, but had no effect on the plasmalemma efflux. The flux measurements therefore identify ion transfer of K/Rb from vacuole to cytoplasm as the sensitive process, and suggest that it is regulated by the degree of tyrosine phosphorylation of a target protein, with the dephosphorylated state required for ion transfer. The reopening on adding inhibitor to closed stomata, still maintained in closing conditions, and the inhibition of efflux on adding PAO after the ABA-induced efflux transient, suggest that there is turnover of phosphate on the target protein, and that initiation of an efflux transient in response to a closing signal involves activation of a PTPase.
The observation that a second inhibitor of PTPases, 3,4 DP, has effects very similar to those of PAO, gives strong support to the hypothesis that the action of PAO should be attributed to its ability to inhibit PTPases. 3,4 DP, like PAO, inhibits stomatal closure in response to ABA, dark and high external Ca2+, and promotes reopening when added to stomata after normal closure in response to these signals. The site of action of 3,4 DP is also clearly located at the tonoplast, inhibiting K/Rb efflux at that membrane without any effect on plasmalemma efflux.
The effects of the two vanadate derivatives tested in a preliminary way, DMHV and bpv(phen), are more complex and more variable, but the effects on aperture are entirely consistent with the effects on fluxes. The inhibition of tonoplast flux, promoting stomatal opening, is clearly seen in the later stages of the efflux curves, but is preceded by a period of stimulated tonoplast efflux, which will promote stomatal closure. Thus the initial partial closure followed by delayed opening seen in the aperture experiments is what would be predicted from the flux experiments. The initial stimulated tonoplast efflux suggests increase in cytoplasmic Ca2+; if the vanadate derivatives share the ability of vanadate to inhibit P-type ATPases, including Ca2+-ATPases, then an effect of this kind is to be expected. The results are entirely consistent with a concurrent inhibition of PTPase, and consequent inhibition of tonoplast (K/Rb) efflux.
Although it is now clear that plants have PTPases, we have no understanding of their role in plants. PAO inhibits both the bending of the touch-sensitive petiole in Mimosa pudica and the tyrosine dephosphorylation of actin associated with this movement (5). The similarity between the changes in pulvinar motor cells associated with the collapse of the petiole, and the changes in guard cells associated with stomatal closure, suggests that the processes may share mechanisms and signaling chains. Actin organization is linked to its degree of tyrosine phosphorylation in Dictyostelium cells (12, 13), and, among its many other effects, ABA induces changes in the state and distribution of actin filaments in guard cells (14–16). In open guard cells actin is organized in thick, radial cables, but in response to ABA or high Ca2+ this organization is disrupted as stomata close. It is not clear whether the two events are causally related or parallel phenomena. More recent work found correlations between stomatal aperture, the organization of guard cell actin and the activity of a small Rho-related GTPase in Arabidopsis, AtRac1 (17). ABA inactivated AtRac1 with the same time course as it induced stomatal closure, and the behavior of dominant-positive and dominant-negative mutants confirmed the association of active Rac1 with organized actin cables in open stomata, contrasting with inactive Rac1 and disorganised actin in closed stomata.
Given the correlation between actin tyrosine dephosphorylation and leaf movement in Mimosa, actin must be a strong potential candidate for the target protein for the action of PAO in guard cells. The possible role for actin remains obscure, and difficult to relate to the specific target process identified in the present paper, the transfer of ions from vacuole to cytoplasm. The organization of actin might determine its interaction with a tonoplast channel (or a channel regulatory protein), affecting channel activity, but it is also possible that the number of channels is regulated by membrane trafficking sensitive to the phosphorylation state of either actin or the channel itself. Recently it has been shown that PAO decreased the activity of the ROMK1 potassium channel in kidney (18); the reduction was the result of increased phosphorylation of ROMK1, stimulating dynamin-dependent endocytosis and internalization of the channel, reducing the number of channels in the plasma membrane. Thus an effect of PAO on tonoplast channels might reflect changes in either their activity or their number. Another possibility would be that the process of ion transfer from vacuole to cytoplasm itself involves actin-dependent vesicle-trafficking, sensitive to the phosphorylation state of actin.
One other effect of PAO has been reported (19), namely the inhibition of ABA-induced rab16 gene expression in barley aleurone. It was shown that ABA activated a MAP kinase, and that both actions were blocked by PAO, suggesting that activation of a PTPase was upstream of the MAP kinase activation in the signaling chain. Activation by ABA of a MAP kinase has also been reported in guard cells, and the inhibitor of the activation of MAP kinase (PD98059) gives a partial inhibition of ABA-induced stomatal closure in the Argenteum mutant of pea (20). However, PD 98059 does not affect either the ABA-induced tonoplast efflux transient, or ABA-induced closure, in Commelina (unpublished observations), which suggests that a MAP kinase signaling pathway is not the one responsible for sensitivity to PAO.
It is important to identify the target protein whose dephosphorylation results in activation of ion release from the vacuole. Apart from actin, other candidates must include a tonoplast ion channel directly, or a regulatory protein controlling the activity of such a channel. Although the target protein remains to be identified, the results clearly identify ion release from the vacuole as the target process—an essential component of the overall process of stomatal closure. The least well understood aspects of the guard cell system are transport at the tonoplast and its regulation, and indications of associated regulatory mechanisms are therefore important. Although the involvement of protein serine/threonine phosphatases in plant cell signaling (including that in guard cells) is well established, the present results provide clear evidence for involvement of tyrosine phosphatases in a major signaling network in plants. The results show that they play a critical role in the process of stomatal closure which is fundamental to the regulation of water loss essential for plant survival.
Acknowledgments
Thanks are due to Dr. Mark Tester and Dr. Alex Webb for discussion and critical comments, Mr. John Banfield for technical assistance, and the Leverhulme Foundation for the support of an Emeritus Fellowship.
Abbreviations
- PTPases
protein tyrosine phosphatases
- PAO
phenylarsine oxide
- ABA
abscisic acid
- 3,4 DP
3,4 dephosphatin
- bpv(phen)
potassium bisperoxo(1,10-phenanthroline)oxovanadate
- DMHV
bis(N,N-dimethylhydroxamido)hydroxovanadate
- MAP
mitogen-activated protein
Footnotes
See commentary on page 11567.
References
- 1.Xu Q, Fu H-H, Gupta R, Luan S. Plant Cell. 1998;10:849–857. doi: 10.1105/tpc.10.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R, Huang Y, Kieber J J, Luan S. Plant J. 1998;16:581–590. doi: 10.1046/j.1365-313x.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- 3.Luan S. Adv Bot Res. 2000;32:67–107. [Google Scholar]
- 4.Neel B G, Tonks N K. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 5.Kameyama K, Kishi Y, Yoshimura M, Kanzawa N, Sameshima M, Tsuchiya T. Nature (London) 2000;407:37. doi: 10.1038/35024149. [DOI] [PubMed] [Google Scholar]
- 6.MacRobbie E A C. Proc Natl Acad Sci USA. 2000;97:12361–12368. doi: 10.1073/pnas.220417197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacRobbie E A C. Plant J. 1995;7:565–576. [Google Scholar]
- 8.MacRobbie E A C. Plant J. 1995;7:835–843. [Google Scholar]
- 9.McAinsh M R, Webb A A R, Taylor J E, Hetherington A M. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doussiere J, Poinas A, Blais C, Vignais P V. Eur J Biochem. 1998;251:649–658. doi: 10.1046/j.1432-1327.1998.2510649.x. [DOI] [PubMed] [Google Scholar]
- 11.Pei Z-M, Murata Y, Benning G, Thomine S, Klüser B, Allen G J, Grill E, Schroeder J I. Nature (London) 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 12.Schweiger A, Michalachie O, Ecke M, Gerisch G. J Cell Sci. 1992;102:601–609. doi: 10.1242/jcs.102.3.601. [DOI] [PubMed] [Google Scholar]
- 13.Kishi Y, Clements C, Mahadeo D C, Cotter D A, Sameshima M. (1998) J Cell Sci. 1998;111:2923–2932. doi: 10.1242/jcs.111.19.2923. [DOI] [PubMed] [Google Scholar]
- 14.Eun S-O, Lee Y. Plant Physiol. 1997;115:1491–1498. doi: 10.1104/pp.115.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang J-U, Eun S-O, Lee Y. In: Actin: A Dynamic Framework for Multiple Plant Cell Functions. Staiger C J, Baluska F, Volkmann D, Barlow P W, editors. Dordrecht, The Netherlands: Kluwer; 2000. pp. 427–436. [Google Scholar]
- 16.Hwang J-U, Eun S-O, Lee Y. Plant Physiol. 2001;125:2120–2128. doi: 10.1104/pp.125.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemichez E, Wu Y, Sanchez J-P, Mettouchi A, Mathur J, Chua N-H. Genes Dev. 2001;15:1808–1816. doi: 10.1101/gad.900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterling H, Lin D-H, Gu R-M, Dong K, Hebert S C, Wang W-H. J Biol Chem. 2002;277:4317–4323. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knetsch M L W, Wang M, Snaar-Jagalska B E, Helmovaara-Dijkstra S. Plant Cell. 1996;8:1061–1067. doi: 10.1105/tpc.8.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnett E C, Desikan R, Moser R C, Neill S J. J Exp Bot. 2000;51:197–205. doi: 10.1093/jexbot/51.343.197. [DOI] [PubMed] [Google Scholar]