Abstract
Practicing a motor skill triggers a process of memory consolidation that continues for hours after practice has ended, and becomes manifest in an improved skill at later testing. We used a sequential motor task (finger-to-thumb opposition task) to show that, in humans, the formation of motor skill memories essentially benefits from sleep. Independent of whether placed during daytime or nighttime, sleep after practice enhanced speed of sequence performance on average by 33.5% and reduced error rate by 30.1% as compared with corresponding intervals of wakefulness. The effect of sleep after learning proved to be stable when retesting was postponed for another night, to exclude effects of sleep loss and to assure that all subjects had sufficient sleep before retrieval testing. Also, the consolidating effect of sleep was specific for the motor sequence learned. It did not generalize to a similar sequence containing identical movement segments in a different order. Retention periods of wakefulness improved performance only moderately and only if placed during daytime. The observations demonstrate a critical role of sleep for storing and optimizing motor skills.
Memory consolidation refers to processes of brain plasticity by which experiences result in more or less enduring changes in adaptive behaviors. In the case of motor skills, practicing a motor task leads to the generation of an internal model representing the different motor outputs in response to the various task stimuli (1–4). Development of the internal model does not stop when practice ends, but continues over hours during which the memory traces of the model are strengthened becoming, for example, increasingly resistant to behavioral interference (5). Most importantly, the internal model becomes also more effective in this process, as indicated by a distinct gain in performance at retesting 24 h later when the task is performed with greater speed and accuracy (6). A critical amount of practice is considered to initiate plastic processes, probably mainly in the primary motor cortex, which gate a shaping of the motor representations, and thereby improve performance in the absence of any further training (7, 8). This latent formation of motor memories has been proposed to be linked to a dynamic reorganization of the respective motor neuronal networks (1), and to require a covert reprocessing of the memory traces (9). Sleep, characterized by largely suppressed overt motor activity and conjunct sensory input, might represent a condition optimal for this reprocessing. However, its possible role for motor skill formation has not been assessed in depth.
Evidence has accumulated supporting the notion that processes during sleep significantly contribute to the formation of different types of memory (9–13). Although earlier human studies suggested facilitating influences of sleep mainly on hippocampus-dependent declarative types of memory, recent studies indicated a similar influence on the formation of procedural memories (11, 14, 15). In contrast to declarative memory, which refers to the knowledge of facts and events, procedural (or “how to”) memory for skills does not require the integrity of the hippocampus. Also, acquisition is slower and shows little generalization to similar behaviors (6, 7). In many cases, procedural learning appears to be linked to discrete changes in low-level representations in the hierarchy of sensory input and motor output processing, taking place in specific sensory and motor cortical areas (7, 8, 16–18). Several recent studies in humans have shown that the consolidation of memories for elementary perceptual skills (texture discrimination) critically depends on sleep (11, 15, 19). Retrieval testing on the texture discrimination task in these studies revealed a significant performance gain only if the learning session was followed by a period of sleep. Deprivation of sleep after practice completely prevented subsequent formation of memory for the trained skill, even when retrieval testing was delayed to allow for recovery sleep, indicating that the first nocturnal sleep period after practice is critical for initiating memory consolidation (11). However, these previous studies exclusively focused on perceptual skills. The present experiments were stimulated by indications that sleep could play a similar critical role for the slow latent consolidation process of motor skills (10, 20).
Methods
Participants.
A total of 52 healthy young student volunteers (18–29 years old; mean ± SD: 23.31 ± 2.69 years) who were nonsmokers, right-handed, and had no history of sleep disturbances participated in the experiments. None of the subjects had practiced playing a musical instrument nor was trained as a typist. All subjects regularly obtained 7–8 h of sleep per night and had no disruptions of the sleep–wake cycle during the 6 weeks before participation. All spent an adaptation night in the sleep laboratory before beginning the experiments. Subjects abstained from caffeine and alcohol the day before the experimental session. They were instructed to get up before 7:00 a.m. and not to take naps during the day. The experiments were approved by the Ethics Committee of the University of Lübeck.
Learning Task.
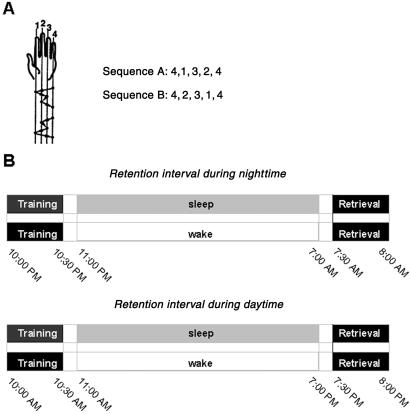
The finger-to-thumb opposition task (Fig. 1A) required the subject to tap with his nondominant hand (left hand) the finger sequence as rapidly and accurately as possible without looking at his hand. To familiarize the subject with the task he first practiced the task, with visual feedback provided through the monitor indicating the next finger to be tapped within a response interval of 400 ms. When a criterion of 10 consecutive correct reactions was met, the training period proper started, consisting of three 5-min blocks interrupted by two 2-min periods of rest. The procedure of retrieval testing after the retention interval was the same as during the training proper before sleep. During task performance the subject sat in a silent and darkened room. All instructions were presented on a 15-inch monitor. To register movements, each fingertip of the left hand was covered with aluminum foil connected to a personal computer. Two different motor sequences were used to allow testing of the same subject on two occasions.
Figure 1.
(A) Finger-to-thumb opposition task. The motor skill task was adopted from Karni et al. (10) and demanded the subject to oppose the fingers of the nondominant hand to the thumb in a certain sequence. Two sequences were used on different conditions, which both were composed of the same five movements but in a mirror-reversed manner. In sequence A, the order of the fingers was 4, 1, 3, 2, 4. In sequence B, the order was 4, 2, 3, 1, 4 (finger numbering is from index to little). During both training and retrieval testing, the subject was asked to tap the given sequence as fast and as accurately as possible without looking at his hand for three 5-min blocks. (B) Protocol for main experiments. Subjects received training (left black fields) on a finger sequence before 8-h retention intervals during which they either slept or stayed awake. Thirty minutes after the end of the retention period, retrieval was tested (right black fields). Retention periods were placed either at night (Upper) or during the day (Lower).
Design and Procedure.
In the main experiments, subjects (n = 20, 9 female) were trained on the finger-to-thumb opposition task in the evening (at 10:00 p.m.) before a nocturnal 8-h retention interval during which they either slept regularly or stayed awake. Thereafter (at 7:30 a.m.), motor performance was retested. To examine effects of sleep independent of circadian factors, possibly affecting learning and motor performance (21, 22), on a further condition learning took place in the morning (at 10:00 a.m.) before 8-h retention intervals of sleep and wakefulness placed during daytime. Retesting took place at 7:30 p.m. (Fig. 1B). Awakening from sleep was always 30 min before retrieval testing. Each subject was assigned to one nocturnal and one daytime retention condition, performing on each of these occasions one of the two motor sequences, respectively. The order of retention conditions and of motor sequences was balanced across subjects. Experimental conditions for each subject were at least 1 week apart. Subjects stayed awake the night before the daytime sleep condition to enable daytime sleep. Three supplementary studies were performed to examine (i) effects of sleep loss (n = 16, 6 female), (ii) effects of sleep versus wakefulness on a delayed retrieval (n = 6, 2 female), and to test (iii) for the specifity of the memory effects for the particular motor sequence (n = 10, 4 female).
Data Reduction and Analysis.
Sleep recordings were visually scored according to standard criteria (23). Performance in the finger-to-thumb opposition task was measured in terms of performance rate (mean number of correctly completed sequences per 30 s) and accuracy (mean number of errors per 30 s). The initial 30-s period of each block served as an adaptation period and was not included in the analysis. Changes in performance rate and accuracy across the retention intervals were also transformed to percentages, with the individual performance value at learning set to 100%. Repeated-measures ANOVA including a “before/after” and a “sleep/wake” factor with subsequent pairwise contrasts were used to analyze performance rate and errors. A P value < 0.05 was considered significant.
Results
Total sleep time was closely comparable during both the nocturnal and daytime retention intervals (Table 1). Expected circadian influences on sleep expressed themselves in a decreased time spent in stage 2 sleep (P < 0.01) and a tendency toward increased time in stage 1 sleep and rapid eye movement (REM) sleep (P < 0.1) during daytime sleep in comparison with nocturnal sleep. Initial learning of the sequence before the 8-hour retention intervals was comparable for all four conditions. Performance rate, i.e., the number of correctly completed sequences per 30 s, at learning was 12.96 ± 1.06 for the nocturnal sleep condition, 14.17 ± 0.8 for the nocturnal wake condition, 13.13 ± 0.72 for the daytime sleep condition, and 13.26 ± 0.58 for the daytime wake condition (P > 0.4, for pairwise comparisons). Also, error rates at learning did not differ among the conditions (nocturnal sleep: 7.68 ± 0.8; nocturnal wake: 6.51 ± 0.98; daytime sleep: 6.46 ± 0.65; daytime wake: 6.59 ± 0.98; P > 0.3 for pairwise comparisons).
Table 1.
Sleep parameters
| Nocturnal sleep | Daytime sleep | t | |
|---|---|---|---|
| Total sleep time, min | 418.9 ± 7.23 | 425.9 ± 14.24 | −0.44 |
| Sleep onset, min | 14.5 ± 3.53 | 6.6 ± 2.11 | 1.93 |
| Sleep efficiency, % | 93.77 ± 1.62 | 91.43 ± 2.94 | 0.70 |
| REM latency, min | 90.4 ± 13.38 | 74.8 ± 5.24 | 1.09 |
| Wake time, min (%) | 11.4 ± 5.40 (2.62 ± 1.24) | 26.6 ± 13.59 (5.85 ± 2.95) | −1.04 |
| Stage 1 sleep, min (%) | 22.1 ± 2.96 (5.08 ± 0.65) | 44.1 ± 11.75 (9.79 ± 2.59) | −1.82 |
| Stage 2 sleep, min (%) | 249.3 ± 8.64 (58.01 ± 2.08) | 203.5 ± 11.49 (44.92 ± 2.35) | 3.19* |
| SWS, min (%) | 73.8 ± 8.48 (17.18 ± 2.0) | 83.2 ± 10.22 (18.41 ± 2.25) | −0.71 |
| REM sleep, min (%) | 73.7 ± 6.13 (17.10 ± 1.33) | 95.2 ± 9.22 (21.01 ± 1.99) | −1.94 |
Sleep during 8-h retention intervals placed during nighttime and daytime. Mean (± SEM) total sleep time, time to sleep onset (after lights off), sleep efficiency (% of total 8-h period not spent awake), latency to first REM sleep period (with reference to sleep onset), wake time, and time spent in stage 1 sleep, stage 2 sleep, SWS, and REM sleep are shown. Time in sleep stages is indicated in min and % of total sleep time. Right column indicates t values for statistical comparisons between the two conditions.
, P < 0.05.
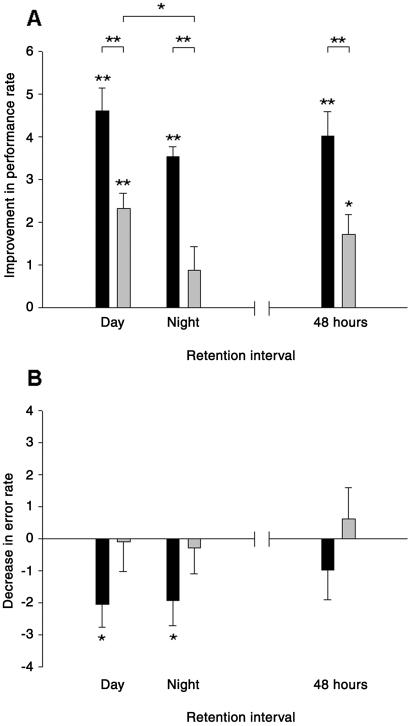
Performance rates were generally improved at retesting after the 8-h retention interval, with this improvement strongly depending on sleep versus wakefulness during the retention interval [F(1,36) = 167.0, P < 0.001 for main effect before/after and F(1,36) = 31.81, P < 0.001 for before/after × sleep/wake ANOVA interaction; Fig. 2A]. Indeed, the benefit for performance rates was considerably stronger after retention intervals of sleep than that revealed for the corresponding nocturnal or daytime retention intervals of wakefulness [F(1,18) = 19.40, P < 0.001 and F(1,18) = 12.93, P < 0.002, for before/after × sleep/wake interaction, respectively, for the nocturnal and daytime retention intervals]. Performance rate improved on average from 13.04 ± 0.63 to 17.12 ± 0.64, i.e., 33.47% ± 3.73% across the sleep intervals, and from 13.72 ± 0.49 to 15.32 ± 0.52, i.e., 12.49% ± 2.79% across the wake retention intervals (Fig. 3). The performance gain for retention intervals of sleep did not differ between nighttime (29.68% ± 3.72%) and daytime sleep (37.26% ± 6.45%; P > 0.3). However, improvements across wake retention intervals were stronger when placed during the day (17.56% ± 2.62%) than at night (7.41% ± 4.49%; P < 0.05, for separate pairwise comparisons). In fact, these analyses revealed that only performance gains across the daytime wake interval were significant (P < 0.001), whereas changes across the nocturnal wake interval were not (P > 0.2).
Figure 2.
Performance gains on the finger-to-thumb opposition task are indicated by the difference between training and retrieval testing (A) for performance rate (mean number of correctly completed sequences per 30 s) and (B) error count (mean number of errors per 30 s). (Left) Mean (± SEM) differences are indicated for 8-h retention intervals of sleep (black bars) and wakefulness (gray bars) placed during daytime and at night. (Right) In additional experiments, effects of a 48-h retention interval were tested which was filled either with two nights of regular sleep (black bars) or a first night of sleep deprivation followed by a night of recovery sleep (gray bars). *, P < 0.05. **, P < 0.001 for tests against zero and for differences between the effects of the retention intervals.
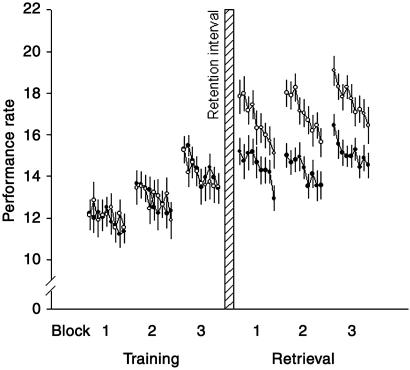
Figure 3.
Progression of performance on the finger-to-thumb opposition task as indicated by the number of correctly completed sequences sampled at 30-s intervals. Three blocks each of 5 min duration were run before (Training) and after (Retrieval) 8-h retention intervals during which subjects slept (open circles), or remained awake (filled circles). Mean (± SEM; adjusted to first block of training) are shown collapsed across both daytime and nighttime condition (refer to text).
Performance rates also improved within the learning sessions, on average by 0.73 sequences per block (P < 0.001, for a comparison between first and third block). Thus, it could be argued that performance gains at retesting after the retention interval reflect a mere repetition effect. This was excluded in supplementary analyses taking into account the estimated repetition effect by linearly extrapolating the individual performance gains within the learning session. This analysis confirmed a significant improvement in performance rates after the retention intervals [F(1,36) = 57.31, P < 0.001 for main effect before/after], in particular when filled with sleep [F(1,18) = 16.54, P < 0.001 and F(1,18) = 8.68, P < 0.01, for before/after × sleep/wake ANOVA interaction for nocturnal and daytime retention intervals, respectively]. Also, separate analysis of the wake retention interval confirmed significance selectively for the daytime retention condition (P < 0.01).
Accuracy of performance, as indicated by a decrease in the number of errors per 30 s, also improved only after retention intervals of sleep [on average by 30.07%, F(1,19) = 14.41, P < 0.001], but remained unchanged across retention intervals of wakefulness regardless of whether placed during the night or daytime [F(1,19) = 0.09, P > 0.8, Fig. 2B]. The improving effects of the sleep intervals on performance accuracy were closely comparable for the nocturnal and daytime sleep conditions (P > 0.9).
For the retention sleep conditions, we also assessed the relationship between time spent in the different sleep stages and performance gains (across daytime and nighttime conditions) by using Pearson's correlation. Improvement in performance rate was proportional to the time spent in REM sleep (r = 0.61, P < 0.004). Correlation with time in slow wave sleep (SWS; r = 0.01, P > 0.9), stage 2 sleep (r = −0.37, P > 0.1) and stage 1 sleep (r = 0.14, P > 0.5) remained nonsignificant. Also, dividing sleep time into four quarters did not reveal evidence that performance gains were correlated with the amount of early SWS or late REM sleep, or with stage 2 sleep during any of these intervals.
Comparing effects of sleep and wake retention intervals could be in principle confounded by unspecific effects of sleep loss on motor performance. Self-reports of mood and feelings of activation revealed that in comparison to normal nocturnal sleep, subjects after nights of sleep deprivation, felt more tired (P < 0.001), less activated (P < 0.01) and less concentrated (P < 0.05) as revealed by an adjective check-list (24). Nevertheless, a substantial contamination of motor performance by these subjective feelings seems unlikely in light of the fact that initial performance at learning was comparable for all retention conditions, regardless of whether this period was preceded by sleep or wakefulness. To further rule out unspecific motor effects of tiredness, particularly on retrieval tested after nocturnal wakefulness, two additional groups of eight naïve subjects each practiced the finger motor sequences in the morning at 7:30 a.m. after a night of regular sleep and sleep deprivation. Performance on the two conditions was indeed closely comparable with regard to both performance rate (regular sleep: 13.34 ± 0.45; sleep deprivation: 13.38 ± 0.34; P > 0.9) and errors (regular sleep: 7.61 ± 0.96; sleep deprivation: 5.63 ± 0.6; P > 0.1).
We also investigated whether the selective gain in performance after nocturnal sleep as compared with a retention period of nocturnal wakefulness is preserved after an additional night, in which sleep deprived subjects had recovery sleep. For this purpose, six additional subjects were trained, as in the main experiment, on one of the finger sequences, and retested after a 48-h retention interval that was filled either with two consecutive nights of regular sleep or with a first night of sleep deprivation followed by a second night of recovery sleep. Initial learning before the retention intervals did not differ between the sleep and sleep deprivation condition with respect to both, performance rates (P > 0.3), and error rates (P > 0.8). At retrieval testing 48 h later, the improvement in performance was distinctly more pronounced when subjects had slept the night after training (from 14.92 ± 1.28 to 18.94 ± 0.96, i.e., 28.99% ± 5.41%) than when they stayed awake in this first night [from 16.20 ± 1.89 to 17.92 ± 1.86, i.e., 11.19% ± 3.03%; F(1,5) = 45.98, P < 0.001, for before/after × sleep/wake interaction; Fig. 2]. Errors also decreased only when subjects were retested after two regular nights of sleep (from 7.97 ± 2.28 to 7.0 ± 2.44, i.e., −15.78% ± 13.07%), and increased when subjects had stayed awake the night after training (from 7.61 ± 1.53 to 8.23 ± 1.74, i.e., 12.76% ± 18.96%) with these differences, however, not reaching significance.
Finally, we tested the specificity of the improving effect of sleep on motor memories. Before an 8-h interval of nocturnal sleep, 10 other subjects were trained on one of the two finger sequences, as in the main experiment. At retrieval testing after the retention interval subjects were first tested on the untrained sequence and, 1 h later, on the sequence trained before the retention interval. As expected, performance improved across sleep only for the trained sequence (from 13.95 ± 1.1 to 17.52 ± 0.93, P < 0.001), whereas performance on the untrained sequence was similar to that at training before sleep (13.95 ± 1.1 versus 14.48 ± 1.17, P > 0.3).
Discussion
Results indicate an improvement in finger motor skills that is distinctly greater and more consistent across retention periods of sleep than of wakefulness. Finger skills after a time of wakefulness were improved only with regard to performance rate, but not to error rate, and only when the retention period took place during daytime. Effects of tiredness do not explain our findings because performance on the task used here was shown to be unaffected by prior sleep deprivation. Moreover, memory for the trained motor sequence was still superior after sleep than after a vigil on the night immediately after training even when retrieval testing was postponed for another 24 h, including a night of sleep for all subjects. Because subjects in these experiments had sufficiently slept before retrieval on both conditions, fatigue and other factors induced by sleep deprivation can be safely ruled out as possible confounds. Importantly, this finding indicates that sleep enhances the formation of memory for the motor skill only within a critical time frame after training. Sleep occurring after training, rather than sleep before recall, appears to be effective. This conclusion complements results of a most recent study employing a similar tapping task (25). Donchin et al. (26) failed to find an influence of sleep versus sleep deprivation on the memory for reaching movements. This outcome could point to an effect of sleep depending on the type of motor memory. Alternatively, sensitivity of different performance measures to the influence of sleep may vary, though this cannot be decided on the basis of the available data. The present result of a sleep-dependent enhancement in motor memories adds to previous evidence indicating a similar essential role of sleep in the formation of perceptual discrimination skills (11, 27, 28).
Performance improvements observed during daytime wakefulness but not during nighttime wakefulness suggest both that the wake state per se is not sufficient to promote memory formation for the trained finger skill, and that circadian factors play a role in consolidation. Further evidence of a circadian influence comes from our observation that, across both the sleep and wake retention conditions, performance gains on average were greater for the retention intervals positioned during daytime than during the night. Moreover, it is conceivable that the circadian influence on the formation of motor memories during wake retention periods differs in quality from that during sleep. After daytime wake periods, the improvement in finger sequence tapping remained restricted to performance rate and was not paralleled by a decrease in errors, suggesting that only sleep leads to changes in internal representations improving motor accuracy. Also, previous studies indicated that performance on motor skills (reaching movements), though becoming more resistant to interference from similar behaviors (2, 5), did not improve in accuracy across wake periods of 6 hours (1). Thus, consolidation during daytime wakefulness appears to spare certain aspects of the internal motor representations which are enhanced only by sleep.
Skill learning is characterized essentially by rather discrete changes in low-level representations within the hierarchical organization of motor systems and, importantly, shows little generalization (6, 29). Here, we found that the enhancing effect of sleep on memory for a motor sequence is highly specific with regard to the trained finger sequence and does not generalize to a control sequence containing the identical finger movements in a mirror-reversed order. This further excludes effects not specifically linked to the task, e.g., on motor fluency, but speaks for a direct influence of sleep on forming the internal model for this particular finger sequence.
At the neuronal level, the consolidation of motor skill memories has been considered to involve a reorganization of motor representations residing predominantly in the primary motor cortex (M1) (7, 16, 30–32). Human studies using functional magnetic resonance imaging and transcranial magnetic stimulation have shown that the amount of motor training in our subjects is sufficient to trigger plastic neuronal changes in M1, whereby the initially fragile motor representations become increasingly stabilized (7, 8). Moreover, findings with positron emission tomography suggested that consolidation of a serial motor task is based on a covert reactivation of brain structures already activated at training, with the signs of reactivation being most obvious during REM sleep (33). In the present study, exploratory calculation of correlation coefficients indicated greater performance gains in subjects with high amounts of REM sleep. Although, in light of the limited size of the subject sample, this result needs to be considered with caution, it would also point to a particular relevance of REM sleep in procedural memory formation.
Nevertheless, the covert reprocessing of newly acquired motor representations during sleep is a concept which could explain the considerable performance gain in the finger motor sequence task seen after sleep. This gain, consisting of increased speed and a reduced number of false reactions, cannot arise from a nonselective strengthening of connectivity within the acquired representations, but implies a reorganization that enhances correct reactions to the exclusion of false ones. This reorganization, at the cellular level, probably involves processes such as synaptic long-term potentiation and depression (34–38), as well as synaptogenesis (39, 40) in the motor cortex, which may particularly benefit from the specific orchestration of neurotransmitters in the different sleep stages (41–43). However, the synaptic factors critically involved in sleep-dependent formation of skill memory remain to be identified. Demonstrating an essential role of sleep in the formation of memory for motor skills, our data extend previous observations of a similar function of sleep with regard to perceptual skills. In generalizing these observations to skills of everyday life (such as learning a musical instrument or sport), we would conclude that sleep is required to achieve optimum performance on any of these skills.
Acknowledgments
We thank Drs. Pierre Maquet and Robert Stickgold for their valuable comments on an earlier version of the manuscript. The study was supported by a grant from the Deutsche Forschungsgemeinschaft (to J.B.).
Abbreviations
- REM
rapid eye movement
- SWS
slow wave sleep
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Shadmehr R, Holcomb H H. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- 2.Shadmehr R, Brashers-Krug T. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolpert D M, Ghahramani Z, Jordan M I. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 4.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Nature (London) 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 5.Brashers-Krug T, Shadmehr R, Bizzi E. Nature (London) 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 6.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams M M, Turner R, Ungerleider L G. Proc Natl Acad Sci USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karni A, Meyer G, Jezzard P, Adams M M, Turner R, Ungerleider L G. Nature (London) 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 8.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Nature (London) 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland G R, McNaughton B. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 10.Smith C. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 11.Stickgold R, James L, Hobson J A. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 12.Plihal W, Born J. J Cognit Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 13.Maquet P. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 14.Karni A, Tanne D, Rubenstein B S, Askenasy J J, Sagi D. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 15.Gais S, Plihal W, Wagner U, Born J. Nat Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 16.Hund-Georgiadis M, von Cramon D Y. Exp Brain Res. 1999;125:417–425. doi: 10.1007/s002210050698. [DOI] [PubMed] [Google Scholar]
- 17.Pantev C, Oostenveld R, Engelien A, Ross B, Roberts L E, Hoke M. Nature (London) 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- 18.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 19.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson J A. J Cognit Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 20.Smith C, MacNeill C. J Sleep Res. 1994;3:206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 21.Carrier J, Monk T H. Chronobiol Int. 2000;17:719–732. doi: 10.1081/cbi-100102108. [DOI] [PubMed] [Google Scholar]
- 22.Stolz G, Aschoff J C, Born J, Aschoff J. J Neurol. 1988;235:308–313. doi: 10.1007/BF00314180. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service; 1968. [Google Scholar]
- 24.Janke W, Debus G. Die Eigenschaftswörterliste EWL. Göttingen, Germany: Hogrefe; 1978. [Google Scholar]
- 25.Walker M P, Brakefield T, Morgan A, Hobson J A, Stickgold R. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 26. Donchin, O., Sawaki, L., Madupu, G., Cohen, L. G. & Shadmehr, R. (2001) Soc. Neurosci. Abstr.27, Program No. 302.2.
- 27.Smith C, Butler S. Physiol Behav. 1982;29:469–473. doi: 10.1016/0031-9384(82)90268-2. [DOI] [PubMed] [Google Scholar]
- 28.Smith C. Behav Brain Res. 1996;78:49–56. doi: 10.1016/0166-4328(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 29.Karni A, Bertini G. Curr Opin Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 30.Sanes J N, Donoghue J P. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 31.Giraux P, Sirigu A, Schneider F, Dubernard J M. Nat Neurosci. 2001;4:691–692. doi: 10.1038/89472. [DOI] [PubMed] [Google Scholar]
- 32.Li C S, Padoa-Schioppa C, Bizzi E. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 33.Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, et al. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 34.Iriki A, Pavlides C, Keller A, Asanuma H. Science. 1989;245:1385–1387. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- 35.Asanuma H, Pavlides C. NeuroReport. 1997;8:i–vi. [PubMed] [Google Scholar]
- 36.Karni A, Sagi D. Proc Natl Acad Sci USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rioult-Pedotti M S, Friedman D, Hess G, Donoghue J P. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 38.Rioult-Pedotti M S, Friedman D, Donoghue J P. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 39.Keller A, Asanuma H. J Comp Neurol. 1993;336:229–242. doi: 10.1002/cne.903360206. [DOI] [PubMed] [Google Scholar]
- 40.Kleim J A, Lussnig E, Schwarz E R, Comery T A, Greenough W T. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graves L, Pack A, Abel T. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- 42.Hobson J A. Curr Opin Neurobiol. 1992;2:759–763. doi: 10.1016/0959-4388(92)90130-d. [DOI] [PubMed] [Google Scholar]
- 43.McGaugh J L. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]