Abstract
Calcineurin, a Ca2+/calmodulin-dependent protein phosphatase, is the common target for two immunophilin–immunosuppressant complexes, cyclophilin A–cyclosporin A (CyPA-CsA) and FKBP–FK506. How the two structurally distinct immunophilin–drug complexes bind the same target has remained unknown. We report the crystal structure of calcineurin (CN) in complex with CyPA-CsA at 2.8-Å resolution. The CyPA-CsA complex binds to a composite surface formed by the catalytic and regulatory subunits of CN, where the complex of FK506 and its binding protein FKBP also binds. While the majority of the CN residues involved in the binding are common for both immunophilin-immunosuppressant complexes, a significant number of the residues are distinct. Unlike FKBP-FK506, CyPA-CsA interacts with Arg-122 at the active site of CN, implying direct involvement of CyPA-CsA in the regulation of CN catalysis. The simultaneous interaction of CyPA with both the composite surface and the active site of CN suggests that the composite surface may serve as a substrate recognition site responsible for the narrow substrate specificity of CN. The comparison of CyPA-CsA-CN with FKBP-FK506-CN significantly contributes to understanding the molecular basis of regulation of CN activity by the immunophilin–immunosuppressant.
Keywords: immunosuppressants, FK506, FK506-binding protein, protein phosphatase, T cell
Calcineurin (CN) is a unique serine/threonine protein phosphatase (PP3 or PP2B) in its regulation by the second messenger Ca2+ and calmodulin. CN is involved in many biological processes, including immune responses (1–5), the second messenger cAMP pathway (3, 6), Na+/K+ ion transportation in the nephron (7), cell cycle progression in lower eukaryotes (8), cardiac hypertrophy (9), and memory formation (10, 11). The most extensively studied function of CN is its role in the signal transduction pathway during T cell activation. CN has been shown to be a common receptor for two immunophilin–immunosuppressant complexes, cyclophilin A-cyclosporin A (CyPA-CsA) and FK506-binding protein (FKBP)-FK506 (12, 13). The binding of CyPA-CsA or FKBP-FK506 inhibits the calcium-dependent dephosphorylation of the transcription factor nuclear factor of activated T cell (NFAT) by CN, thus blocking T cell receptor-mediated cytokine transcription and T-cell activation (14, 15).
Since the discovery of CN as a common target for both CyPA-CsA and FKBP-FK506 complexes, how two structurally distinct immunophilin–immunosuppressant complexes recognize a common target has remained a mystery. While the crystal structures of CN and its complex with FKBP-FK506 have shed light on the molecular recognition of CN by FKBP-FK506 (16, 17), the CyPA-CsA-CN structure has remained elusive. We report here the crystal structure of CyPA-CsA-CN at 2.8-Å resolution. The structure revealed that CyPA-CsA and FKBP-FK506 are bound to the same surface of CN, thus providing a molecular basis for understanding the regulation of CN activity by the immunophilin–immunosuppressant.
Methods
Protein Purification.
The catalytic subunit (CNA, 61 kDa) and regulatory subunit (CNB, 19 kDa) of the α isoform of human CN were coexpressed in Escherichia coli and purified by using a previously described procedure with slight modifications (18). The recombinant human CyPA was expressed as a glutathione S-transferase (GST) fusion protein in E. coli BL21 and purified by glutathione-agarose beads (Sigma). The CyPA-CsA-CN complex was prepared by a four-step procedure: (i) purification of recombinant human CN by Ni NTA (Qiagen, Valencia, CA) and Q-Sepharose (Pharmacia) columns, (ii) purification of GST-CyPA by glutathione beads, (iii) incubation of CN with GST-CyPA in the presence of CsA and cleavage of the CyPA-CsA-CN complex from GST by thrombin, and (iv) further purification of the CyPA-CsA-CN complex by a Superdex 200 gel filtration column (Pharmacia). This procedure produced a truncated CNA subunit with an apparent molecular mass of 43 kDa.
Crystallization and Data Collection.
Two crystal forms were obtained by vapor diffusion, using the CyPA-CsA-CN complex at a concentration of about 15 mg/ml in a storage buffer consisting of 0.1 M NaCl, 20 mM Tris⋅HCl (pH 7.5), 1 mM 2-mercaptoethanol, and 2 mM CaCl2. The trigonal crystals were grown against a well buffer of 0.1 M Hepes (pH 7.5) and 1.5 M Li2SO4, and they are in the space group P3221 with cell dimensions of a = b = 135.0 and c = 121.0 Å. A diffraction data set of the trigonal crystal was collected on beamline X12C at Brookhaven National Laboratory and has Rmerge of 0.088 and completeness of 87.9% to 2.8-Å resolution.
The tetragonal crystals of CyPA-CsA-CN were grown by hanging drop against a well buffer containing 0.1 M sodium cacodylate (pH 6.5), 0.2 M MgCl2, 13% PEG 8000, and 2.5% ethanol. The protein drop was made up of protein and well buffer in a 2:1 ratio. The CyPA-CsA-CN crystals are in the space group P41212 with cell dimensions of a = b = 108.7 and c = 316.6 Å. The diffraction data of the tetragonal CyPA-CsA-CN crystal were collected on beamline BioCars 14C of the Advanced Photon Source at Argonne National Laboratory at 100 K and were processed with the hkl software (Table 1).
Table 1.
Statistics of diffraction data and structure refinement of CyPA-CsA-CN
| Data collection | |
| Space group | P41212 |
| Unit cell, Å | a = b = 108.7, c = 316.6 |
| Total measurements | 183,803 |
| Unique reflections | 43,551 |
| Rmerge | 0.136 (0.326) |
| Resolution, Å | 2.8 |
| Completeness, % | 90.9 (62.9) |
| 〈I/σ〉 | 7.1 (1.5) |
| Structure refinement | |
| R factor | 0.260 |
| Rfree | 0.322 |
| Reflections | 42,377 |
| Resolution | 50 to 2.8 |
| rms deviation | |
| Bond length, Å | 0.013 |
| Angle, ° | 1.67 |
| Average B factor, Å2 | |
| CNA | 60.6 |
| CNB | 84.6 |
| CyPA | 54.6 |
| CsA | 34.8 |
| Fe | 48.7 |
| Zn | 48.5 |
| Ca | 85.4 |
| All atoms | 64.3 |
The numbers in parentheses are the statistics for the 2.8- to 2.9-Å resolution shell.
Structure Determination.
The crystal in the space group P3221 contains one complex of CyPA-CsA-CN in the crystallographic asymmetric unit. The CyPA-CsA-CN structure in P3221 was solved by molecular replacement program AMoRe using individual subunits of CyPA (19) and CNA and CNB from the structure of FKBP-FK506-CN (16, 17). The orientation of CNA was first located, yielding a correlation coefficient of 0.37 and R factor of 0.39 for the data between 4- and 8-Å resolution. When CyPA was added, the correlation coefficient and R factor were improved to 0.52 and 0.35, respectively. However, the location for CNB was ambiguous in the rotation and translation search. The orientation of CNB was determined by superposition of the CNA subunit over the CN-FKBP structure. CsA was built into the electron density map that was calculated from the model of CyPA-CN without CsA. The structure of CyPA-CsA-CN in P3221 was refined by x-plor to R factor of 0.23 and Rfree of 0.36 for 13,586 reflections between 20.0- and 3.5-Å resolution.
The refined structure of the CyPA-CsA-CN complex in P3221 was then used to solve the structure in the space group P41212. Two solutions from the rotation and translation search were easily recognized and corresponded to two molecules in the crystallographic asymmetric unit. The translational search using two molecules of CyPA-CsA-CN yielded an R factor of 0.44 and correlation coefficient of 0.45 for 14,453 reflections between 8- and 4-Å resolution. The orientations of individual subunits of CyPA, CNA, and CNB were optimized by rigid-body refinement of cns (20). The atomic model was rebuilt by using the program o (21) and refined by cns (Table 1).
Results and Discussion
Architecture of the CyPA-CsA-CN Structure.
The current CyPA-CsA-CN structure includes full-length CyPA (residues 1–165), CsA (11 residues), truncated CNA (residues 1–372), and CNB residues 10–169. The electron density was excellent for CNA, CyPA, and CsA, as shown in an example of the electron density of CsA (Fig. 1). The N-terminal residues 1–13 of one of two CNAs in the crystallographic asymmetric unit can be traced in the electron density without difficulty. The electron density for the N-terminal domain of CNB (residues 10–85) is relatively weak, but the secondary structure elements and two calcium sites in the domain are clearly visible in the map calculated from the CyPA-CsA-CN structure omitting the domain. The CNB subunit has average B factors of 96.6 Å2 for the N-terminal domains and 74.1 Å2 for the C-terminal domains (residues 86–169), in comparison to the average of 64.3 Å2 for all atoms in the entire structure (Table 1). The relatively large B factor and the weak electron density for the N-terminal domain of CNB likely reflect the conformational flexibility of this region. We speculate that this conformational flexibility may play a role in the regulation of the CN phosphatase activity by CNB and calcium.
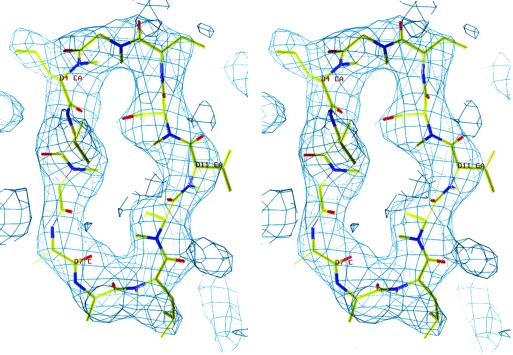
Fig 1.
Stereoview of electron density for CsA. The (2Fo − Fc) map was calculated from the structure-omitted CsA and contoured at 1.5σ.
The truncated CNA subunit (residues 1–372) contains a catalytic domain and the CNB-binding helix (BBH) (Fig. 2). The catalytic domain consists of a central β-sheet flanked by α-helices and has folding similar to other serine/threonine protein phosphatases such as PP1 and PP2A (22). The active site metal ions were assumed as Fe3+ and Zn2+ without further verification because of the resolution limit. The N-terminal residues 1–13 of one CNA in the crystallographic asymmetric unit interact with the active site residues of the second CNA by crystallographic symmetry. These interactions are likely the consequence of the crystal packing without biological relevance. The BBH of CNA embeds in CNB and forms a composite surface together with CNB for binding of CyPA-CsA as well as FKBP-FK506. CNB comprises two domains, each of which has two calcium-binding sites with a folding pattern similar to calmodulin. CyPA in the CyPA-CsA-CN complex is an eight-stranded β-barrel with the same folding as the unligated CyPA (Fig. 2).
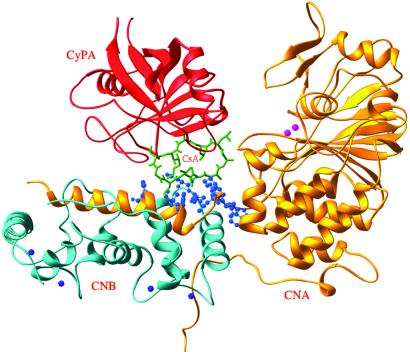
Fig 2.
Ribbon representation of CyPA-CsA-CN. Color codes are CNA, gold; CNB, cyan; CsA, green; CyPA, red; Zn2+ and Fe3+, pink; and calcium, blue. The residues from CN involved in binding of CyPA-CsA are shown as blue balls.
Subtle Conformational Changes of CN upon CyPA-CsA Binding.
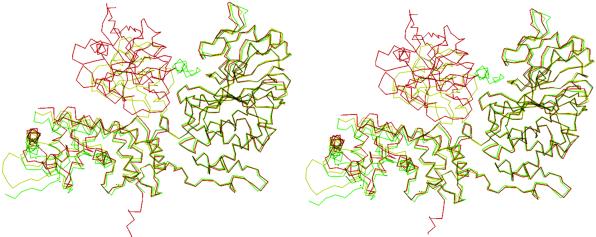
The conformations of CyPA, CsA, CNA, and CNB in the CyPA-CsA-CN complex are similar to the corresponding subunits in the CyPA-CsA binary complex (19, 23), unbound CN (17), and the FKBP-FK506-CN complex (16, 17). Superposition of the Cα atoms of the corresponding subunits revealed average displacements of 0.39 Å for CyPA between the binary CyPA-CsA and tertiary CyPA-CsA-CN complexes, and 0.66 and 0.79 Å for CNA and CNB between CyPA-CsA-CN and unligated CN, indicating no substantial change of overall molecular conformations upon formation of the CyPA-CsA-CN complex. In contrast, significant local migration was observed for BBH of CNA (Fig. 3). The averaged displacement for the Cα atoms of residues 354–372 of CNA between the unbound CN and CyPA-CsA-CN structures was 2.64 Å, about 4 times the overall average of 0.66 Å. The corresponding residues in FKBP-FK506-CN also showed significant migration of the Cα atoms of the same domain with an average displacement 1.60 Å, more than 3 times the overall average 0.49 Å upon FKBP-FK506 binding. Thus, the movement of BBH appears as a consequence of binding of CN to either immunophilin–drug complex.
Fig 3.
Stereoview of the superposition of Cα atoms of CyPA-CsA-CN (red) over unligated CNA (green) and FKBP-FK506-CN (yellow). The complex structures were overlaid with optimal overlap of the catalytic domain of CNA.
The Common and Distinct Recognition Elements in CN for CyPA-CsA and FKBP-FK506.
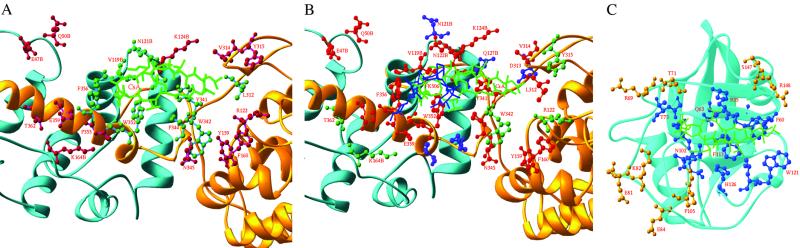
A comparison of CyPA-CsA-CN with FKBP-FK506-CN revealed that both CyPA-CsA and FKBP-FK506 bind to the same region of CN and share major common recognition elements on CN (Fig. 4). Trp-352 of CNA is a key residue commonly recognized by both immunosuppressive drugs, as its Nɛ2 forms a hydrogen bond with the backbone carbonyl oxygen of Val-5 of CsA in CyPA-CsA-CN or with O7 and O8 of FK506 in FKBP-FK506-CN. In addition, Trp-352 and Phe-356 of CNA and Met-118 and Val-119 of CNB make multiple hydrophobic contacts with residues 2–6 of CsA or a group of atoms of FK506 (Fig. 4). At the protein level, Oɛ1 of Glu-47, Nɛ2 of Gln-50, and Nδ2 of Asn-122 of CNB form hydrogen bonds with O of Gly-80, Oɛ2 of Glu-81, and O of Ala-103 of CyPA, respectively. These three residues also form hydrogen bonds with Gln-53, Glu-54, and Lys-47 of FKBP. For the common van der Waals interactions, residues Tyr-159, Phe-160, Leu-312, Val-314, Asn-345, and Pro-355 in CNA make contact with Pro-50, Arg-148, Ser-147, Trp-121, Asn-149, and Thr-73 of CyPA, or Asp-32, Lys-34, Lys-35, Gly-89, and Pro-88 of FKBP (16, 17).
Fig 4.
Interfacial interactions between CN and immunophilins–immunosuppressants. CNA and CNB are shown as yellow and cyan ribbons. (A) The CN composite surface for CyPA-CsA binding. A total of 25 residues of CN are involved in interaction with CyPA-CsA: Arg-122, Tyr-159, Phe-160, Leu-312, Val-314, Tyr-315, Tyr-341, Trp-342, Pro-344, Asn-345, Trp-352, Ser-353, Pro-355, Phe-356, and Glu-359 of CNA, and Glu-47, Gln-50, Met-118, Val-119, Asn-122, Leu-123, Lys-124, and Lys-164 of CNB. Red balls represent residues interacting with CyPA and green balls represent residues interacting with CsA (green sticks). The residues from CNB are marked with the letter “B” attached to the residue number. (B) The CN surface for binding of CyPA-CsA and FKBP12-FK506. CN residues in red interact with both CyPA-CsA and FKBP12-FK506. Green residues are unique for CyPA-CsA binding and blue residues are unique for FKBP-FK506. CsA and FK506 are shown as green and blue sticks. (C) CyPA residues for recognition of CN (gold) and CsA (blue). CsA is shown in green sticks.
On the other hand, a significant number of CN residues interacting with CyPA-CsA differ from those with FKBP-FK506. First, the majority of the hydrogen bonds between CN and CyPA-CsA are different from those between CN and FKBP-FK506. Thus, five hydrogen bonds were uniquely observed between CN and CyPA-CsA: Arg-122 (CNA)⋅⋅⋅Arg-148 (CyPA), Tyr-341 (CNA)⋅⋅⋅Ala-7 (CsA), Glu-359 (CNA)⋅⋅⋅Arg-69 (CyPA), Gln-50 (CNB)⋅⋅⋅Lys-82 (CyPA), and Lys-164 (CNB)⋅⋅⋅Asn-71 (CyPA) (Fig. 4 A and C). In comparison, four hydrogen bonds are unique for CN and FKBP-FK506: Tyr-159 (CNA)⋅⋅⋅Asp-32 (FKBP), Leu-312 (CNA)⋅⋅⋅Lys-35 (FKBP), Asn-121 (CNB)⋅⋅⋅Lys-44 (FKBP), and Gln-127 (CNB)⋅⋅⋅Arg-42 (FKBP) (16, 17). Particularly interesting is the hydrogen bond between Arg-148 of CyPA and the active site residue Arg-122 of CAN, which may force the reorientation of the side chain of Arg-122, thus directly affecting the catalytic activity of CN. In contrast, the nearest residue from FKBP, Lys-34, is situated more than 5 Å away from Arg-122, too far to exert the same effect as Arg-148 of CyPA. This hydrogen bonding difference might imply a potentially different mechanism on regulation of CN catalysis by the CyPA-CsA complex. In addition, a group of residues from CN involved in van der Waals interactions with CyPA-CsA are distinct from those interacting with FKBP-FK506. Thus, Arg-122, Tyr-315, Trp-342, and Thr-362 of CNA, and Lys-164 of CNB take part in van der Waals interactions only with CyPA-CsA, whereas Asp-313, Met-347, and Thr-351 of CNA and Asn-121 and Gln-127 of CNB uniquely contact FKBP-FK506 (Fig. 4).
In summary, among the 25 residues of CN involved in hydrogen bonds or hydrophobic interactions with CyPA-CsA, 20 residues are common to those of FKBP-FK506, 5 (Arg-122, Tyr-315, Trp-342, and Thr-362 of CNA and Lys-164 of CNB) uniquely interact with CyPA-CsA. Similarly, 5 residues of Asp-313, Met-347, and Thr-351 of CNA and Asn-121 and Gln-127 of CNB come in contact only with FKBP-FK506. Although the majority of the CN residues are common for binding of the two structurally distinct immunophilin–drug complexes, the patterns of recognition are dramatically different. For example, Tyr-341 forms a hydrogen bond with CsA, but takes part in a van der Waals interaction with Arg-42 of FKBP. Another interesting feature is lower degree of conservation of hydrogen bonds. Of the nine hydrogen bonds between CyPA-CsA and CN, only four are common to FKBP-FK506. This character of recognition diversity of the composite surface may imply its capacity for binding of a variety of protein substrates.
The common and distinct interactions of CN with the two immunophilin–drug complexes correlate with the properties of many, but not all, known CN mutants. The unique hydrogen bond between Tyr-341 of CNA and CsA can account for the observation that mutation of Tyr-341 to Phe rendered T lymphocytes and yeast resistant to CsA, but not to FK506 (24, 25). Similarly, mutation of Thr-350 of yeast CN CMP-1 to either Lys or Arg, which corresponds to Val-314 in human CNA, leads to selective resistance to CsA. The resistance to CsA may be explained by the disruption of the van der Waals contact between Val-314 of CNA and Trp-121 of CyPA upon introduction of positively charged Lys or Arg. However, it is less apparent why the same mutant remained sensitive to FK506, as Val-314 also interacts with Lys-35 of FKBP. Trp-352 is another residue whose mutant phenotype is not obvious from the structure. The W352C equivalent mutant of yeast CMP-1 exhibited FK506 resistance, but no change in sensitivity to CsA (25). As Trp-352 has hydrophobic contacts and forms a hydrogen bond with both CsA and FK506 (Fig. 4), one would have predicted that its mutation to Cys should lead to a decrease in binding of CyPA-CsA as well as FKBP-FK506. One possible explanation for the selective resistance of the W352C mutant to FK506 is that Trp-352 is the only residue forming a hydrogen bond with FK506, and thus removal of the Trp-352 side chain causes a net loss of binding energy between FKBP-FK506 and CN. In contrast, CsA has two hydrogen bonds with Tyr-341 and Trp-352, and thus loss of contacts with Trp-352 upon its mutation may be compensated by the interactions with Tyr-341. In addition to the drug-resistant mutants isolated from the yeast genetic screen, mutagenesis of Val-119 and Leu-123 of CNB or both together significantly decreases the affinity for CyPA-CsA (26). This can be explained by the contacts of both residues with CsA. The CNB mutant of Cryptococcus neoformans with an insertion of two amino acids between Asn-121 and Gln-127 has reduced binding affinity for FKBP-FK506 both in vivo and in vitro (27). The crystal structures show that the loop 121–127 forms three hydrogen bonds and numerous van der Waals contacts with FKBP and FK506. As a result, the two-residue insertion will dramatically perturb the FKBP binding. In contrast, CyPA-CsA has only minor interactions with the loop so that its binding is not substantially affected by the mutation.
Subtle Conformational Changes of CsA upon Complex Formation and Their Pharmacological Implications.
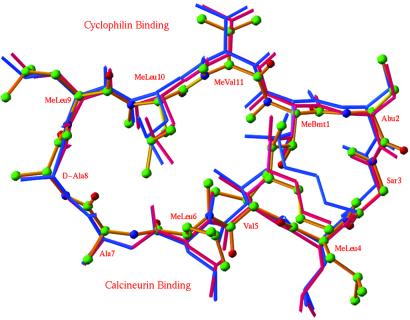
The conformation of the CsA backbone and the side chains interacting with CyPA in the ternary complex of CyPA-CsA-CN are very similar to those in the binary CyPA-CsA complex (Fig. 5). In addition, four pairs of hydrogen bonds between CsA and CyPA: MeBmt-1⋅⋅⋅Gln-63, Abu-2⋅⋅⋅Asn-102, MeLeu-9⋅⋅⋅Trp-121, and MeLeu-10⋅⋅⋅Arg-55, are conserved in both binary CyPA-CsA (19) and ternary CyPA-CsA-CN complexes. However, the side chains of CN-binding moiety of CsA undergo significant conformational changes. For example, the torsion angles of the side chain of MeLeu-4 change from (180°, 60°) to (60°, 180°) upon ternary complex formation to make hydrophobic contacts with CNA and CNB.
Fig 5.
Superposition of CsA in the binary CyPA-CsA complex (purple), CsA in the ternary CyPA-CsA-CN complex (gold), and MeBm2t-CsA in the binary CyPA-MeBm2tCsA complex (blue). MeBmt, N-methyl-(4R)-4-[(E)-2-butenyl]-4-methyl-l-threonine; MeBm2t, N-methyl-4-[(E)-2-butenyl]-4,4-dimethyl-l-threonine. Color codes for atoms: carbons as green balls, oxygen as red, and nitrogen as blue. Residues of MeLeu-9 to 2-aminobutyrate (Abu)-2 (top) bind CyPA, whereas MeLeu-4 to Ala-7 bind CN (bottom).
The CyPA-CsA-CN structure can help us to understand the structure–activity relationships of a large number of CsA analogs that were synthesized in attempts to improve the pharmacological properties of CsA over the past few decades. Unique among them are MeBm2t-CsA and MeAla6-CsA (28–30). MeBm2t-CsA showed a 100-fold decrease in cyclophilin binding but only 3-fold decrease in potency for inhibition of CN and T cell activation. The backbone conformation of MeBm2t-CsA is almost identical to that of CsA in their binary complexes with CyPA, but the MeBm2t side chain of MeBm2t-CsA deviates from that of CsA by up to 1.8 Å (Fig. 5). This subtle conformational change may be explained by the extra methyl group in the MeBm2t side chain that takes part in intramolecular interactions with MeLeu-6, MeLeu-10, and MeVal-11, thus rigidifying the conformation of MeBm2t-CsA. This rigidification of conformation of MeBm2t-CsA may disfavor its binding to CyPA, but once MeBm2t-CsA is bound to CyPA, favor the ternary complex formation with CN. In contrast to MeBm2t-CsA, MeAla6-CsA, in which Leu-6 is changed to a methylalanine, has a 2-fold decrease in CyPA binding activity, but a 25-fold decrease in CN inhibition and about 250-fold decrease in immunosuppressive activity (28, 30). Given that the side chain of MeLeu-6 in CsA makes extensive contacts with Tyr-341, Pro-344, and Trp-352 of CNA and Leu-123 of CNB, it is expected that removal of the three-carbon isopropyl group from MeLeu-6 of CsA will result in loss of the most contacts of this residue with CNA and CNB, thus reducing affinity for CN.
Among the different positions on CsA, the side chain of d-Ala-8 has been shown to be most tolerant of modifications without significant loss of immunosuppressive activity. Thus, SDZ IMM125, the Ala-8 → Ser analog of CsA with an additional ethanol unit on the Ser-8 hydroxyl group, possesses immunosuppressive activity similar to that of CsA (31). Two related analogs, d-Dap8-CsA and d-Dab8-CsA, also retained significant activity (29). Interestingly, a CsA analog, PL-CS with attachment of a photoaffinity probe to the hydroxyl group of Ser-8, is active as evidenced by its photodependent irreversible inhibition of T cell activation (32), and apparently forms a ternary complex with CyPA and CN. Examination of the CyPA-CsA-CN complex indicated that d-Ala-8 of CsA does not have direct contacts with either CN or CyPA. An open space was found around d-Ala-8 of CsA in the ternary complex, consistent with the tolerance of the side chain for modifications without significant impact on binding.
Direct Interactions of CyPA-CsA with an Active Site Residue of CN and Its Mechanistic Implications.
On the basis of the structure of FKBP-FK506-CN, it has been proposed that the inhibition of CN activity by FKBP-FK506 results from spatial blockage of access for protein substrates to the active site (16). This idea is strongly supported by the observation that both FKBP-FK506 and CyPA-CsA bind to the CNA-CNB composite surface near the active site of CN. Examination of the CyPA-CsA-CN structure, however, suggests that modulation of CN activity by the immunophilin–drug complexes might be more complicated than the spatial blockade model. The CyPA-CsA-CN structure revealed the hydrogen bond and hydrophobic interactions between Arg-148 of CyPA and Arg-122 of CNA. These contacts are likely to place a conformational constraint on the catalytic residue Arg-122, thus implying direct involvement of CyPA-CsA in regulation of CN activity. Whereas CyPA-CsA and FKBP-FK506 were shown to inhibit the phosphatase activity of CN toward a 19-amino acid phosphopeptide substrate, they stimulated the phosphatase activity of CN toward a small substrate, para-nitrophenyl phosphate (PNPP; refs. 12 and 33). These paradoxical observations can now be rationalized, at least in part, by the conformational change of Arg-122, which may favor PNPP but disfavor the peptide substrate for binding and catalysis.
The CNA-CNB Composite Surface May Be a Recognition Site Defining the Narrow Substrate Specificity of CN.
CN has relatively narrow substrate specificity in comparison with other protein phosphatases. The molecular basis of the substrate specificity of CN has remained largely unknown. We have shown that CyPA-CsA and the structurally distinct FKBP-FK506 complex share their binding sites on the composite surface formed by CNA and CNB. The ability of the CNA-CNB composite surface to bind two structurally distinct immunophilin–drug complexes suggests the capacity of the surface to accommodate different protein–ligand complexes. On the basis of the observation that CyPA-CsA interacts simultaneously with the CNA-CNB composite surface and the active site residue, we speculate that the CNA-CNB composite surface may serve as a general substrate recognition site, defining substrate specificity of CN. The nearly orthogonal extension of the composite surface from one end of the catalytic domain forms an L-shaped clamp, restricting the binding of phosphoprotein substrates that match the shape and size. Inherent in this model is the existence of a separate substrate-binding domain from the active site of CN, as observed in the case of NFAT (34). This model is also consistent with the fact that BBH of CN represents a unique C-terminal extension of the phosphatase catalytic domain of CN among protein serine/ threonine phosphatases.
The crystal structure of CyPA-CsA-CN in this paper, together with the previously determined FKBP-FK506-CN complex, reveals how the same composite surface achieves the molecular recognition of two structurally distinct immunophilin–drug complexes. It is reminiscent of the complex between the human growth hormone and the homodimer of its receptor, in which two identical receptor subunits used two largely distinct sets of residues to bind two different surfaces of the growth hormone (35). Another example is the Fc fragment of human IgG, where a single protein surface is capable of binding multiple proteins or peptides (36). With the structural puzzle of immunophilin–drug recognition by CN solved, how two unrelated immunosuppressive natural products, CsA and FK506, have evolved from different microorganisms to target the same mammalian protein phosphatase on the same surface remains a mystery.
Acknowledgments
We thank Frank Rusnak for initiating this work, Eric Griffith and Larry Stern for helpful comments on the manuscript, and Yongquan Chen and Yuanhong Xia for protein purification and crystallization. Beamlines of BioCars 14C at the Advanced Photon Source and X12C at Brookhaven are thanked for collection of diffraction data. This work was supported in part by National Institutes of Health grants (GM55783 to J.O.L. and AI33072 to H.K.). A.M. was supported by a Graduate Fellowship from the Ford Foundation.
Abbreviations
CN, calcineurin
CNA, the catalytic subunit of calcineurin
CNB, the regulatory subunit of calcineurin
CyPA, cyclophilin A
CsA, cyclosporin A
FKBP, FK506- binding protein
NFAT, nuclear factor of activated T cell
MeBm2t, N-methyl-4-[(E)-2-butenyl]-4,4-dimethyl-l-threonine
Abu, 2-aminobutyrate
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates of CyPA-CsA-CN have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code ).
References
- 1.Klee C. B., Ren, H. & Wang, X. (1998) J. Biol. Chem. 273, 13367-13370. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber S. L. (1992) Cell 70, 365-368. [DOI] [PubMed] [Google Scholar]
- 3.Kincaid R. L. (1995) J. Allergy Clin. Immunol. 96, 1170-1177. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas M. E., Sanfridson, A., Cutler, N. S. & Heitman, J. (1998) Trends Biotechnol. 16, 427-433. [DOI] [PubMed] [Google Scholar]
- 5.Hemenway C. S. & Heitman, J. (1999) Cell Biochem. Biophys. 30, 115-151. [DOI] [PubMed] [Google Scholar]
- 6.Antoni F. A., Smith, S. M., Simpson, J., Rosie, R., Fink, G. & Paterson, J. M. (1998) Adv. Second Messenger Phosphoprotein Res. 32, 153-172. [DOI] [PubMed] [Google Scholar]
- 7.Tumlin J. A. (1997) Am. J. Kidney Dis. 30, 884-895. [DOI] [PubMed] [Google Scholar]
- 8.Nanthakumar N. N., Dayton, J. S. & Means, A. R. (1996) Prog. Cell Cycle Res. 2, 217-228. [DOI] [PubMed] [Google Scholar]
- 9.Molkentin J. D., Lu, J. R., Antos, C. L., Markham, B., Richardson, J., Robbins, J., Grant, S. R. & Olson, E. N. (1998) Cell 93, 215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winder D. G., Mansuy, I. M., Osman, M., Moallem, T. M. & Kandel, E. R. (1998) Cell 92, 25-37. [DOI] [PubMed] [Google Scholar]
- 11.Mansuy I. M., Mayford, M., Jacob, B., Kandel, E. R. & Bach, M. E. (1998) Cell 92, 39-49. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Farmer, J. D., Jr., Lane, W. S., Friedman, J., Weissman, I. & Schreiber, S. L. (1991) Cell 66, 807-815. [DOI] [PubMed] [Google Scholar]
- 13.Friedman J. & Weissman, I. (1991) Cell 66, 799-805. [DOI] [PubMed] [Google Scholar]
- 14.Clipstone N. A. & Crabtree, G. R. (1992) Nature (London) 357, 695-697. [DOI] [PubMed] [Google Scholar]
- 15.Jain J., McCaffrey, P. G., Miner, Z., Kerppola, T. K., Lambert, J. N., Verdine, G. L., Curran, T. & Rao, A. (1993) Nature (London) 365, 352-355. [DOI] [PubMed] [Google Scholar]
- 16.Griffith J. P., Kim, J. L., Kim, B. E., Simchak, N. D., Thomson, J. A., Fitzgibbon, M. J., Flaming, M. A., Caron, P. R., Haiao, K. & Navia, M. A. (1995) Cell 82, 507-522. [DOI] [PubMed] [Google Scholar]
- 17.Kissinger C. R., Parge, N. E., Knighton, D. R., Lewis, C. T., Pelletier, L. A., Tempczyk, A., Kallish, V. J., Tucker, K. D., Showalter, R. E., Moomaw, E. W., et al. (1995) Nature (London) 378, 641-644. [DOI] [PubMed] [Google Scholar]
- 18.Mondragon A., Griffith, E. C., Sun, L., Xiong, F., Armstrong, C. & Liu, J. O. (1997) Biochemistry 36, 4934-4942. [DOI] [PubMed] [Google Scholar]
- 19.Ke H. M., Mayrose, D., Belshaw, P. J., Alberg, D., Schreiber, S. L., Chang, Z. Y., Etzkorn, F. A., Ho, S. & Walsh, C. T. (1994) Structure (London) 2, 33-44. [DOI] [PubMed] [Google Scholar]
- 20.Brünger A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 21.Jones T. A, Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. (1991) Acta Crystallogr. A 47, 110-119. [DOI] [PubMed] [Google Scholar]
- 22.Lohse D. L., Denu, J. M. & Dixon, J. E. (1995) Structure (London) 3, 987-990. [DOI] [PubMed] [Google Scholar]
- 23.Pflugl G., Kallen, J., Schirmer, T., Jansonius, J. N., Zurini, M. G. & Walkinshaw, M. D. (1993) Nature (London) 361, 91-94. [DOI] [PubMed] [Google Scholar]
- 24.Zhu D., Cardenas, M. E. & Heitman, J. (1996) Mol. Pharmacol. 50, 506-511. [PubMed] [Google Scholar]
- 25.Cardenas M. E., Muir, R. S., Breuder, T. & Heitman, J. (1995) EMBO J. 14, 2772-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milan D., Griffith, J., Su, M., Price, E. R. & McKeon, F. (1994) Cell 79, 437-447. [DOI] [PubMed] [Google Scholar]
- 27.Fox D. S., Cruz, M. C., Sia, R. A. L., Ke, H., Cox, G. M., Cardenas, M. E. & Heitman, J. (2001) Mol. Microbiol. 39, 835-849. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Albers, M. W., Wandless, T. J., Luan, S., Alberg, D. G., Belshaw, P. J., Cohen, P., MacKintosh, C., Klee, C. B. & Schreiber, S. L. (1992) Biochemistry 31, 3896-3901. [DOI] [PubMed] [Google Scholar]
- 29.Nelson P. A., Akselband, Y., Kawamura, A., Su, M., Tung, R. D., Rich, D. H., Kishore, V., Rosborough, S. L., DeCenzo, M. T., Livingston, D. J., et al. (1993) J. Immunol. 150, 2139-2147. [PubMed] [Google Scholar]
- 30.Sigal N. H., Dumont, F., Durette, P., Siekierka, J. J., Peterson, L., Rich, D. H., Dunlap, B. E., Staruch, M. J., Melino, M. R., Koprak, S. L., et al. (1991) J. Exp. Med. 173, 619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann G., Andersen, E., Quesniaux, V. & Eberle, M. K. (1992) Transplant. Proc. 24, 43-48. [PubMed] [Google Scholar]
- 32.Ryffel B., Woerly, G., Quesniaux, V. F. J., Husi, H. & Foxwell, B. M. (1992) Biochem. Pharmacol. 43, 953-960. [DOI] [PubMed] [Google Scholar]
- 33.Swanson S. K.-H., Born, T., Zydowsky, L. D., Cho, H., Chang, H. Y., Walsh, C. T. & Rusnak, F. (1992) Proc. Natl. Acad. Sci. USA 89, 3741-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aramburu J., Garcia-Cozar, F., Raghavan, A., Okamura, H., Rao, A. & Hogan, P. G. (1998) Mol. Cell 1, 627-637. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham B. C., Ultsch, M., De Vos, A. M., Mulkerrin, M. G., Clauser, K. R. & Wells, J. A. (1991) Science 254, 821-825. [DOI] [PubMed] [Google Scholar]
- 36.DeLano W. L., Ultsch, M. H., de Vos, A. M. & Wells, J. A. (2000) Science 287, 1279-1283. [DOI] [PubMed] [Google Scholar]