Abstract
Corticotropin-releasing factor (CRF), recognized as an important stress factor, binds to a CRF receptor and a CRF-binding protein (CRFBP) that represents a reservoir of endogenous CRF. Although CRFBP was observed to dimerize, at least in part, the ligand was found to be exclusively bound to the monomer—as indicated by photoaffinity labeling. We localized the CRF binding site by using photoaffinity labeling in combination with different mass spectrometric techniques. The amino acid residues Arg-23 and Arg-36 of CRFBP were identified as the sites of photoincorporation of monofunctional and bifunctional photoprobes designed on the basis of the amino acid sequence of human/rat CRF6–33. It was, therefore, concluded that the sequence of amino acid residues 23–36 of CRFBP is involved in ligand binding. Our data are in support of an antiparallel alignment of the photoprobe with the amino acid residues 23–36 of the CRFBP monomer.
Corticotropin-releasing factor (CRF) (1, 2), the key mediator of the mammalian responses to stress stimuli, has also been recognized as an important neuromodulator of brain functions such as learning and anxiety (3). The central actions of CRF are mediated through at least two different subtypes of CRF receptors (CRFRs), CRFR1 and CRFR2 (4), and are modulated by a 37-kDa CRF-binding protein (CRFBP) (5), which is localized in several distinct brain regions, including the cerebral cortex and the hippocampus (6). Consistent with its proposed role to reduce the ligand's availability for CRFR-mediated actions (6, 7), it has been shown that 40–60% of the human brain CRF is bound by CRFBP (8). Thus, the binding protein can be considered as a physiologically relevant reservoir of endogenous CRF.
CRFR1 and -2 mediate opposite effects on learning and anxiety. Learning is enhanced through hippocampal CRFR1, whereas it is impaired through septal CRFR2 (9). Anxiety-like behavior is increased by activation of CRFR1 and predominantly decreased by activation of CRFR2—as indicated by CRFR1 and CRFR2 gene deletions (10–13). In this complex situation, selective activation of CRFR-dependent brain functions could be achieved on the basis of the distinct distribution of CRFBP in the brain. Thus, the release of endogenous ligand from hippocampal CRFBP could increase memory consolidation under physiologic and pathophysiologic conditions (8, 9) without producing anxiety-like effects through CRFR2 of the lateral septum void of CRFBP. Displacement of CRF from its binding protein may be achieved by CRFBP-selective peptides (CRFBP inhibitors) such as human/rat (h/r)-CRF6–33 (8, 14), a synthetic fragment of h/rCRF. A detailed knowledge on the ligand-binding site of CRFBP, whose three-dimensional structure has not been elucidated so far, may facilitate the design of new peptidic and nonpeptidic CRFBP inhibitors.
We identified, using a photoaffinity label, the ligand-binding site of the rat CRFBP (rCRFBP). New photoreactive analogs of h/rCRF6–33 were used in combination with different mass spectrometric techniques to directly determine contact sites between residues of CRF and its binding protein. In view of the finding that human CRFBP dimerizes after association with ligand (15), the subunit structure of rCRFBP was investigated with mono- and bifunctional photoprobes, as well as by chemical cross-linking.
Methods
Peptide Synthesis.
All peptides were synthesized by standard solid phase synthesis, using fluorenylmethoxycarbonyl (Fmoc) chemistry as described (16). Fmoc-para-benzoyl-Phe (Bachem) was used to introduce the benzophenone photophore into the polypeptides. For N-terminal modification, para-benzoylbenzoic acid N-hydroxysuccinimide ester (Molecular Probes) was coupled to the deprotected α-amino group of the resin-linked peptide. The reaction was carried out overnight at room temperature in dimethyl formamide with a 5-fold molar excess of the activated ester.
Production of rCRFBP and Binding Assay.
Recombinant rCRFBP, containing a C-terminal His-tag, was produced in human embryonic kidney (HEK) 293 cells under serum-free conditions (16). Binding of peptides to rCRFBP was determined in PBS/0.02% Nonidet P-40, using a scintillation proximity assay with [125I-Tyr0]h/rCRF as radioligand (17).
Generation and Detection of the Photoadducts.
For photoaffinity labeling, medium that contained rCRFBP was incubated with the photoprobe (100 nM) under the conditions of the binding assay. Unless otherwise stated, activation was carried out for 30 min at 0°C with an Ultratech 400 W halogen metal vapor lamp (Osram). Protein-damaging wavelengths below 300 nm were filtered with a B270 glass screen (Schott). Unlabeled rCRFBP and the photoadduct were copurified by adsorption to a nickel-chelate matrix under denaturing conditions. The eluted fraction was analyzed by SDS/9% PAGE (16) and by reversed-phase HPLC coupled on-line to mass spectrometry (HPLC-MS) on a Vydac C4 column (0.3 × 150 mm; LC Packings). Western blot analysis with chemoluminescence detection, using the polyclonal antibodies anti-rCRFFP (16) or anti-h/rCRF (Sigma), was carried out as described (16).
Chemical Cross-Linking.
rCRFBP was cross-linked by incubation with or without ligand (50 nM) under the conditions of the binding assay and subsequent treatment with 1 mM sulfo-DST (Pierce) for 1 h at 20°C.
Peptide Mapping of the Photoadducts by HPLC-MS.
S-carboxamidomethylation of Cys residues by iodoacetamide and peptide mapping of rCRFBP monitored by mass spectrometric analysis were performed as described (16). The photoadduct was separated from unlabeled rCRFBP by HPLC on a Vydac C4 column (1.0 × 150 mm; LC Packings). Both protein species were first digested with endoprotease AspN and subsequently with TPCK-trypsin. The resulting peptide mixtures were analyzed by HPLC-MS either on a PepMap C18 or a Vydac LowTFA C4 column (0.3 × 150 mm; LC Packings). Gradients formed by 0.07% formic acid and 0.05% formic acid, containing 80% acetonitrile, were applied. These columns were also used to fractionate enzymatic digests for off-line tandem MS.
Tandem MS.
The high-energy collision-induced dissociation (CID) mass spectra were recorded on an Autospec-T four-sector tandem mass spectrometer (Micromass, Manchester, U.K.) equipped with a nanoelectrospray (NanoES) ion source and a multichannel array detector. The NanoES glass capillaries (Protana) were filled with 1 μl of the peptide samples. Argon was used as collision gas with an adjusted pressure to provide an attenuation of the ion beam by 70% (singly charged precursor ions) or by 90% (multiply charged precursor ions). The ion-accelerating voltage was 4 kV; the gas cell was operated at 2 kV above ground potential. The fragment ions were annotated as proposed by Tuinman and Pettit (18).
Prediction of Secondary Structure.
Secondary structure predictions were performed, using the jnet prediction method (19), which is available in the jpred server from http://barton.ebi.ac.uk. A multiple-sequence alignment of the four known mammalian CRFBP amino acid sequences from different species (rat, mouse, human, sheep) was submitted to the server.
Results
Design of the Photoprobes.
The photoprobes were designed on the basis of the amino acid sequence of h/rCRF6–33 that represents the minimal sequence required for high-affinity binding to CRFBP (14). The benzophenone (Bp) photophore was introduced into h/rCRF6–33 either at the N terminus by modification of the α-amino group to generate [Bp6]h/rCRF6–33, or at the C terminus by replacement of the bulky His residue in position 32 by a para-benzoyl-Phe residue to generate [Bp32]h/rCRF6–33. By combining these modifications, the bifunctional photoprobe [Bp6,32]h/rCRF6–33 was obtained to be used as a specific cross-linker to covalently connect two remote contact points between h/rCRF6–33 and CRFBP. The affinities of the monofunctional photoprobes [Bp6]h/rCRF6–33 [IC50 = 3.2 nM; 95% confidence interval (CI), 2.3–4.0 nM] and [Bp32]h/rCRF6–33 (IC50 = 2.1 nM; 95% CI, 0.7–3.5 nM) were not significantly different from the affinity of h/rCRF6–33 to rCRFBP (IC50 = 1.9 nM; 95% CI, 1.3–2.5 nM). The affinity of the bifunctional photoprobe [Bp6,32]h/rCRF6–33 (IC50 = 14 nM; 95% CI, 11–18 nM) was decreased by a factor of seven compared with that of h/rCRF6–33; however, despite this reduction, neither the specificity nor the yield of photoadduct formation was detectably affected.
Detection of the Photoadducts.
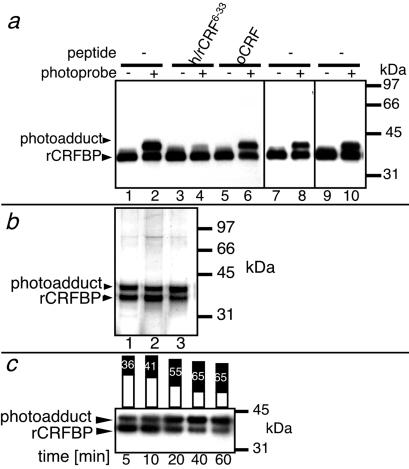
By Western blot analysis, using specific polyclonal antibodies directed against rCRFBP (anti-rCRFBP) (16), 41-kDa species that corresponded to the photoadducts of rCRFBP were detected (Fig. 1a). As representatively shown for [Bp6]h/rCRF6–33 (Fig. 1a), the formation of all photoadducts was completely suppressed in the presence of an excess of h/rCRF6–33, but not in the presence of a low-affinity ligand such as ovine CRF (oCRF); those data demonstrated the specificity of the photoprobe binding.
Fig 1.
Detection of the photoadducts. (a) Immunodetection of the photoadducts formed by [Bp6]h/rCRF6–33 (lanes 1–6), [Bp32]h/rCRF6–33 (lanes 7 and 8), and [Bp6,32]h/rCRF6–33 (lanes 9 and 10) and rCRFBP. The absence (−) or presence (+) of photoprobe is indicated. h/rCRF6–33 or oCRF (1 μM) were added to the photoprobes (100 nM) and rCRFBP—as indicated. The antibodies anti-rCRFBP were used for detection. (b) Silver-stained SDS gel, showing unlabeled rCRFBP and photoadducts both purified by Ni-chelate chromatography. All lanes show the products of photoaffinity labeling with three different photoprobes. Lane 1, [Bp6]h/rCRF6–33; lane 2, [Bp32]h/rCRF6–33; lane 3, [Bp6,32]h/rCRF6–33. (c) Immunodetection of the photoadduct formed by [Bp6]h/rCRF6–33 and rCRFBP. Aliquots of the same sample were analyzed at different time points of irradiation. The bar graphs represent the densitometric evaluation of the intensities of the rCRFBP bands (white) and of the photoadduct bands (black). The photoadduct yields expressed in percentage are included. Prolongation of the irradiation time to 60 min resulted in photodestruction of the proteins as indicated by decreasing signal intensities. The antibodies anti-rCRFBP were used for detection.
rCRFBP and its respective photoadduct were copurified by nickel-chelate chromatography, employing the C-terminal His-tag fused to recombinant rCRFBP (16). Analysis of the obtained fractions with SDS/PAGE followed by silver staining (Fig. 1b), or with HPLC-MS (data not shown), revealed photoadduct yields of approximately 50%, which were similar to those observed by immunodetection (Fig. 1a). This markedly high yield of photoincorporation may have resulted from the reported slow dissociation of ligands from CRFBP (20) in combination with the reversible activation of benzophenone photophores via excitation–relaxation cycles (21).
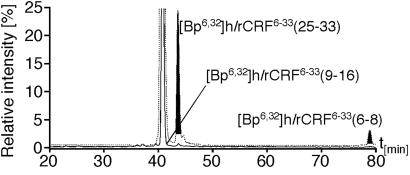
It has been proposed that CRF induces the formation of homodimers of CRFBP (15). Thus, it was conceivable that the ligand was bound to one or both subunits of this putative CRFBP complex. These possibilities were investigated with the bifunctional photoprobe [Bp6,32]h/rCRF6–33. It was demonstrated that the sites of photoincorporation of the monofunctional photoprobes [Bp6]h/rCRF6–33 and [Bp32]h/rCRF6–33 resided in the same subunit of the putative rCRFBP dimer. By SDS/PAGE, only a 41-kDa species, corresponding to the photoadduct formed by rCRFBP and [Bp6,32]h/rCRF6–33, was detected (Fig. 1 a and b). The absence of a dimer species indicated that the bifunctional photoprobe did not interlink different subunits. To verify that the photoprobe's benzophenone groups had both reacted with rCRFBP, proteolytic digests of the photoadduct formed by rCRFBP and [Bp6,32]h/rCRF6–33, and of the bifunctional photoprobe alone, were analyzed by the extraction of multiple ion chromatograms from full-scan HPLC-MS recordings (ref. 22; Fig. 2). Thereby, the photophore-containing ligand fragments were detected in the digest of [Bp6,32]h/rCRF6–33, but not in the digest of the photoadduct. By this finding, it was demonstrated that the N-terminal as well as the C-terminal benzophenone groups of [Bp6,32]h/rCRF6–33 reacted almost quantitatively with rCRFBP.
Fig 2.
Peptide mapping of the photoadduct by HPLC-MS. Multiple ion chromatograms (MICs) were extracted from full scan HPLC-MS recordings. The solid trace represents the MIC of the digested photoadduct of rCRFBP and [Bp6,32]h/rCRF6–33 on the basis of the following masses: 540.6 = [M+H]+ of [Bp6,32]h/rCRF6–33(6–8); 507.6 = [M + 2H]2+ of [Bp6,32]h/rCRF6–33(9–16); 1,127.2 = [M+H]+ of [Bp6,32]h/rCRF6–33(25–33). The superimposed dashed trace represents the MIC of the digested bifunctional photoprobe [Bp6,32]h/rCRF6–33 on the basis of the same set of masses. The signals corresponding to the photophore-containing fragments of [Bp6,32]h/rCRF6–33 are shaded in gray. The mass-selective chromatograms were scaled to give the same signal height for the internal proteolytic fragment [Bp6,32]h/rCRF6–33(9–16) present in both digests.
When the photoadduct formation of rCRFBP and [Bp6]h/rCRF6–33 was analyzed at different time points of irradiation, yields of up to ≈65% were observed (Fig. 1c). These results were suggestive that the photoadduct formation took place on the level of a CRFBP monomer labeled by one ligand molecule.
Subunit Structure of rCRFBP.
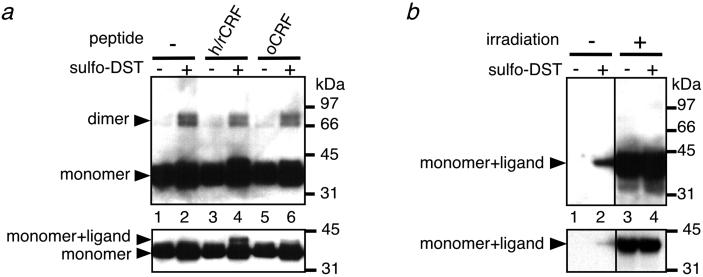
The subunit structure of rCRFBP under the conditions of photoaffinity-labeling was further analyzed by chemical cross-linking, using sulfo-disuccinimidyl tartrate (sulfo-DST) in the absence or presence of different ligands. In agreement with the observed different affinities to rCRFBP, h/rCRF, but not oCRF, was found to be cross-linked to the binding protein, as indicated by Western blot analysis using anti-rCRFBP for detection (Fig. 3a). In addition, treatment of rCRFBP with sulfo-DST generated species of 65–80 kDa—consistent with the size of dimeric species (Fig. 3a). The appearance of the dimer in the gel as a doublet with an apparent difference in size of approximately 10 kDa was probably not due to glycosylation differences, because protein chemical characterization revealed that recombinant rCRFBP carried a single Asn-linked carbohydrate moiety with an average mass of 1.5 kDa (16). The doublet may be rather easily explained by the two distinct protein species formed during cross-linking that displayed different electrophoretic mobilities in SDS/PAGE. No evidence for significant amounts of cross-linked oligomers larger than dimers was observed with SDS/PAGE. It was, therefore, concluded that dimerization was not due to nonphysiological high concentrations of rCRFBP. On the basis of Bmax values derived from homologous competition binding curves (16), the concentration of rCRFBP in the cross-linking experiment was estimated to be in the range of 1–10 nM—in agreement with the plasma levels of CRFBP in humans (23, 24).
Fig 3.
Subunit structure of rCRFBP. (a) Immunodetection of the cross-linked rCRFBP forms using the antibodies anti-rCRFBP. rCRFBP was incubated without ligand (lanes 1 and 2) or with 50 nM h/rCRF (lanes 3 and 4) or with 50 nM oCRF (lanes 5 and 6) and then cross-linked with sulfo-DST (1 mM). The absence (−) or presence (+) of sulfo-DST is indicated. (Lower) Part of the immunoblot after shortening the exposure time of the same blotting membrane to the x-ray film from 2 min to 10 sec. (b) Immunodetection of the cross-linked rCRFBP forms using the antibodies anti-h/rCRF. rCRFBP was incubated with 50 nM [Bp1]h/rCRF1–41 without (lanes 1 and 2) or with subsequent irradiation (lanes 3 and 4), and then cross-linked with sulfo-DST (1 mM). The absence (−) or presence (+) of sulfo-DST is indicated. (Lower) Part of the immunoblot after shortening the exposure time of the same blotting membrane to the x-ray film from 2 min to 5 sec.
Surprisingly, the intensity of the dimer bands did not depend on the presence of h/rCRF or oCRF, and dimerization was even detected in the absence of any added ligand (Fig. 3a). These results were suggestive that the dimer formation took place on the basis of a ligand-independent mechanism. For further analysis of the composition of the dimeric species, chemical cross-linking was carried out in the presence of the photoprobe [Bp1]h/rCRF1–41 without and with prior irradiation. The cross-linked species were visualized by immunodetection of the photoprobe, using polyclonal antibodies directed against h/rCRF25–41 (anti-h/rCRF). In agreement with the photoprobe's high affinity to rCRFBP (IC50 = 0.67 nM; 95% CI, 0.40–0.93 nM), a 42-kDa species that corresponded to [Bp1]h/rCRF1–41 chemically cross-linked to rCRFBP was detected after treatment with sulfo-DST (Fig. 3b, lane 2). When [Bp1]h/rCRF1–41 was covalently linked to rCRFBP by irradiation before treatment with sulfo-DST, the signal intensity of this 42-kDa band was significantly increased (Fig. 3b, lane 4). However, despite the covalent attachment of the ligand to rCRFBP before chemical cross-linking, no signals were detected in the range of 65–80 kDa—where a dimeric species would have been expected to appear (Fig. 3b). Thus, it was demonstrated that the ligand was not part of the rCRFBP dimer. It was, therefore, concluded that the dimer was not involved in ligand binding.
Identification of the Ligand-Binding Site.
After S-carboxamidomethylation of the Cys residues with iodoacetamide, the photoadduct was separated from unlabeled rCRFBP by reversed-phase HPLC on the basis of the significantly increased hydrophobic properties of the photoadduct due to covalent attachment of the photoprobe (data not shown). The isolated photoadduct was digested with endoprotease AspN and subsequently with trypsin. By combining these two proteases, relatively small protein fragments (<3 kDa) were obtained, which were analyzed with HPLC-MS and thus characterized on the basis of their molecular masses. Unlabeled rCRFBP obtained from the same labeling experiment was digested similarly and used as control. Peptides with observed molecular masses that were incompatible with the calculated rCRFBP and photoprobe fragments, respectively, were considered as photoadduct-specific fragments and were further analyzed.
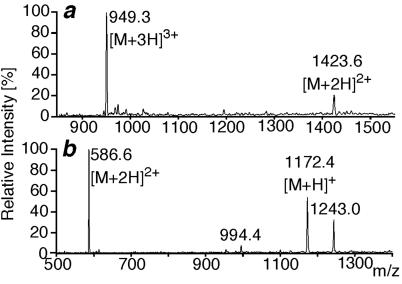
By HPLC-MS analysis of the digested photoadduct formed by rCRFBP and the bifunctional photoprobe [Bp6,32]h/rCRF6–33, two characteristic molecular masses were identified and assigned to proteolytic photoadduct fragments. A mass of Mobs = 2,845.1 (Fig. 4a) was assigned to rCRFBP(12–26) labeled by [Bp6,32]h/rCRF6–33(25–33) (Mcalc = 2,845.24; ΔM = 50 ppm). A mass of Mobs = 1,171.3 (Fig. 4b) was assigned to rCRFBP(34–38) photolabeled by [Bp6,32]h/rCRF6–33(6–8) (Mcalc = 1,171.43; ΔM = 110 ppm). Thus, rCRFBP(12–26) and rCRFBP(34–38) were identified as the binding sites of the C- and the N-terminal part of the bifunctional photoprobe, respectively. In agreement with this finding, the monofunctional photoprobes [Bp32]h/rCRF6–33 and [Bp6]h/rCRF6–33 labeled rCRFBP(12–26) and rCRFBP(34–38), respectively (data not shown). The tryptic cleavage sites Lys-22, Arg-23, and Arg-36, which were found to be cleaved in the control digest of unlabeled rCRFBP, were blocked as a result of the photolabeling; those cleavages indicated that the modification occurred directly at, or in close proximity to, the respective amino acid residue.
Fig 4.
Mass spectra of the photoadducts. (a) Mass spectrum of the photoadduct fragment rCRFBP(12–26)x[Bp6,32]h/rCRF6–33(25–33). (b) Mass spectrum of the photoadduct fragment rCRFBP(34–38)x[Bp6,32]h/rCRF6–33(6–8). The additional signals at m/z = 994.4 ([M + 5H]5+) and 1,243.0 ([M + 4H]4+) were assigned to the fragment rCRFBP(126–170) coeluting with the photoadduct fragment.
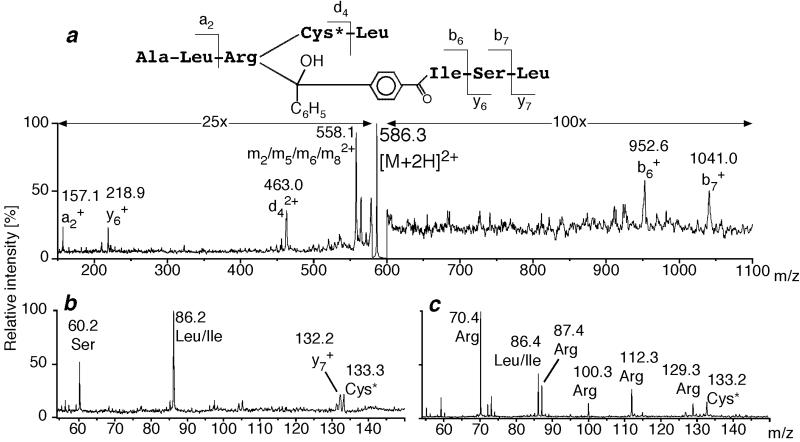
For further characterization, proteolytic digests of the photoadducts were fractionated by HPLC. Fractions that contained the labeled peptides were analyzed by tandem MS. The amino acid sequence of the photoadduct fragment rCRFBP(34–38)×[Bp6,32]h/rCRF6–33(6–8) (×, the covalent linkage of fragments) was deduced from its high-energy CID mass spectrum (Fig. 5a). Thereby, a covalent linkage of rCRFBP at Arg-36 to [Bp6,32]h/rCRF6–33(6–8) was demonstrated. The same result was obtained for the photoadduct fragment rCRFBP(34–38)×[Bp6]h/rCRF6–33(6–8) formed by the monofunctional photoprobe [Bp6]h/rCRF6–33 (data not shown). Consistent with the modification of the side chain of Arg-36, the low-mass fragment ions at m/z = 70, 87, 100, 112, and 129 (25)—indicative for the presence of Arg—were absent in the spectrum of the photoadduct fragment (Fig. 5b). In contrast, all low-mass ion signals were present in the spectrum of the corresponding synthetic peptide rCRFBP(34–38) that contained unmodified Arg-36 (Fig. 5c).
Fig 5.
Analysis of photoadduct fragments by tandem mass spectrometry. (a) High-energy CID mass spectrum of [M + 2H]2+ of rCRFBP(34–38)x[Bp6,32]h/rCR6–33(6–8). The derived structure is shown above the spectrum. Cys is marked by an asterisk to indicate S-carboxamidomethylation. Note that the side chain of Cys* is lost with the fragment ion d4 and thereby excluded as the site of photoincorporation. (b) Low-mass ion region of the high-energy CID mass spectrum shown in a. (c) Low-mass ion region of the high-energy CID mass spectrum of [M+H]+ of the synthetic peptide Ala-Leu-Arg-Cys*-Leu representing unlabeled rCRFBP(34–38).
The high-energy CID mass spectrum of the photoadduct fragment rCRFBP(12–26)×[Bp6,32]h/rCRF6–33(25–33) did not reveal any fragment ion signals that would be indicative of the cleavage of the peptide backbone of rCRFBP(12–26). Therefore, the CID mass spectra of the photoadduct fragment and of synthetic rCRFBP(12–26) were compared with respect to their low-mass ion regions—as described above (data not shown). The lack of the low-mass ion signals at m/z = 100, 112, and 129 indicated the labeling of the side chain of Arg-23 by [Bp6,32]h/rCRF6–33(25–33). In agreement with the presence of Pro-13 and Asn-20,24 in rCRFBP(12–26), the low-mass ion signals at m/z = 70 and 87 (25) were not absent, but their intensities were significantly decreased. The same observations held for the photoadduct fragment rCRFBP(12–26)×[Bp6,32]h/rCRF6–33(25–33) obtained with the monofunctional photoprobe [Bp32]h/rCRF6–33 (data not shown).
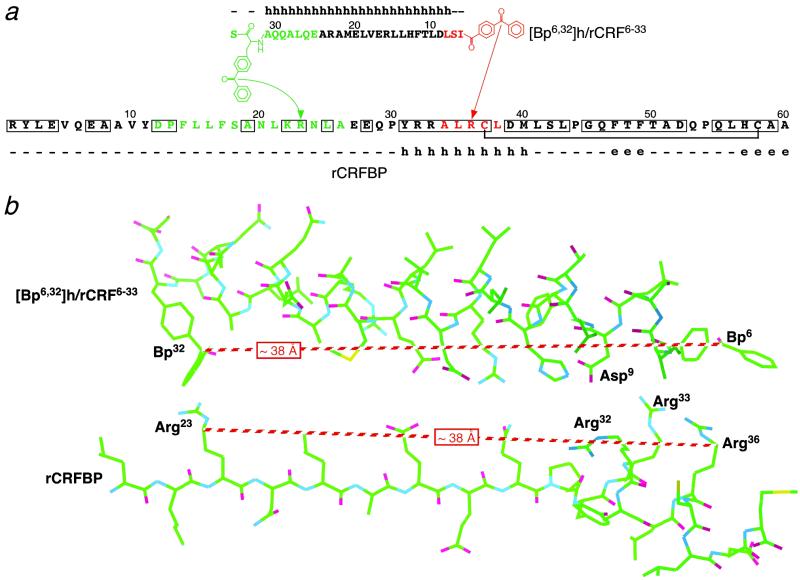
Within the amino acid side chains, methyl or methylene groups adjacent to heteroatoms are known to be particularly reactive sites for photoincorporation of benzophenone groups (21). In agreement with this preference, labeling of the Met side chain was found in the majority of photoaffinity-labeling studies that used benzophenone-derivatized peptides (26–28). However, the labeling of the Arg side chain observed here was also consistent with the reaction preference of benzophenones. Despite the uncertainty that resulted from this preference, the photoaffinity-labeling of rCRFBP was considered to be highly regio-specific—as indicated by the finding that only one arginine, Arg-36, of three Arg residues within the sequence of amino acid residues 32–36, was labeled (Fig. 6a).
Fig 6.
Proposed interaction of the bifunctional photoprobe with rCRFBP. (a) Schematic representation of the photoaffinity labeling results. Only the N-terminal 60 of totally 299 amino acid residues of rCRFBP are shown. The amino acid sequence of the ligand including the chemical structures of the photophores is depicted from the C to the N terminus to display the antiparallel alignment. The C-terminal photophore-containing fragment [Bp6,32]h/rCRF6–33(25–33) and its site of labeling, rCRFBP(12–26), are shown in green. The N-terminal photophore-containing fragment [Bp6,32]h/rCRF6–33(6–8) and its site of labeling, rCRFBP(34–38), are shown in red. The arrows indicate the photolabeled amino acids, Arg-23 and Arg-36. The residues conserved between all mammalian CRFBP sequences known to date are boxed. The disulfide bond between Cys-37 and Cys-58 is indicated by a bracket. The secondary structure predicted by the jnet algorithm is shown above the ligand sequence and below the rCRFBP sequence, respectively. h, helix; e, extended (sheet); –, other (loop). (b) Wire frame model of [Bp6,32]h/rCRF6–33 interacting with the stretch of amino acids 20–40 of rCRFBP. The proposed α-helices were constructed by applying the torsion angles φ = −57°, ψ = −47°, and ω = 180°. The extended stretch of amino acids 20–30 of rCRFBP was constructed by applying the torsion angles for a planar polypeptide chain (φ = −180°, ψ = 180°, and ω = 180°). In the shown conformations, the distances between Bp6 and Bp32 of [Bp6,32]h/rCRF6–33, and Arg-23 and Arg-36 of rCRFBP were both approximately 38 Å. Asp-9 of [Bp6,32]h/rCRF6–33 may bind to either Arg-32 or Arg-33 of rCRFBP by an electrostatic interaction.
Discussion
In view of the composition of the CRFBP–CRF complex, we concluded from our data that one molecule of h/rCRF was bound to a rCRFBP monomer. This conclusion contrasts with the assumption of the ligand-induced dimerization of human CRFBP—as was proposed on the basis of gel filtration data (15). However, on the basis of the available data, we cannot exclude the possibility that human CRFBP interacts differently with its ligand than rodent CRFBP.
On the basis of the results of photoaffinity labeling, we propose an antiparallel alignment of [Bp6,32]h/rCRF6–33 with the N-terminal domain of rCRFBP during binding (Fig. 6). Our data indicated that, in the bound state, Ile-6 and para-benzoyl-Phe-32 of [Bp6,32]h/rCRF6–33 were in close proximity to Arg-36 and Arg-23 of rCRFBP, respectively. Thus, it was probable that the 28-aa residue photoprobe spanned over the 14-residue polypeptide chain of rCRFBP. By using CD and NMR spectroscopic methods, evidence has been provided that h/rCRF forms a defined amphiphilic α-helix in the central part of the molecule (29, 30). Therefore, a similar helical structure was assumed for [Bp6,32]h/rCRF6–33. To fit into the proposed alignment, the interacting polypeptide chain of rCRFBP needed to adopt a more stretched conformation, which was compatible with the secondary structure predicted by the jnet prediction method (ref. 19; Fig. 6).
Recently, we demonstrated that Ala-22 of h/rCRF plays a crucial role in high-affinity binding to rCRFBP (17). Because this amino acid is part of the hydrophobic patch of the amphiphilic α-helix of h/rCRF, we proposed an involvement of the hydrophobic patch in binding to rCRFBP (17). In the present study, we identified a relatively hydrophilic sequence of rCRFBP as a contact site between h/rCRF and its binding protein (Fig. 6). In agreement with the model developed here, it is suggested that the hydrophilic patch of h/rCRF was in close contact with the amino acid residues 23–36 of rCRFBP (Fig. 6), whereas the hydrophobic patch of h/rCRF remained available for interaction with a different—presumably more hydrophobic—part of rCRFBP. Interestingly, the stretch of amino acids 31–40 of rCRFBP was the unique part of the entire protein predicted to form an α-helical secondary structure. Because residues 31–40 are highly conserved between the mammalian CRFBP sequences known to date (31), it was likely that a helix–helix interaction may be involved in ligand binding of CRFBP. In view of this assumption, an ionic interaction of Asp-9 of [Bp6,32]h/rCRF6–33 with Arg-32 or Arg-33 of rCRFBP may have contributed to the stability of the complex. Asp-9 is completely conserved by all high-affinity ligands of CRFBP, and its absence in the CRF antagonist astressin, designed on the basis of the sequence of h/rCRF12–41 (3), may be responsible for the low affinity of this peptide compared with the high affinity of the CRF antagonist α-helical CRF9–41 (17).
Acknowledgments
We thank Lars van Werven and Thomas Liepold for expert technical help. Dr. Jelena Radulovic is gratefully acknowledged for helpful discussion.
Abbreviations
Bp, benzophenone
CID, collision-induced dissociation
CRF, corticotropin-releasing factor
CRFBP, CRF-binding protein
h/rCRF, human/rat CRF
oCRF, ovine CRF
rCRFBP, rat CRFBP
References
- 1.Spiess J., Rivier, J., Rivier, C. & Vale, W. (1981) Proc. Natl. Acad. Sci. USA 78, 6517-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vale W., Spiess, J., Rivier, C. & Rivier, J. (1981) Science 213, 1394-1397. [DOI] [PubMed] [Google Scholar]
- 3.Eckart K., Radulovic, J., Radulovic, M., Jahn, O., Blank, T., Stiedl, O. & Spiess, J. (1999) Curr. Med. Chem. 6, 1035-1053. [PubMed] [Google Scholar]
- 4.Perrin M. H. & Vale, W. W. (1999) Ann. N.Y. Acad. Sci. 885, 312-328. [DOI] [PubMed] [Google Scholar]
- 5.Potter E., Behan, D. P., Fischer, W. H., Linton, E. A., Lowry, P. J. & Vale, W. W. (1991) Nature (London) 349, 423-426. [DOI] [PubMed] [Google Scholar]
- 6.Potter E., Behan, D. P., Linton, E. A., Lowry, P. J., Sawchenko, P. E. & Vale, W. W. (1992) Proc. Natl. Acad. Sci. USA 89, 4192-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seasholtz A. F., Burrows, H. L., Karolyi, I. J. & Camper, S. A. (2001) Peptides 22, 743-751. [DOI] [PubMed] [Google Scholar]
- 8.Behan D. P., Heinrichs, S. C., Troncoso, J. C., Liu, X. J., Kawas, C. H., Ling, N. & De Souza, E. B. (1995) Nature (London) 378, 284-287. [DOI] [PubMed] [Google Scholar]
- 9.Radulovic J., Rühmann, A., Liepold, T. & Spiess, J. (1999) J. Neurosci. 19, 5016-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith G., Aubry, J.-M., Dellu, F., Contarino, A., Bilezikjian, L., Gold, L., Chen, R., Marchuk, Y., Hauser, C., Bentley, C., et al. (1998) Neuron 20, 1093-1102. [DOI] [PubMed] [Google Scholar]
- 11.Timpl P., Spanagel, R., Sillaber, I., Kresse, A., Reul, J., Stalla, G. K., Blanquet, V., Steckler, T., Holsboer, F. & Wurst, W. (1998) Nat. Genet. 19, 162-166. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T., Radulovic, J., Radulovic, M., Lin, C. R., Schrick, C., Hooshmand, F., Hermanson, O., Rosenfeld, M. G. & Spiess, J. (2000) Nat. Genet. 24, 415-419. [DOI] [PubMed] [Google Scholar]
- 13.Bale T. L., Contarino, A., Smith, G. W., Chan, R., Gold, L. H., Sawchenko, P. E., Koob, G. F., Vale, W. W. & Lee, K.-F. (2000) Nat. Genet. 24, 410-414. [DOI] [PubMed] [Google Scholar]
- 14.Sutton S. W., Behan, D. P., Lahrichi, S. L., Kaiser, R., Corrigan, A., Lowry, P., Potter, E., Perrin, M. H., Rivier, J. & Vale, W. W. (1995) Endocrinology 136, 1097-1102. [DOI] [PubMed] [Google Scholar]
- 15.Woods R. J., Kennedy, K. M., Gibbins, J. M., Behan, D., Vale, W. W. & Lowry, P. J. (1994) Endocrinology 135, 768-773. [DOI] [PubMed] [Google Scholar]
- 16.Jahn O., Eckart, K., Sydow, S., Hofmann, B. A. & Spiess, J. (2001) Peptides 22, 47-56. [DOI] [PubMed] [Google Scholar]
- 17.Eckart K., Jahn, O., Radulovic, J., Tezval, H., van Werven, L. & Spiess, J. (2001) Proc. Natl. Acad. Sci. USA 98, 11142-11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuinman A. A. & Pettit, G. R. (1990) Int. J. Peptide Protein Res. 36, 331-334. [DOI] [PubMed] [Google Scholar]
- 19.Cuff J. A. & Barton, G. J. (1999) Proteins 34, 508-519. [DOI] [PubMed] [Google Scholar]
- 20.Henriot S., Dautzenberg, F. M. & Kilpatrick, G. J. (1999) Eur. J. Pharmacol. 376, 321-324. [DOI] [PubMed] [Google Scholar]
- 21.Dormán G. & Prestwich, G. D. (1994) Biochemistry 33, 5661-5673. [DOI] [PubMed] [Google Scholar]
- 22.Jahn O., Hofmann, B., Brauns, O., Spiess, J. & Eckart, K. (2002) Int. J. Mass Spectrom. 214, 37-51. [Google Scholar]
- 23.Linton E. A., Perkins, A. V., Woods, R. J., Eben, F., Wolfe, C. D., Behan, D. P., Potter, E., Vale, W. W. & Lowry, P. J. (1993) J. Clin. Endocrinol. Metab. 76, 260-262. [DOI] [PubMed] [Google Scholar]
- 24.Behan D. P., Khongsaly, O., Liu, X. J., Ling, N., Goland, R., Nasman, B., Olsson, T. & Desouza, E. B. (1996) J. Clin. Endocrinol. Metab. 81, 2579-2586. [DOI] [PubMed] [Google Scholar]
- 25.Papayannopoulos I. A. (1995) Mass Spectrom. Rev. 14, 49-73. [Google Scholar]
- 26.O'Neil K. T., Erickson-Viitanen, S. & DeGrado, W. F. (1989) J. Biol. Chem. 264, 14571-14578. [PubMed] [Google Scholar]
- 27.Behar V., Bisello, A., Bitan, G., Rosenblatt, M. & Chorev, M. (2000) J. Biol. Chem. 275, 9-17. [DOI] [PubMed] [Google Scholar]
- 28.Li H., Macdonald, D. M., Hronowski, X., Costello, C. E., Leeman, S. E. & Boyd, N. D. (2001) J. Biol. Chem. 276, 10589-10593. [DOI] [PubMed] [Google Scholar]
- 29.Pallai P. V., Mabilia, M., Goodman, M., Vale, W. & Rivier, J. (1983) Proc. Natl. Acad. Sci. USA 80, 6770-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romier C., Bernassau, J., Cambillau, C. & Darbon, H. (1993) Protein Eng. 6, 149-156. [DOI] [PubMed] [Google Scholar]
- 31.Valverde R. A., Seasholtz, A. F., Cortright, D. N. & Denver, R. J. (2001) Mol. Cell. Endocrinol. 173, 29-40. [DOI] [PubMed] [Google Scholar]