Abstract
Enzyme-catalyzed β-elimination of sugar uronic acids, exemplified by the degradation of plant cell wall pectins, plays an important role in a wide spectrum of biological processes ranging from the recycling of plant biomass through to pathogen virulence. The three-dimensional crystal structure of the catalytic module of a “family PL-10” polysaccharide lyase, Pel10Acm from Cellvibrio japonicus, solved at a resolution of 1.3 Å, reveals a new polysaccharide lyase fold and is the first example of a polygalacturonic acid lyase that does not exhibit the “parallel β-helix” topology. The “Michaelis” complex of an inactive mutant in association with the substrate trigalacturonate/Ca2+ reveals the catalytic machinery harnessed by this polygalacturonate lyase, which displays a stunning resemblance, presumably through convergent evolution, to the tetragalacturonic acid complex observed for a structurally unrelated polygalacturonate lyase from family PL-1. Common coordination of the −1 and +1 subsite saccharide carboxylate groups by a protein-liganded Ca2+ ion, the positioning of an arginine catalytic base in close proximity to the α-carbon hydrogen and numerous other conserved enzyme–substrate interactions, considered in light of mutagenesis data for both families, suggest a generic polysaccharide anti-β-elimination mechanism.
Polysaccharide lyases (EC 4.2.2.x) are carbon–oxygen lyases that harness β-elimination chemistry (reviewed in ref. 1) to bring about degradation of C5 uronic acid containing pyranoside substrates such as polygalacturonates, alginates, hyaluronan, and chondroitin. They play a pivotal role in a wide range of processes ranging from the recycling of plant material, a process essential for biosphere maintenance (2), through to virulence of pathogens (3, 4). In contrast to the 87 sequence-derived families of glycoside hydrolases, polysaccharide lyases have been classified into just 12 families on the basis of amino acid sequence similarities (5), reflecting the requirement for substrate uronic acid groups in the elimination mechanism. Three-dimensional structures have been reported for enzymes from polysaccharide lyase (PL) families 1, 3, 5, 6, 8, and 9 and have thus far revealed just two catalytic module topographies: the “(α/α)6” barrel seen in families PL-5 and 8, or the “parallel β-helix” revealed by the first structure determination for a polysaccharide lyase, that of Pel1C from family PL-1, and observed subsequently in structures from families PL-3, 6, and 9. A catalytic mechanism featuring proton abstraction from C5 of the +1 subsite sugar residue, termed the α-carbon, and proton donation to the glycosidic oxygen, with the elimination of the leaving group from C4, termed the β-carbon (1, 6) seems the most plausible.
Polygalacturonic acid lyases (EC 4.2.2.2; polygalacturonate transeliminases) are extracellular enzymes found in plants and also secreted by both pathogenic and saprophytic microorganisms. They cleave polymeric α-1,4-linked galacturonic acids (GalA) generating 4,5-unsaturated oligogalacturonates as products (6). In addition to their role in the carbon cycle, polygalacturonic acid lyases are important virulence factors of plant pathogens, such as Erwinia chrysanthemi (3), whereas the enzymes from Pseudomonas fluorescens and Pseudomonas viridiflava are responsible for the majority of fresh fruit and vegetable spoilage (7). The critical role of polygalacturonate lyases in plant development is emphasized by the dedication of at least 34 ORFs for this function in Arabidopsis thaliana (8).
The CAZy classification (5) describes 12 families of polysaccharide lyases with polygalacturonate-active enzymes found in families PL-1, 2, 3, 9, and 10. Family PL-10 currently comprises just seven sequences and is a family for which no structural or mechanistic data exist. Here we report the 1.3-Å resolution three-dimensional structure of the competent catalytic module of the polygalacturonic acid lyase Pel10A (Pel10Acm), from Cellvibrio japonicus, together with analysis of the activity of wild-type and mutant enzymes. The enzyme topology reveals a predominantly α-helical enzyme with a distorted (α/α)3 barrel quite unlike the parallel β-helix displayed by other pectate lyases (Pel). The “Michaelis complex” of an inactive mutant of Pel10A with the substrate trigalacturonic acid GalA3 reveals the catalytic machinery and supports catalysis via an E1cb or concerted E2 elimination mechanism with Brønsted base catalysis provided by arginine. The active center provides a stunning example of convergent evolution. The location of three substrate-binding arginines, a main-chain carbonyl-O3 interaction, the Ca2+ coordinating carboxylates and the potential Brønsted base itself are isostructural with the catalytic center of the totally unrelated family PL-1 enzyme Pel1C from E. chrysanthemi.
Materials and Methods
Production of SeMet and Native Protein.
Protein production and purification were achieved essentially as described (9) except the methionine auxotroph Escherichia coli B834 (DE3, Novagen), transformed with p4.2.1 (10), was used for both native and SeMet preparations. Matrix-assisted laser desorption ionization-time of flight mass spectrometric analysis of native and SeMet-containing protein confirmed the identity of the polypeptides and indicated that the N-terminal methionine residue had been processed by the host bacterium (data not shown). The QuikChange Site-Directed Mutagenesis kit (Stratagene) was used to mutate plasmid p4.2.1.
Kinetic Analyses.
Kinetic parameters were determined for native and derivative forms of Pel10Acm against Na-polygalacturonic acid and trigalacturonic acid (Sigma-Aldrich). Release of the 4-deoxy-α-D-gluc-4-enuronosyl-containing products was followed on a Helios Alpha UV-visible spectrometer (ThermoSpectronic) at 232 nm, with a 1-cm light path quartz cuvette. The reaction mixture, 0.5 ml, comprised substrate in 50 mM CAPS buffer, pH 10.0, containing calcium chloride at a concentration of 2 mM (with GalA3 as substrate) or 0.1 mM (with polygalacturonic acid as substrate; the viscosity of polygalacturonic acid in high [Ca2+] preventing utilization of higher concentrations). The reaction components were prewarmed to, and the assay performed at, 310 K. Individual kinetic parameters were calculated by using grafit version 4 (Erithacus Software, Surrey, U.K.). To investigate bond cleavage frequencies by using tri- and tetragalacturonic acid, substrate consumption and product appearance were followed over time by using high-pressure anion-exchange chromatography as described (10) with the unsaturated nonreducing end of the product used to establish the location of the scissile bond.
Crystallization, Data Collection, and Processing.
Native and SeMet Pel10Acm crystals were grown as described (9). Crystals belong to space group P21, with unit cell dimensions a = 47.9, b = 106.7, c = 55.6 Å, β = 92.0° and have two molecules in the asymmetric unit. Native crystals grown in the presence of 25 mM CaCl2 and mutant D389A crystals cocrystallized with 20 mM GalA3, belong to closely related space group P21212, with unit cell dimensions a = 106.3, b = 55.2, c = 47.7 Å, and have one molecule in the asymmetric unit. Before data collection a rayon-fiber loop was used to transfer single crystals to a cryoprotectant solution consisting of the growth buffer supplemented with 25% (vol/vol) glycerol.
A three-wavelength MAD experiment, at 100 K, was conducted on beamline BM14 at the European Synchrotron Radiation Facility at Grenoble, France, with a MAR CCD detector. The wavelengths for the MAD experiment were chosen by scanning through the absorption edge of the Se-Pel10Acm crystal. Data sets were collected at the wavelength corresponding to the minimum ƒ′ (0.97930Å), the maximum ƒ′′ (0.97889Å) and a reference wavelength at an energy above the absorption edge (0.88560Å) to maximize dispersive differences. Native data were collected on a single crystal belonging to space group P21, at 100 K, on beamline ID14–4 at the European Synchrotron Radiation Facility, Grenoble; data for the native crystals in space group P21212 on station PX9.6 at the SRS, Daresbury, U.K.; and data for the complex with GalA3 on beamline ID29 of the European Synchrotron Radiation Facility, Grenoble. All data were processed by using the HKL2000 suite of programs (11).
Structure Solution.
Unmerged data for the selenium-MAD experiment were input to SOLVE (12) to locate 12 Se positions, corresponding to two molecules of Pel10Acm in space group P21. The Se positions were refined and phases calculated by using SOLVE. These phases were used as a starting set for phase improvement, incorporating the native data initially at 1.6 Å resolution, with DM (13). ARP (14) and REFMAC (15) were used to generate an electron density map which allowed tracing of a single molecule with the X-AUTOFIT module in QUANTA (Accelrys, San Diego). The second molecule was located by using amore (16). The two molecules were refined with refmac, initially with the phases from DM included as experimental restraints. The final model has a crystallographic R factor of 0.130 (Rfree = 0.162) and has 98% of residues in “allowed” regions of the Ramachandran plot [calculated by using MOLEMAN2 (17)]. One molecule from the native structure in space group P21 was used as a starting model for refinement in space group P21212, because the unit cells are very closely related. After 10 initial cycles of rigid body refinement, individual coordinates and temperature factors were refined as above. The final model has a crystallographic R factor of 0.129 (Rfree = 0.157). The P21212 native model was used as the starting model for the complex with GalA3 which, with the same Rfree reflections, has a crystallographic R factor of 0.17 and Rfree 0.23.
Results
Pel10A from Cellvibrio japonicus comprises an N-terminal carbohydrate-binding module (CBM family 2a), a central X4 module of unknown function, and a C-terminal polygalacturonic acid lyase catalytic module classified into family PL-10 (10). This C-terminal module (residues 327–649) had previously been expressed as a separate entity, termed Pel10Acm, and shown to be an endo-acting polygalacturonic acid lyase with activity solely against the homogalacturonic acid backbone. Catalytic activity is optimal at pH 10.3 and is absolutely dependent on Ca2+ with maximal activity at ≈2 mM [Ca2+] (10), as observed for many other polysaccharide lyases (6, 18–20) and supported by three-dimensional analysis of enzymes from family PL-1 (3, 6, 21–23).
Quantitative analysis of the kinetics of Pel10Acm, after the release of the unsaturated products from polygalacturonate degradation yields kcat and KM for the wild-type enzyme of 408 s−1 and 0.074 mg ml−1, respectively. Pel10Acm had no detectable activity against GalA2. Against GalA3, the enzyme exclusively cleaved the substrate in the −1 to +2 subsite-binding mode (nomenclature according to ref. 24), releasing galacturonic acid and the 4,5-unsaturated GalA2 with a kcat and KM of 1,075 s−1 and 0.6 mM, respectively. GalA4 was cleaved both in the −2 to +2 (64%) or the −1 to +3 (36%) subsite-binding modes but the scarcity of tetrasaccharide substrate prevented determination of accurate kinetic parameters for GalA4.
Three-Dimensional Structure of Pel10Acm.
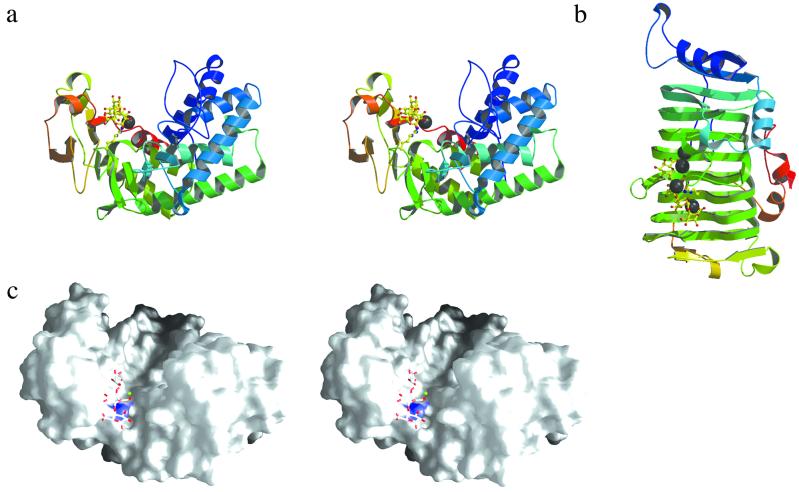
The three-dimensional structure of Pel10Acm was solved at a resolution of 1.65 Å (see Materials and Methods). Native data allow the resolution to be extended to 1.32 Å. The three-dimensional structure (Fig. 1a), extending from residues 339 to 648, does not adopt the parallel β-helix fold displayed both by polygalacturonic acid lyases from families PL-1 (Fig. 1b), -3, and -9 and other enzymes active on polygalacturonates. Instead, it is a predominantly α-helical structure. The topography of the protein reveals two “domains” the interface of which forms a wide central chasm, the location for the substrate-binding and catalytic residues (Fig. 1c). The N-terminal, helical domain is of the α-toroid form, displaying a compact (α/α)3 barrel. The sixth helix (residues 479–487) of the toroid crosses over to the N-terminal domain, which is predominantly formed by irregular coil, a sheet of short β-strands, and a further three helices. The P21 crystal form contains two molecules that are extremely similar, except for a small rigid-body closure of the N-terminal domain of molecule B compared with A. The segment from residues 368–381 shows the greatest movement resulting in 3- to 4-Å shifts in the position of the side chains of Asn370 and Asp372, which are part of a loop that sites above one wall of the substrate-binding cleft, described below, with Asn370 some 5–7 Å distant from the trisaccharide ligand.
Fig 1.
(a) Three-dimensional structure of the catalytic domain of Pel10 in divergent (wall-eyed) stereo; (b) Pel1C ligands and the potential base arginine residues in “ball-and-stick” representation, with the Ca2+ ions as shaded spheres, and the structures color-ramped from N to C terminus [figure prepared by using MOLSCRIPT/BOBSCRIPT (32, 33)]. (c) Divergent (wall-eyed) stereo surface representation of Pel10Acm with the ligand as licorice, the Ca2+ as a sphere, and the guanidinium group of Arg524 shaded blue (prepared by using GRASP 34).
Although the predominantly α-helical structure of Pel10Acm bares no relationship to other polygalacturonic acid lyases, limited topological similarity to other α-toroidal folds exists, including the (α/α)6 and (α/α)7 toroids displayed by other classes of carbohydrate-active enzymes. Glycoside hydrolases from family GH-47, α-1,2-mannosidases (for example, Protein Data Bank code ), possess an (α/α)7 topology six of whose helices correspond well with Pel10Acm with 231 Cα atoms overlapping with rms 3.2 Å. The (α/α)6 barrels of other glycoside hydrolases including family GH-8 cellulases, GH-15 glucoamylases, and family GH-65 maltose phosphorylases are also similar, as are some sugar epimerases, such as GlcNAc 2-epimerases (PDB shares 227 overlapping Cα atoms with rms 3.4 Å). Many polysaccharide lyases acting on other uronic acid polymers, including the family PL-5 alginate and PL-8 chondroitin and hyaluronan lyases, also display variants on the (α/α)6 fold, which suggests that the α-toroid fold, in its many guises, is a powerful and adaptable scaffold for carbohydrate-active enzymes upon which a diverse array both of specificities and catalytic mechanisms may be grafted.
GalA3/Ca2+ Complex of Pel10Acm and Site-Directed Mutagenesis at the Catalytic Center.
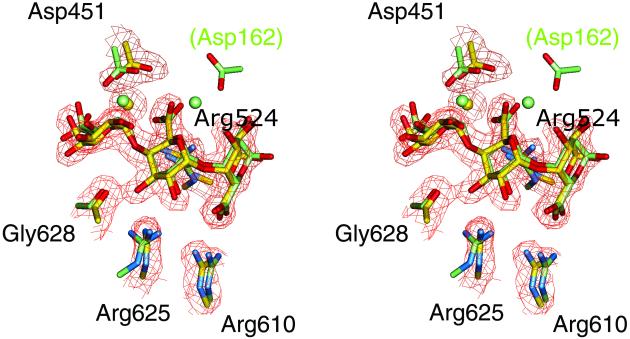
Cocrystallization of native Pel10Acm with substrates revealed neither ligand nor Ca2+ (data not shown). An inactive mutant, D389A, was used to obtain a “Michaelis” complex with Ca2+/GalA3 to a resolution of 2.15 Å. Continuous electron density for the trisaccharide substrate, each sugar unit present in its 4C1 (chair) conformation, and additional density relating to an active-site-liganded Ca2+ were clear (Fig. 2). The trisaccharide occupies subsites −1 to +2, consistent with the unique mode of bond cleavage for this substrate, and allows description of the enzyme–substrate interactions.
Fig 2.
Observed electron density for the GalA3/Ca2+ complex of Pel10Acm. The map shown is a maximum-likelihood/σA weighted 2Fobs − Fcalc syntheses contoured at 0.33 electrons per Å3. The Pel10A structure is in yellow and the convergent evolution of unrelated pectate lyases revealed by the active-center overlap of R218K mutant of Pel1C shown in dark green. (Arg218 has been reintroduced, in its native location, for reference.) The relative locations of the two putative catalytic base arginines within the protein framework are shown in Fig. 1 a and b for Pel10 and Pel1, respectively.
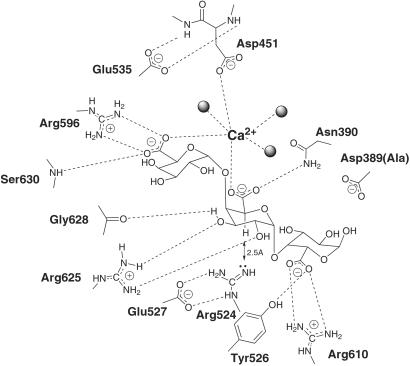
At the −1 (leaving-group) subsite, Arg596 forms a salt bridge with the substrate carboxylate group, which in turn also forms a coordinate bond with the Ca2+ ion (Fig. 3). The R596A derivative displayed very little detectable activity against GalA3 although it maintained ≈0.5% activity against polymeric substrates (Table 1). The deleterious effects of some mutations being mitigated by the additional binding energy derived from long polymeric substrates are a common feature in polysaccharide degradation (see, for example, refs. 25 and 26). The Ca2+ ion is also coordinated by an oxygen atom from the +1 subsite sugar carboxylate and one carboxylate oxygen atom from Asp451 with three water molecules completing hexacoordination. Substitution of Asp451, the only close protein ligand of the Ca2+ ion, led to a total loss of activity on both GalA3 and polygalacturonic acid (Table 1). The side chain of Asp451 lies at the end of a helical segment that is held in appropriate main-chain conformation through direct hydrogen bonds from both its flanking main-chain amide groups through to the side-chain carboxylate of Glu535. Mutation of Glu535 indeed reduces activity approximately 200-fold even on GalA3 from which it lies some 7–10 Å distant, suggesting that it does indeed play a role in maintaining the structural integrity of the catalytic center groups.
Fig 3.
Schematic diagram of the interactions of the mutant D389A Pel10Acm with trigalacturonate. The approximate location of Asp389 from the native structure is indicated for reference.
Table 1.
Relative activities for native and mutant forms of Pel10Acm
| Polygalacturonic acid | GalA3 | |
|---|---|---|
| Native | 100% | 100% |
| D389A | ND | ND |
| N390A | 5.7 | 1.1 |
| D451A | ND | ND |
| R524A | ND | ND |
| R524K | ND | ND |
| Y526F | 48.9 | 30.5 |
| E527A | ND | ND |
| E527Q | ND | ND |
| E535A | 0.38 | 0.47 |
| R596A | 0.44 | 0.04 |
| R610A | 21.8 | 3.7 |
| R625A | 0.41 | 0.02 |
| R625K | 3.32 | 0.14 |
, ND, not detectable, activity <0.05 s−1.
In the +1 subsite, where the enzyme chemistry occurs, Arg625 forms a hydrogen-bonding interaction with both the C2 and C3 hydroxyl groups of the +1 galacturonic acid moiety, reminiscent of a similar interaction observed in family 11 xylanases (27). O3 makes a further interaction with the main-chain carbonyl of Gly628 (discussed below). The side chain of Arg524 is positioned 2.5 Å from the hydrogen atom on C5 of the galacturonic residue at subsite +1, stabilized through two hydrogen bonds with Glu527. Arg524 is thus the only potential catalytic base in close proximity to H5 in the +1 subsite. The side-chain hydroxyl group of Tyr526 is a hydrogen bond donor to the +2 sugar carboxylate, whereas Arg610 forms a salt bridge with this group. The involvement of the −1 and +2 subsite carboxylate groups in salt-bridge formation, and the coordination of −1 and +1 subsite carboxylate oxygen atoms with Ca2+, may contribute to the specificity of Pel10A for homopolygalacturonic acid. At the +1 subsite, the majority of mutations result in complete loss of catalytic activity. D389A, R524A, R524K, E527A, and E527Q derivatives were all inactive on both GalA3 and polygalacturonic acid. Mutation of Asn390 to Ala resulted in 20- to 100-fold reductions in activity on polygalacturonate and GalA3 respectively. R625A and R625K mutations resulted in almost total loss of activity against small soluble substrates, with substantially reduced catalytic efficiency against polygalacturonate. In the +2 subsite, the Y526F mutation led to 2- to 3-fold reductions in specific activity on both polygalacturonic acid and GalA3 whereas the R610A mutant is five times less active against polymeric substrates but 25 times less active against the trisaccharide. The catalytic inactivity of the D389A mutant, located between the +1 and +2 subsites, is superficially surprising, given that the mutant is isomorphous with the native structure and that this residue lies some 5–7 Å from the nearest atom of the substrate. This inactivity is discussed further below in light of the similarity with other pectate-active enzymes.
Discussion
Evidence for Convergent Evolution of Catalytic Mechanism.
Pel10Acm reveals a stunning example of the convergent evolution of catalytic mechanism. Comparison of the Pel10Acm GalA3/Ca2+ complex with the inactive mutant R218K GalA4/Ca2 complex of Pel1C (kindly provided by F. Jurnak) (6) (Fig. 2), reveals an essentially identical disposition of six active-center groups despite no topological similarity between these enzymes (Fig. 1 a and b). In both cases substrate carboxylates at the putative −1 and +1 subsites are coordinated with a Ca2+ ion that is in turn liganded to a strictly conserved acidic amino acid; Asp451 in Pel10 and Glu166 in Pel1C. The interactions of the +1 subsite sugar are identical including a main-chain carbonyl interaction with O3 and an arginine interaction with both O3 and O2. An identical location also exists for the putative catalytic base Arg524 of Pel10A with the well characterized base of Pel1C, Arg218. Asp162 of Pel1C, which coordinates a second calcium ion to the +1 sugar carboxylate, finds no direct equivalent in the D389A mutant of Pel10A. This position, however, is isostructural with the position of the Asp389 side chain of native Pel10A and a similar role for this residue may be envisaged. The trisaccharide itself is also found in an essentially identical conformation to the −1 to +2 subsites of the GalA4 complex of Pel1C, and the structural similarity even extends to the +2 subsite where the only close interaction of Pel10A, that from the carboxylate of the substrate to Arg610, is also invariant in Pel1C.
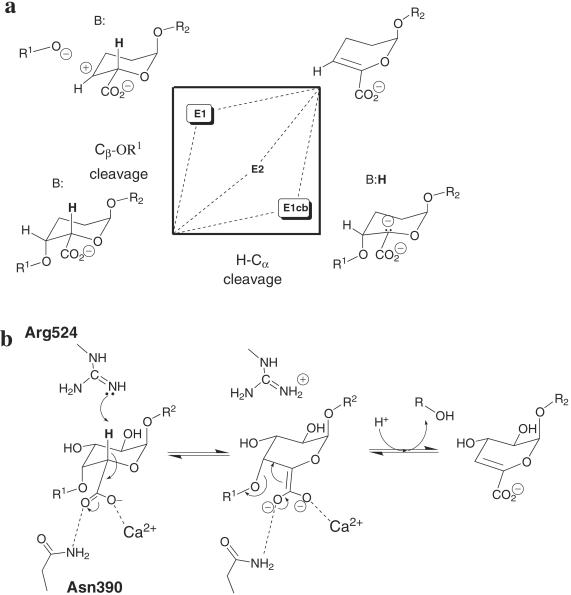
Both Pel10Acm and Pel1 perform anti β-elimination characterized by Brønsted base-catalyzed abstraction of the H5 proton (acidified by the electronegative carboxylate substituent at C5) and elimination of the substituent at O4 generating a 4,5-unsaturated product (Fig. 4). The mechanistic landscape for this reaction is best considered in light of the More O'Ferrall diagram (Fig. 4a), in which the courses of the three pathways, referred to as E1, E2, and E1cb, are determined by the order of bond cleavage (1). In the E1-type reaction, the Cβ—X bond is cleaved before Cα—H, a stabilized carbocation intermediate formed followed by subsequent elimination of the proton from the α-carbon; such reactions in aqueous solution or enzyme active sites are widely considered unlikely (1). Alternatively, the E1cb reaction involves a carbanion intermediate preceded by α-carbon proton abstraction with leaving-group elimination occurring subsequently. The E2 pathway reflects concerted proton abstraction and leaving-group elimination, although mechanistic boundaries may be rather blurred (28). The catalytic mechanism is further classified to reflect the location of the two leaving groups either on the same face of the incipient double bond (syn elimination) or on opposite sides (anti elimination). Crystallography alone may not discriminate between these mechanistic pathways but the active-center similarity of this enzyme, with structurally unrelated anti-eliminases from family PL-1 and the apparent absence of any Brønsted acid in the vicinity of O4, allows some description of the likely reaction trajectory.
Fig 4.
(a) More O'Ferral diagram for β-elimination of galacto-configured uronic acids. (b) Putative E1cb/asynchronous E2 reaction mechanism for Pel10 and related enzymes in which proton abstraction by arginine is followed by leaving-group elimination. The essential role of Asp389 may involve a role in binding a second Ca2+ ion as observed in Pel1C.
The only potential catalytic base in close proximity to a substrate C5 hydrogen atom is Arg524, located at the putative +1 subsite (Figs. 2–4), which is in a location identical to the putative arginine base of PL-1 enzymes (6). We therefore conclude that a deprotonated arginine functions as the catalytic base in both systems which, given the pH optima of about 10.5 this function represents only a 2-unit pKa perturbation for this arginine, similar to the well characterized pKa shifts of carboxylate groups in glycoside hydrolases. Calculations on the Pel1C system indeed revealed appropriate perturbation of the arginine pKa by virtue of its interactions with adjacent carboxylates (3). In Pel10A Arg524 interacts with both substrate and side-chain carboxylates. Arginine is a rare base in enzymatic reactions, the reverse reaction of quinol:fumarate reductase providing another example (29), but it is perhaps not surprising given the high pH optima of pectate lyases. The enolase superfamily operates at a much lower pH and instead utilizes lysine for proton abstraction (30, 31).
The notable absence of a classical Brønsted acid (either enzyme or substrate-derived) in proximity to the oxygen of the scissile glycosidic bond in both complexes suggests that little or no buildup of negative charge may occur at this center at the rate-limiting transition state, which implies either an E1cb reaction by a resonance-stabilized putative aci-acid carbanion intermediate (Fig. 4b) or an E2 reaction with concerted but asynchronous bond cleavage. In either case, elimination of the leaving group would not be wholly rate-limiting, and proton donation to the leaving group may subsequently be achieved through solvent water. The nearest group to either of the lone pairs of the glycosidic oxygen is in fact the O3 hydroxyl of the +1 subsite sugar at 2.9 Å (but with C3—O3—O4 angle 55°). Given the total structural invariance of the coordination of O3 in Pel1 and Pel10, with arginine and main-chain carbonyl ligands, it may be that leaving-group protonation is achieved from Arg625 in a shuttle involving the O3 hydroxyl and main-chain carbonyl moieties. It is also formally possible that Lewis acid assistance to leaving-group departure, facilitated by Ca2+, plays a role, but no metal ion seems appropriately positioned in either the PL-1 or PL-10 enzyme families.
The critical role played by Asp389 in catalysis is intriguing given the 5- to 6-Å distance between this residue and the nearest substrate atoms. The overlap with the inactive R218K GalA4/Ca2+ complex of Pel1C (6) reveals that each oxygen of the α-carbon carboxylate in subsite +1 of Pel1C is liganded to a separate Ca2+ ion, suggestive of a role played by electrophilic catalysis and somewhat reminiscent of the divalent metal μ-bridges observed for other eliminases such as the enolase superfamily. Twin metal ions function both through the acidification of the adjacent α-proton and stabilization of the subsequent aci-acid carbanion intermediate (30, 31). Given the mechanistic similarities observed between PL-1 and PL-10 enzymes, and the similar location of the carboxylate of Asp389 with Asp162 of Pel1C, it is possible that the D389A mutant acts by way of disruption of an analogous second Ca2+-coordinating system leading to complete loss of enzyme activity as is witnessed when the first Ca2+ coordination sphere is mutated in the D451A mutant.
Despite the availability of both native and complexed three-dimensional structures of PL-8 chondroitin and hyaluronate lyases and corresponding mutagenesis data, no consistent catalytic mechanism exists. Two of the hypothesized mechanisms feature a histidine as the catalytic base, whereas others implicate tyrosine. The 12% residual activity for the H225A mutant of the Streptococcus pneumoniae hyaluronate lyase seems to rule out its role as a base, whereas mutation of the tyrosine residue results in a complete loss of activity (4). Complexes of family PL-8 enzymes do, however, identify a conserved arginine residue analogous in position to that of Pel10Acm, which lies in close proximity to the α-carbon of bound substrate (20). Cleavage of the β-glycosidic bonds of hyaluronan and chondroitin substrates with equatorial C4 substituents involves syn elimination, which is chemically more challenging. PL-5 and 8 enzymes need not necessarily share a similar catalytic mechanism to those from PL-1 and 10 and may indeed feature a less concerted reaction pathway (Fig. 4a). Many polysaccharide lyases play a role in the virulence of pathogens of eukaryotes. Hyaluronate lyases spreading and infection factors for highly pathogenic bacteria such as S. pneumoniae, for example. Polygalacturonic acid lyases themselves are involved in pathogenic infection of plants and foodstuff degradation. The design of inhibitors on the basis of the likely transition state(s) for the E2/E1cb reaction mechanisms is, therefore, of great importance. The Pel10Acm structure and its revealing active-center similarities with Pel1C begin to shed light on the mechanistic conundrums and should, in harness with appropriate physical-chemical studies, lead to the design of anti-infective agents against both human and plant pathogens.
Note Added in Proof.
Recent work has shown Pseudomonas fluorescens subsp. cellulosa, sometimes also described as Pseudomonas cellulosa, to be a Cellvibrio species (35, 36). It has been proposed that the organism be renamed Cellvibrio japonicus to reflect this fact.
Acknowledgments
This work was supported by the Higher Education Funding Council for England, the Biotechnology and Biological Sciences Research Council, the Yorkshire Agricultural Society, and the Wellcome Trust. G.J.D. is a Royal Society University Research Fellow.
Abbreviations
GalA, galacturonic acid
Pel, pectate lyase
PL, polysaccharide lyase
Data deposition: The coordinates and observed structure factor amplitudes for the structures described in this paper have been deposited in Protein Data Bank, www.rcsb.org [PDB ID codes: (P21 native), (P21212 native), and (P21212 GalA3 complex)].
References
- 1.Anderson V. E. (1998) in Comprehensive Biological Catalysis: A Mechanistic Reference, ed. Sinnott, M. (Academic, London), Vol. 2, pp. 115–133. [Google Scholar]
- 2.Tamaru Y. & Doi, P. H. (2001) Proc. Natl. Acad. Sci. USA 98, 4125-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scavetta R. D., Herron, S. R., Hotchkiss, A. T., Kita, N., Keen, N. T., Benen, J. A. E., Kester, H. C. M., Visser, J. & Jurnak, F. (1999) Plant Cell 11, 1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S. L., Kelly, S. J., Lamani, E., Ferraroni, M. & Jedrzejas, M. J. (2000) EMBO J. 19, 1228-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho P. M. & Henrissat, B. (1999) in Recent Advances in Carbohydrate Engineering, eds. Gilbert, H. J., Davies, G. J., Svensson, B. & Henrissat, B. (R. Soc. Chem., Cambridge, U.K.), pp. 3–12.
- 6.Herron S. R., Benen, J. A. E., Scavetta, R. D., Visser, J. & Jurnak, F. (2000) Proc. Natl. Acad. Sci. USA 97, 8762-8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao C. H. (1989) Appl. Environ. Microbiol. 55, 1677-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrissat B., Coutinho, P. M. & Davies, G. J. (2001) Plant Mol. Biol. 47, 55-72. [PubMed] [Google Scholar]
- 9.Charnock S. J., Brown, I. E., Turkenburg, J. P., Black, G. W. & Davies, G. J. (2001) Acta Crystallogr. D 57, 1141-1143. [DOI] [PubMed] [Google Scholar]
- 10.Brown I. E., Mallen, M. H., Charnock, S. J., Davies, G. J. & Black, G. W. (2001) Biochem. J. 355, 155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otwinowski Z. & Minor, W. (1997) Methods Enzymol. 276, 307-326. [DOI] [PubMed] [Google Scholar]
- 12.Terwilliger T. C. & Berendzen, J. (1999) Acta Crystallogr. D 55, 849-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowtan K. D. & Main, P. (1996) Acta Crystallogr. D 49, 148-157. [DOI] [PubMed] [Google Scholar]
- 14.Lamzin V. S. & Wilson, K. S. (1993) Acta Crystallogr. D 49, 129-147. [DOI] [PubMed] [Google Scholar]
- 15.Murshudov G. N., Vagin, A. A. & Dodson, E. J. (1997) Acta Crystallogr. D 53, 240-255. [DOI] [PubMed] [Google Scholar]
- 16.Navaza J. & Saludijan, P. (1997) Methods Enzymol. 276, 581-594. [DOI] [PubMed] [Google Scholar]
- 17.Kleywegt G. J. & Jones, T. A. (1996) Structure (London) 4, 1395-1400. [DOI] [PubMed] [Google Scholar]
- 18.Bekri M. A., Desair, J., Keijers, V., Proost, P., Searle-van Leeuwen, M., Vanderleyden, J. & Vande Broek, A. (1999) J. Bacteriol. 181, 2440-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benen J. A., Kester, H. C., Parenicova, L. & Visser, J. (2000) Biochemistry 39, 15563-15569. [DOI] [PubMed] [Google Scholar]
- 20.Huang W., Boju, L., Tkalec, L., Su, H., Yang, H. O., Gunay, N. S., Linhardt, R. J., Kim, Y. S., Matte, A. & Cygler, M. (2001) Biochemistry 40, 2359-2372. [DOI] [PubMed] [Google Scholar]
- 21.Pickersgill R., Jenkins, J., Harris, G., Nasser, W. & Robert-Baudouy, J. (1994) Nat. Struct. Biol. 1, 717-723. [DOI] [PubMed] [Google Scholar]
- 22.Lietzke S. E., Scavetta, R. D., Yoder, M. D. & Jurnak, F. (1996) Plant Physiol. 111, 73-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lietzke S. E., Yoder, M. D., Keen, N. T. & Jurnak, F. (1994) Plant Physiol. 106, 849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies G. J., Wilson, K. S. & Henrissat, B. (1997) Biochem. J. 321, 557-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charnock S. J., Lakey, J. H., Virden, R., Hughes, N., Sinnott, M. L., Hazlewood, G. P., Pickersgill, R. & Gilbert, H. J. (1997) J. Biol. Chem. 272, 2942-2951. [DOI] [PubMed] [Google Scholar]
- 26.Hogg D., Woo, E.-J., Bolam, D. N., McKie, V. A., Gilbert, H. J. & Pickersgill, R. W. (2001) J. Biol. Chem. 276, 31186-31192. [DOI] [PubMed] [Google Scholar]
- 27.Sabini E., Sulzenbacher, G., Dauter, M., Dauter, Z., Jørgensen, P. L., Schülein, M., Dupont, C., Davies, G. J. & Wilson, K. S. (1999) Chem. Biol. 6, 483-492. [DOI] [PubMed] [Google Scholar]
- 28.Jencks W. P. (1980) Acc. Chem. Res. 13, 161-169. [Google Scholar]
- 29.Lancaster C. R. D., Gross, R. & Simon, J. (2001) Eur. J. Biochem. 268, 1820-1827. [PubMed] [Google Scholar]
- 30.Babbitt P. C., Mrachko, G. T., Hasson, M. S., Huisman, G. W., Kolter, R., Ringe, D., Petsko, G. A., Kenyon, G. L. & Gerlt, J. A. (1995) Science 267, 1159-1161. [DOI] [PubMed] [Google Scholar]
- 31.Babbitt P. C., Hasson, M. S., Wedekind, J. E., Palmer, D. R. J., Barrett, W. C., Reed, G. H., Rayment, I., Ringe, D., Kenyon, G. L. & Gerlt, J. A. (1996) Biochemistry 35, 16489-16501. [DOI] [PubMed] [Google Scholar]
- 32.Kraulis P. J. (1991) J. Appl. Crystallogr. 24, 946-950. [Google Scholar]
- 33.Esnouf R. M. (1997) J. Mol. Graphics 15, 133-138. [DOI] [PubMed] [Google Scholar]
- 34.Sharp K., Fine, R. & Honig, B. (1987) Science 236, 1460-1463. [DOI] [PubMed] [Google Scholar]
- 35.Humphry, D. R., Black, G. W. & Cummings, S. P. (2002) Int. J. Syst. Evol. Biol., in press.
- 36.Nagy T., Emami, K., Fontes, C. M. G. A., Ferreira, L. M. A. & Gilbert, H. J. (2002) J. Bacteriol. 184, 4925-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]