Abstract
FMRFamide and FMRFamide-related neuropeptides are extremely widespread and abundant in invertebrates and have numerous important functions. Here, we have cloned a Drosophila orphan receptor, and stably expressed it in Chinese hamster ovary cells. Screening of a peptide library revealed that the receptor reacted with high affinity to FMRFamide (EC50, 6 × 10−9 M). The intrinsic Drosophila FMRFamide peptides are known to be synthesized as a large preprohormone, containing at least 13 related FMRFamide peptides (8 distinct FMRFamides). Screening of these intrinsic Drosophila FMRFamides showed that the receptor had highest affinity to Drosophila FMRFamide-6 (PDNFMRFamide) (EC50, 9 × 10−10 M), whereas it had a somewhat lower affinity to Drosophila FMRFamide-2 (DPKQDFMRFamide) (EC50, 3 × 10−9 M) and considerably less affinity to the other Drosophila FMRFamide-related peptides. To our knowledge, this article is the first report on the molecular identification of an invertebrate FMRFamide receptor.
The invertebrate neuropeptide FMRFamide was originally isolated from clam ganglia, because of its prominent cardioexcitatory actions (1). Since then, FMRFamide and FMRFamide-related peptides have been isolated from a wide variety of invertebrate species, ranging from primitive metazoans, such as cnidarians, to higher invertebrates, such as insects (2–5). FMRFamide-related peptides occur in all classes of cnidarians, suggesting that these peptides were among the first transmitters used in evolution (6). After their discovery in invertebrates, FMRFamide-related substances have also been purified from mammals and other vertebrates (7–9), suggesting that these peptides occur in all animals having a nervous system.
The preprohormones of the FMRFamide-related peptides in invertebrates are characterized by a large number of FMRFamide peptide copies, ranging from 38 copies (36 identical peptides) in cnidarians (6, 10), to 29 copies (28 FMRFamides and one FLRFamide copy) in molluscs (11) and 13 (eight different peptides) in insects (12, 13). FMRFamide-related peptides may act excitatory or inhibitory on their target cells (depending on the target cell type) and they are involved in reproduction, feeding, and many other behaviors (3, 5, 14–18). The abundance, complexity, and importance of FMRFamide peptides within an invertebrate species, is illustrated by the fact that the nematode worm Caenorhabditis elegans contains at least 20 FMRFamide preprohormone genes, and that the deletion of only one of these genes causes severe behavioral defects (5, 17, 19).
It might be that not all genes coding for FMRFamide-related peptides are evolutionarily related—i.e., that some genes evolved independently of others by coevolution (20). For example, some FMRFamide-related peptides have only the C-terminal sequence RFamide in common with the FMRFamides, and these peptides might represent a separate, evolutionarily unrelated group.
The actions of the FMRFamide-related peptides are mediated by G protein-coupled receptors (21–23), although in snails (24) and mammals (25) FMRFamide peptides also can directly activate ligand-gated ion channels and, thus, act rapidly. A G protein-coupled receptor from the pond snail Lymnaea stagnalis was recently described to be activated by the Lymnaea cardioexcitatory peptide (LyCEP), a neuropeptide that has the C-terminal RFamide sequence in common with the FMRFamides (26). This receptor, however, was not activated by “genuine” FMRFamides—i.e., by peptides having the C-terminal sequence FMRFamide—or by FMRFamide itself (26). The invertebrate G protein-coupled FMRFamide receptor, therefore, has remained elusive, so far. Here, we report on the cloning of the FMRFamide receptor from the fruitfly Drosophila melanogaster. This G protein-coupled receptor reacts with high affinity to FMRFamide and the intrinsic Drosophila FMRFamide-related peptides. This paper, therefore, is the first report on the cloning and characterization of a “genuine” invertebrate FMRFamide receptor.
Materials and Methods
Cloning of the Receptor and Northern Blot Analyses.
Primers were constructed based on the proposed exons of the annotated gene, CG2114 (www.flybase.org), and used in PCR with cDNA from D. melanogaster third instar larvae (Canton S) as a template. The sense primer was 5′-AGGGCCCAACGGTACGCTACGA-3′ (corresponding to nucleotide positions 140–161 of Fig. 1) and the antisense primer was 5′-AGTAAACCACTGGACCAGGCGAGGGA-3′ (corresponding to nucleotide positions 1497–1522 of Fig. 1). The PCR parameters were one cycle of the following step program: 95°C for 3 min, 56°C for 10 s, 72°C for 2 min, then 35 cycles of the following program step: 95°C for 30 s, 56°C for 10 s, 72°C for 2 min, and a final extension step of 72°C for 10 min. pCR4-TOPO (Invitrogen) was used for the cloning and the SMART RACE cDNA kit (CLONTECH) was used for the rapid amplification of cDNA ends (RACE) reactions. The 3′-RACE reactions were made with the sense primer, 5′-GCTCCATATCGAACAACGGCGATGGAACTCTGAACCA-3′ (corresponding to nucleotide positions 1274–1310 of Fig. 1), followed by the nested sense primer, 5′-ACTGACCCAGGTCTCGGGATCACCCGGTCTGGTCA-3′ (corresponding to nucleotide positions 1443–1477 of Fig. 1). All PCR products were cloned into pCR4-TOPO (Invitrogen), using the TOPO TA cloning method (Invitrogen). Northern blots were prepared, using the NorthernMax-Formaldehyde kit (Ambion, Austin, TX) and BrightStar-Plus membranes (Ambion). A cDNA probe (nucleotide positions 1274–2713 of Fig. 1) was labeled using the Strip-EZ DNA kit (Ambion). The Drosophila ribosomal protein 49 (RP-49) probe was generated as described in ref. 27.
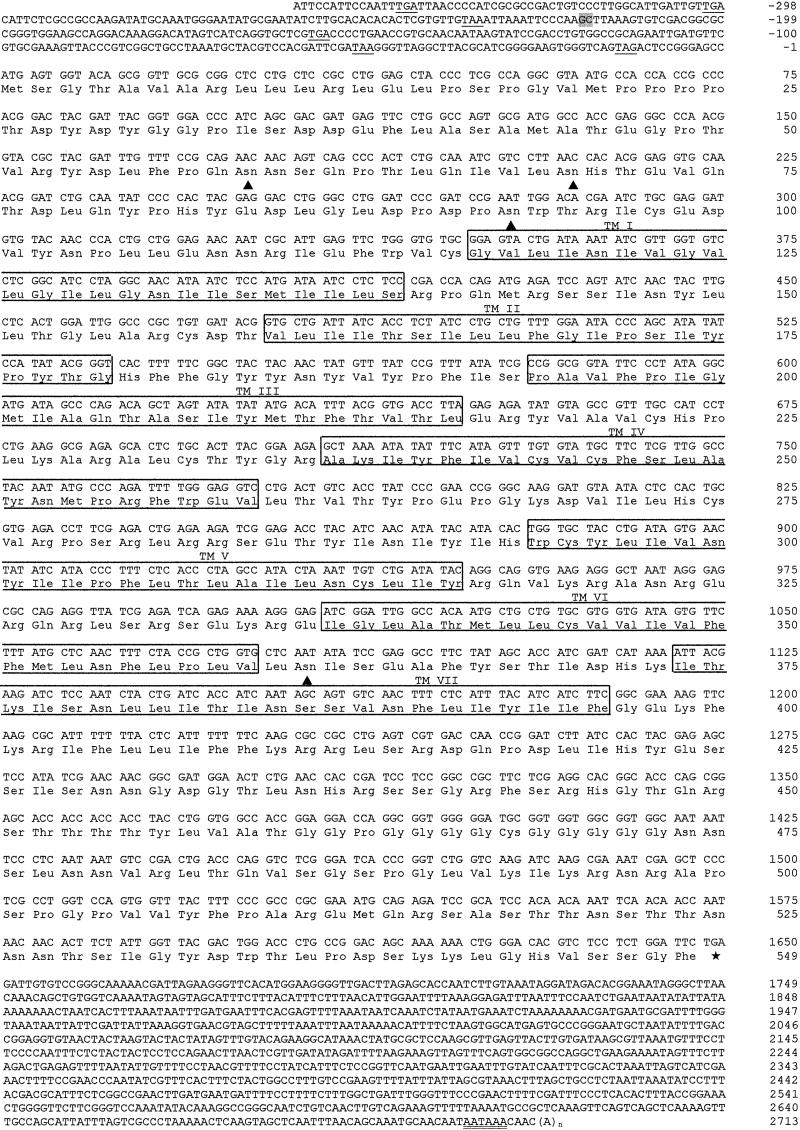
Fig 1.
cDNA and deduced amino acid sequence of the Drosophila receptor CG2114. Nucleotides are numbered from 5′ to 3′ end and the amino acid residues are numbered starting with the first ATG codon in the ORF. The two nucleotides bordering the single intron found to be present in the gene are highlighted in gray. The seven membrane spanning domains are boxed and labeled TM I–VII. The translation termination codon is indicated by an asterisk. In-frame stop codons in the 5′-noncoding region are underlined. The putative polyadenylylation signal in the 3′-noncoding region is underlined twice. Putative glycosylation sites in the extracellular N terminus and in the third extracellular loop, following the N-X-S/N-X-T consensus sequence, are indicated by a triangle.
Cell Culture, Transfection, and Bioluminescence Assay.
Chinese hamster ovary (CHO) cells were grown as described (28). To amplify a full-length cDNA coding for the receptor, the following primers were applied: sense primer 5′-GGTACCAAGATGAGTGGTACAGCGGTTGCGCGG-3′ (corresponding to nucleotide positions 1–24 of Fig. 1) and antisense primer 5′-GATATCTCAGAATCCAGAGGAGACGTGTCCCAG-3′ (corresponding to nucleotide positions 1624–1650 of Fig. 1). The product was cloned into pCR4-TOPO vector (Invitrogen). Several independent clones were sequenced to verify the correct amplification of the cDNA. The KpnI and EcoRV restriction sites that had been incorporated into the above primers facilitated the subcloning into pcDNA3.1 (Invitrogen). cDNA from third instar D. melanogaster larvae was used as a template. The same transfection method was used as described earlier (28). The bioluminescence assay is described in refs. 28 and 29.
Sequence Analysis.
DNA sequence compilation, and nucleotide and amino acid sequence comparisons were performed using the lasergene dna software package (DNAstar, Madison, WI). The secondary structure of the receptor protein was analyzed using the TMHMM V.2.0 prediction server from the Center for Biological Sequence Analysis, Danish Technical University (www.cbs.dtu.dk).
Peptides.
The peptides used in this paper were either custom synthesized by Genemed Synthesis, San Francisco (D. melanogaster FMRFamides 1–8; drostatins-A4, -B2, -C; D. melanogaster myosuppressin; D. melanogaster short neuropeptide F1; D. melanogaster tachykinin-3; and D. melanogaster adipokinetic hormone), or purchased from Bachem, Bubendorf, Switzerland (FMRFamide, D. melanogaster crustacean cardioactive peptide, cockroach perisulfakinin, cockroach leucokinin III, and cockroach leucopyrokinin).
Results
We and others have previously cloned and functionally characterized two Drosophila allatostatin receptors (30–34). To find additional Drosophila allatostatin receptors, we used the blast algorithm to screen the Drosophila Genome Project database (www.flybase.org) and found among the highest scores the sequence of gene CG2114, which was annotated to be a G protein-coupled receptor. We subsequently designed primers against the proposed exons of this receptor gene and performed PCR, using cDNA of larval D. melanogaster as a template. This PCR yielded a band of the expected size and sequence, and after 3′- and 5′-RACE, we obtained the full-length sequence of the receptor cDNA (Fig. 1).
The cDNA of Fig. 1 is 3,061 nucleotides long. It has a 5′-untranslated region of 356 nucleotides, which contains various stop codons, and a long 3′-untranslated region of 1,063 nucleotides, containing a polyadenylylation signal. The cDNA sequence codes for a protein of 549 amino acid residues, which contains seven transmembrane domains. The extracellular N terminus has three potential N-glycosylation sites, whereas the third extracellular loop contains one (Fig. 1).
Comparison of the cDNA with the genomic sequence of the annotated gene CG2114 (www.flybase.org) revealed the presence of one intron located within the 5′-untranslated region (Fig. 1, Table 1). This alignment also showed that the genomic organization of the gene had been correctly predicted. Furthermore, no nucleotide differences existed between the annotated exons and our cloned cDNA (Fig. 1). The gene CG2114 maps in chromosome 3L, position 63 B2. There are no existing mutations available in the gene (www.flybase.org).
Table 1.
Intron/exon boundaries of D. melanogastergene CG2114
| Intron | 5′ donor | Intron size, bp | 3′ acceptor |
|---|---|---|---|
| 1 | AAG gtaagca. . . | 6246 | . . .tttccag CTT |
Comparison of the amino acid sequence of the receptor with that of other proteins from the GenBank database showed that the receptor has a remarkably high sequence identity with a recently released Anopheles gambiae gene product, agCP12601 (53% amino acid residue identity, 68% conserved residues; Fig. 2). Only a much lower amount of sequence identity exists with the Drosophila allatostatin receptors DAR-1 and -2 (23%), the Drosophila neuropeptide Y receptor (24%), the Drosophila tachykinin receptors (23%), the mouse TSH-releasing-hormone receptor (24%), and the rat kappa opioid receptor-1 (24%). All other proteins from the database showed less structural resemblance with the receptor.
Fig 2.
Amino acid sequence comparison between the Drosophila FMRFamide receptor (DFR) from Fig. 1 and the annotated Anopheles protein, agCP12601 (the putative protein encoded by the annotated gene agCG53608), which shows the highest score during a blast search of the GenBank database. Amino acid residues that are identical between DFR and agCP12601 are highlighted in gray. The seven membrane spanning domains are indicated by TM I–VII.
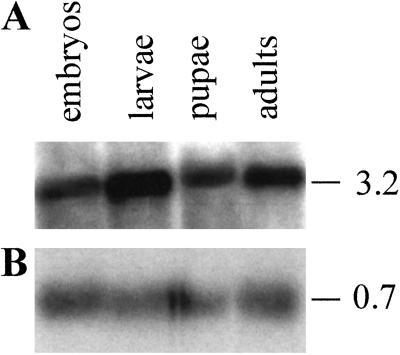
A Northern blot of the various developmental stages of Drosophila showed that the Drosophila receptor was expressed in all stages, but mainly in larvae and adult flies (Fig. 3). The size of the transcript (3.2 kb) corresponded well with that of the cloned cDNA (Fig. 1).
Fig 3.
Northern blots of mRNA isolated from the various developmental stages of Drosophila. The sizes of the transcripts are given at the right (in kb). (A) Each lane contained 5 μg of mRNA from embryos (0–24 h), larvae (1st, 2nd, and 3rd instar), pupae, and adult flies (mixed male and female), which was hybridized with a cDNA probe, coding for a part of the Drosophila receptor (corresponding to nucleotide positions 1274–2713 of Fig. 1). (B) The Northern blot from A was stripped and subsequently incubated with a cDNA probe, coding for RP-49. This blot gives the loading efficiency in each lane.
We stably expressed the receptor in CHO cells that also stably expressed the promiscuous G protein, G16 (29). Two days before the assay (see below), we transiently transfected these cells with DNA, coding for apoaequorin, and 3 h before the assay we added coelenterazine to the cell medium. Activation of the receptor in these pretreated cells would result in a Ca2+-induced bioluminescence response that could easily be measured and quantified (28, 29, 33, 35).
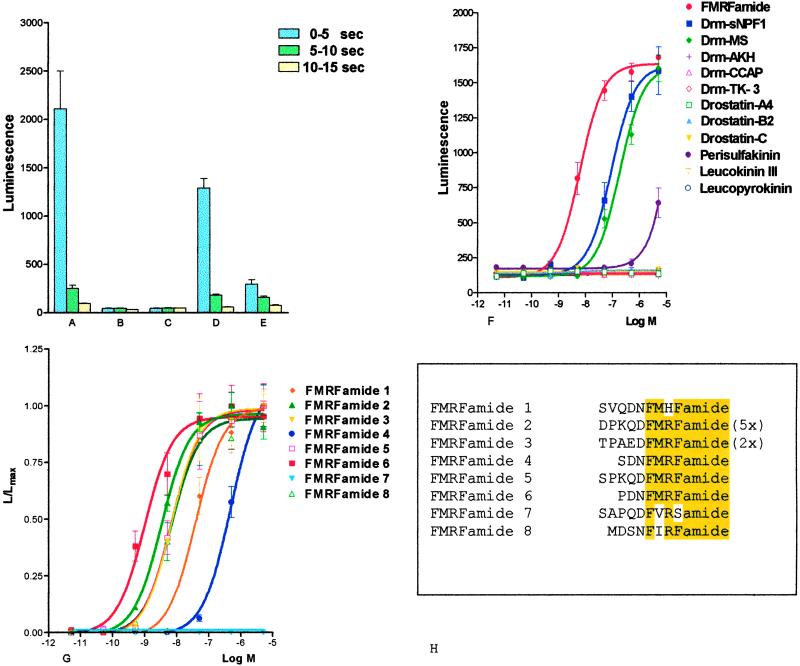
We applied the “reverse pharmacology” strategy (36) during our attempts to find the endogenous ligand for the receptor—i.e., we tested a peptide library of Drosophila and other insect or invertebrate peptide hormones. Addition of 10−5 or 10−6 M of these peptides to the pretreated CHO cells gave negative results for many of these peptides, but peptides resembling FMRFamide at their C termini, and FMRFamide itself, gave clear bioluminescence responses (Fig. 4 A–F). Because FMRFamide was the most potent peptide in inducing the bioluminescence response (Fig. 4F), we synthesized all eight Drosophila FMRFamide-related peptides that are known to be contained in the Drosophila FMRFamide preprohormone (12, 13). After testing these peptides in our bioluminescence assay, we found that Drosophila FMRFamide-6 (PDNFMRFamide) was the most potent intrinsic Drosophila peptide (EC50, 9 × 10−10 M), whereas the other peptides were less effective (Fig. 4G). Drosophila FMRFamide-4 (SDNFMRFamide), for example, showed only a very low efficacy (EC50, > 5 × 10−7 M), whereas Drosophila FMRFamide-7 (SAPQDFVRSamide) showed no activity at all.
Fig 4.
Bioluminescence response of a CHO/G16 cell line transfected with cDNA coding for the receptor shown in Fig. 1 (CHO/G16/DFR); and of a nontransfected cell line (CHO/G16). The vertical bars represent SEM, which are sometimes lower than the symbols (circles, squares, triangles, etc.) used. In these cases, only the symbols are given. (A) Bioluminescence response of CHO/G16/DFR after addition of 5 × 10−8 M of Drm-FMRFamide-6. (B) Response of CHO/G16 cells after addition of 5 × 10−8 M Drm-FMRFamide-6. (C) Response of CHO/G16/DFR cells after addition of PBS alone. (D) Response of CHO/G16/DFR cells after addition of 5 × 10−8 M FMRFamide. (E) Response of CHO/G16/DFR cells after addition of 5 × 10−8 M Drosophila myosuppressin (Drm-MS). (F) Dose–response curves of the bioluminescence responses of CHO/G16/DFR cells induced by various Drosophila or other invertebrate peptides: FMRFamide (1), Drosophila short neuropeptide F1 (Drm-sNPF1; ref. 39), Drm-MS (39), Drosophila adipokinetic hormone (Drm-AKH; refs. 28 and 43), Drosophila crustacean cardioactive peptide (Drm-CCAP; ref. 39), Drosophila tachykinin-3 (Drm-TK-3; ref. 44), drostatin-A4 (45), drostatin-B2 (46), drostatin-C (47), cockroach perisulfakinin (48), cockroach leucokinin III (49), and cockroach leucopyrokinin (49). Of all peptides tested, only FMRFamide, Drm-sNPF1, Drm-MS, and (at only very high concentrations) perisulfakinin induce bioluminescence. (G) Dose–response curves of the bioluminescence responses of CHO/G16/DRF cells induced by the peptides that are contained in the Drosophila FMRFamide preprohormone (12, 13). Drosophila FMRFamide-6 is the most potent peptide (EC50, 9 × 10−10 M). (H) Amino acid sequences of the FMRFamide-related peptides contained in the Drosophila FMRFamide preprohormone. FMRFamide-2 is present with five copies, FMRFamide-3 is present with two copies in the preprohormone. The common C-terminal FMRFamide moieties are highlighted. DFR, Drosophila FMRFamide receptor; Drm-AKH, Drosophila adipokinetic hormone; Drm-CCAP, Drosophila crustacean cardioactive peptide; Drm-MS, Drosophila myosuppressin; Drm-sNPF1, Drosophila short neuropeptide F1; Drm-TK-3, Drosophila tachykinin-3; G16, G protein-16.
Discussion
From invertebrates, only one receptor for an FMRFamide-related peptide has been cloned so far, which is the receptor for the L. stagnalis cardioexcitatory peptide (LyCEP) (TPHWRPQGRF-NH2; ref. 26). LyCEP, however, has only the last two amino acid residues (RFamide) in common with FMRFamide and might, therefore, not be a “genuine” FMRFamide peptide. This conclusion is supported by the finding that the LyCEP receptor does not react with FMRFamide itself (26), a neuropeptide that is present in L. stagnalis and probably all other molluscs (1, 18). Our cloning of a “genuine” FMRFamide receptor from Drosophila (Figs. 1 and 4), therefore, is the first report on the cloning of an invertebrate FMRFamide receptor.
Dose–response curves for the intrinsic Drosophila FMRFamide-related peptides showed that the Drosophila peptide FMRFamide-6 (PDNFMRFamide) has the highest potency to activate the receptor (EC50, 9 × 10−10 M; Fig. 4G), whereas the other Drosophila FMRFamides are clearly less active: Drosophila FMRFamide-2 (DPKQDFMRFamide) by a factor of 3; Drosophila FMRFamides-3 (TPAEDFMRFamide), -5 (SPKQDFMRFamide), and -8 (MDSNFIRFamide) by a factor of 8; and Drosophila FMRFamide-1 (SVQDNFMHFamide) by a factor of 42 (Fig. 4G). In addition, two other peptides showed only a very low affinity for the receptor: Drosophila FMRFamide-4 (SDNFMRFamide) needed more than thousand times higher concentrations to give the same response as Drosophila FMRFamide-6, whereas Drosophila FMRFamide-7 (SAPQDFVRSamide) did not react at all (Fig. 4G). These results indicate that the new Drosophila receptor is the intrinsic receptor for Drosophila FMRFamide-6, but that it cannot be the physiologically relevant receptor for all eight Drosophila FMRFamides that are known to be contained within the Drosophila FMRFamide preprohormone (12, 13). Drosophila FMRFamide-2, however, is present with five copies in the Drosophila FMRFamide precursor (12, 13). Thus, it could be expected that the concentration of Drosophila FMRFamide-2 in the hemolymph or synapses is about five times higher than that of Drosophila FMRFamide-6, which would compensate for the somewhat lower affinity of the Drosophila FMRFamide-2 peptide for the receptor. Under in vivo conditions, therefore, the new receptor could be the cognate receptor for both FMRFamide-6 and -2. The situation for Drosophila FMRFamide-3 and -5, however, is unclear. If all Drosophila FMRFamides are released simultaneously and their concentrations are in accordance to their stoichiometry in the preprohormone, then the receptor would already be fully activated by FMRFamide-6 (and -2), before the FMRFamides-3 and -5 start to be active (around 10−9 M; Fig. 4G). Therefore, if no differential processing of the preprohormone, or alternative splicing of its gene transcript occurs (ref. 37; see, however, ref. 38), the new receptor would not be the cognate receptor for FMRFamides-3 and -5. The same holds for FMRFamide-1. Furthermore, the novel receptor is clearly not the cognate receptor for Drosophila FMRFamide-4 and -7 (Fig. 4G).
The above arguments, therefore, suggest that Drosophila has additional FMRFamide receptors. One would expect that these additional receptors are structurally related to the one cloned in this paper (Fig. 1). Screening of the Drosophila Genome Project database, using the blast algorithm, however, did not yield additional G protein-coupled receptors that were closely related to the one from Fig. 1. The additional FMRFamide receptors from Drosophila, therefore, might have amino acid residue identities of below 23% with the first Drosophila FMRFamide receptor, which would make them difficult to be recognized as FMRFamide receptors.
We have found that the Drosophila FMRFamide receptor can also be activated by peptides that are not “genuine” FMRFamides, such as Drosophila short neuropeptide F-1 (AQRSPSLRLRFamide; ref. 39) and Drosophila myosuppressin (TDVDHVFLRFamide; ref. 39). These peptides, however, can only activate the receptor at concentrations above 10−8 M (Fig. 4F), and again, are not likely to be ligands under normal physiological conditions.
From the dose–response curves of Fig. 4G, it is clear that the receptor recognizes more amino acid residues than the C-terminal FMRFamide sequence and, therefore, is able to discriminate between the various intrinsic Drosophila peptides. For two peptides, this discrimination is extreme: FMRFamide-6 (PDNFMRFamide) is recognized very well, whereas FMRFamide-4 (SDNFMRFamide), where the first amino acid residue proline has been exchanged for a serine residue, is only very poorly recognized. Furthermore, the C-terminal sequence FMRFamide alone is much more effective than, for example, Drosophila FMRFamide-4, but much less effective than Drosophila FMRFamide-6, which both are peptides containing the C-terminal FMRFamide moiety. These findings suggest that some N-terminal extensions of FMRFamide hamper the activation of the receptor, whereas others have the opposite effect.
The Drosophila FMRFamides have been reported to activate larval neuromuscular junctions and to inhibit heartbeat (3, 38, 40, 41). At the larval neuromuscular junctions, all FMRFamides acted similarly (except for FMRFamide-7, which was inactive), suggesting that these peptides were functionally redundant (3, 40). At the heart, however, only FMRFamide-4 was active, whereas FMRFamide-2 and -3 were without effects (38, 41). A comparison of these peptide effects with the characteristics of our FMRFamide receptor (Fig. 4G) makes clear that this receptor cannot be responsible for the actions on the neuromuscular synapses and heart. This finding, again, suggests the existence of multiple Drosophila FMRFamide receptors.
In conclusion, we have cloned the first insect FMRFamide receptor. This receptor is a “genuine” FMRFamide receptor and is, therefore, also the first invertebrate FMRFamide receptor to be identified. The sequence of the Drosophila FMRFamide receptor might provide the basis for the cloning of many other invertebrate FMRFamide receptors. The Anopheles gene product agCP12609 (Fig. 2), for example, might also be an FMRFamide receptor. These findings will lead to a much better understanding of the heterogeneity and actions of invertebrate FMRFamides and, thereby, to an important advancement of invertebrate neuroendocrinology. Furthermore, the availability of a cloned and functionally expressed insect FMRFamide receptor (Fig. 4) may also lead to the development of a new selective and environmentally safe nonpeptide insecticide for use in agriculture or medicine (42). The putative FMRFamide receptor in Anopheles (Fig. 2), for example, is a new and potentially important drug target that might help us to combat malaria. Finally, the possible discovery of an FMRFamide receptor in nematodes will supply us with an essential drug target to fight parasitic nematodes, such as Brugia malayi, that causes lymphatic filariasis (also known as elephantiasis), which is the second leading cause of permanent disability worldwide (130 million people infected; 1.1 billion people, 20% of the world's population, are at risk of infection). All these examples illustrate that our work might also result in useful societal benefits.
Acknowledgments
We thank Drs. S. Rees and J. Stables (Glaxo Wellcome, Stevenage, U.K.) for supplying cell line CHO/G16, L. Steffensen for typing the manuscript, M. Williamson for critically reading the manuscript, and the Lundbeck Foundation, the Danish Natural Science Research Council (equipment grant), the Carlsberg Foundation (equipment grant), and the Novo Nordisk Foundation for financial support.
Abbreviations
CHO, Chinese hamster ovary
DFR, Drosophila FMRFamide receptor
References
- 1.Price D. A. & Greenberg, M. (1977) Science 197, 670-671. [DOI] [PubMed] [Google Scholar]
- 2.Grimmelikhuijzen C. J. P. & Graff, D. (1986) Proc. Natl. Acad. Sci. USA 83, 9817-9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taghert P. H. (1999) Microsc. Res. Tech. 45, 80-95. [DOI] [PubMed] [Google Scholar]
- 4.Geary T. G., Marks, N. J., Maule, A. G., Bowman, J. W., Alexander-Bowman, S. J., Day, T. A., Larsen, M. J., Kubiak, T. M., Davis, J. P. & Thompson, D. P. (1999) Ann. N.Y. Acad. Sci. 897, 212-227. [DOI] [PubMed] [Google Scholar]
- 5.Li C., Kim, K. & Nelson, L. S. (1999) Brain Res. 848, 26-34. [DOI] [PubMed] [Google Scholar]
- 6.Grimmelikhuijzen C. J. P., Leviev, I. & Carstensen, K. (1996) Int. Rev. Cytol. 167, 37-89. [DOI] [PubMed] [Google Scholar]
- 7.Yang H. Y., Fratta, W., Majane, E. A. & Costa, E. (1985) Proc. Natl. Acad. Sci. USA 82, 7757-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinuma S., Habata, Y., Fujii, R., Kawamata, Y., Hosoya, M., Fukusumi, S., Kitada, C., Masuo, Y., Asano, T., Matsumoto, H., et al. (1998) Nature (London) 393, 272-276. [DOI] [PubMed] [Google Scholar]
- 9.Hinuma S., Shintani, Y., Fukusumi, S., Lijima, N., Matsumoto, Y., Hosoya, M., Fujii, R., Watanabe, T., Kikuchi, K., Terao, Y., et al. (2000) Nat. Cell Biol. 2, 703-708. [DOI] [PubMed] [Google Scholar]
- 10.Reinscheid R. K. & Grimmelikhuijzen, C. J. P. (1994) J. Neurochem. 62, 1214-1222. [DOI] [PubMed] [Google Scholar]
- 11.Taussig R. & Scheller, R. H. (1986) DNA 5, 453-461. [DOI] [PubMed] [Google Scholar]
- 12.Schneider L. E. & Taghert, P. H. (1988) Proc. Natl. Acad. Sci. USA 85, 1993-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nambu J. R., Murphy-Erdosh, C., Andrew, P. C., Feistner, G. J. & Scheller, R. H. (1988) Neuron 1, 55-61. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell G. A. (1993) Experientia 63, 279-285. [Google Scholar]
- 15.Van Golen F. A., Li, K. W., de Lange, R. P., Jespersen, S. & Geraerts, W. P. M. (1995) J. Biol. Chem. 270, 28487-28493. [DOI] [PubMed] [Google Scholar]
- 16.Scott M. L., Brezina, V. & Weiss, K. R. (1997) J. Neurophysiol. 78, 2372-2387. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Nelson, L. S., Kim, K., Nathoo, A. & Hart, A. C. (1999) Ann. N.Y. Acad. Sci. 897, 239-252. [DOI] [PubMed] [Google Scholar]
- 18.Santama N. & Benjamin, P. R. (2000) Microsc. Res. Tech. 49, 547-556. [DOI] [PubMed] [Google Scholar]
- 19.Nelson L. S., Rosoff, M. L. & Li, C. (1998) Science 281, 1686-1690. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg M. J. & Price, D. A. (1992) Prog. Brain Res. 92, 25-37. [DOI] [PubMed] [Google Scholar]
- 21.Van Tol-Steye H., Lodder, J. C., Mansvelder, H. D., Planta, R. J., van Heerikhuizen, H. & Kits, K. S. (1999) J. Neurosci. 19, 3739-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiba O., Sasaki, K., Higuchi, H. & Takashima, K. (1992) Neurosci. Res. 15, 255-264. [DOI] [PubMed] [Google Scholar]
- 23.Volterra A. & Siegelbaum, S. A. (1988) Proc. Natl. Acad. Sci. USA 85, 7810-7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottrell G. A. (1997) J. Exp. Biol. 200, 2377-2386. [DOI] [PubMed] [Google Scholar]
- 25.Lingueglia E., Champigny, G., Lazdunski, M. & Barbry, P. (1995) Nature (London) 378, 730-733. [DOI] [PubMed] [Google Scholar]
- 26.Tensen C. P., Cox, K. J. A., Smit, A. B., van der Schors, R. C., Meyerhof, W., Richter, D., Planta, R. J., Hermann, P. M., van Minnen, J., Geraerts, W. P. M., et al. (1998) J. Neurosci. 18, 9812-9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauser F., Nothacker, H.-P. & Grimmelikhuijzen, C. J. P. (1997) J. Biol. Chem. 272, 1002-1010. [DOI] [PubMed] [Google Scholar]
- 28.Staubli F., Jørgensen, T. J. D., Cazzamali, G., Williamson, M., Lenz, C., Søndergaard, L., Roepstorff, P. & Grimmelikhuijzen, C. J. P. (2002) Proc. Natl. Acad. Sci. USA 99, 3446-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stables J., Green, A., Marshall, F., Fraser, N., Knight, E., Sautel, M., Milligan, G., Lee, M. & Rees, S. (1997) Anal. Biochem. 252, 115-126. [DOI] [PubMed] [Google Scholar]
- 30.Birgül N., Weise, C., Kreienkamp, H.-J. & Richter, D. (1999) EMBO J. 18, 5892-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenz C., Søndergaard, L. & Grimmelikhuijzen, C. J. P. (2000) Biochem. Biophys. Res. Commun. 269, 91-96. [DOI] [PubMed] [Google Scholar]
- 32.Lenz C., Willamson, M. & Grimmelikhuijzen, C. J. P. (2000) Biochem. Biophys. Res. Commun. 273, 571-577. [DOI] [PubMed] [Google Scholar]
- 33.Lenz C., Williamson, M., Hansen, G. N. & Grimmelikhuijzen, C. J. P. (2001) Biochem. Biophys. Res. Commun. 286, 1117-1122. [DOI] [PubMed] [Google Scholar]
- 34.Larsen M. J., Burton, K. J., Zantello, M. R., Smith, V. G., Lowery, D. L. & Kubiak, T. M. (2001) Biochem. Biophys. Res. Commun. 286, 895-901. [DOI] [PubMed] [Google Scholar]
- 35.Secher T., Lenz, C., Cazzamali, G., Sørensen, G., Williamson, M., Hansen, G. N., Svane, P. & Grimmelikhuijzen, C. J. P. (2001) J. Biol. Chem. 276, 47052-47060. [DOI] [PubMed] [Google Scholar]
- 36.Civelli O., Nothacker, H. P., Saito, Y., Wang, Z., Lin, S. H. & Reinscheid, R. K. (2001) Trends Neurosci. 24, 230-237. [DOI] [PubMed] [Google Scholar]
- 37.Schneider L. E. & Taghert, P. H. (1990) J. Biol. Chem. 265, 6890-6895. [PubMed] [Google Scholar]
- 38.Merte J. & Nichols, R. (2002) Peptides 23, 209-220. [DOI] [PubMed] [Google Scholar]
- 39.Vanden Broeck J. (2001) Peptides 22, 241-254. [DOI] [PubMed] [Google Scholar]
- 40.Hewes R. S., Snowdeal, E. C., III, Saitou, M. & Taghert, P. H. (1998) J. Neurosci. 18, 7138-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols R., McCormick, J., Cohen, M., Howe, E., Jean, C., Pasley, K. & Rosario, C. (1999) J. Neurogenet. 13, 89-104. [DOI] [PubMed] [Google Scholar]
- 42.Lange A. B., Orchard, I., Wang, Z. & Nachman, R. J. (1995) Proc. Natl. Acad. Sci. USA 92, 9250-9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaffer M. H., Noyes, B. E., Slaughter, C. A., Thorne, G. C. & Gaskell, S. J. (1990) Biochem. J. 269, 315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siviter R. J., Coast, G. M., Winther, E. M. E., Nachman, R. J., Taylor, C. A. M., Shirras, A. D., Coates, D., Isaac, R. & Nässel, D. R. (2000) J. Biol. Chem. 275, 23273-23280. [DOI] [PubMed] [Google Scholar]
- 45.Lenz C., Williamson, M. & Grimmelikhuijzen, C. J. P. (2000) Biochem. Biophys. Res. Commun. 273, 1126-1131. [DOI] [PubMed] [Google Scholar]
- 46.Williamson M., Lenz, C., Winther, Å. M. E., Nässel, D. R. & Grimmelikhuijzen, C. J. P. (2001) Biochem. Biophys. Res. Commun. 281, 544-550. [DOI] [PubMed] [Google Scholar]
- 47.Williamson M., Lenz, C., Winther, Å. M. E., Nässel, D. R. & Grimmelikhuijzen, C. J. P. (2001) Biochem. Biophys. Res. Commun. 282, 124-130. [DOI] [PubMed] [Google Scholar]
- 48.Veenstra J. A. (1989) Neuropeptides 14, 145-149. [DOI] [PubMed] [Google Scholar]
- 49.Gäde G., Hoffmann, K. H. & Spring, J. H. (1997) Physiol. Rev. 77, 963-1032. [DOI] [PubMed] [Google Scholar]