Abstract
Semaphorins are axon guidance molecules that signal through the plexin family of receptors. Semaphorins also play a role in other processes such as immune regulation and tumorigenesis. However, the molecular signaling mechanisms downstream of plexin receptors have not been elucidated. Semaphorin 4D is the ligand for the plexin-B1 receptor and stimulation of the plexin-B1 receptor activates the small GTPase RhoA. Using the intracellular domain of plexin-B1 as an affinity ligand, two Rho-specific guanine nucleotide exchange factors, leukemia-associated Rho GEF (LARG; GEF, guanine nucleotide exchange factors) and PSD-95/Dlg/ZO-1 homology (PDZ)-RhoGEF, were isolated from mouse brain as plexin-B1-specific interacting proteins. LARG and PDZ-RhoGEF contain several functional domains, including a PDZ domain. Biochemical characterizations showed that the PDZ domain of LARG is directly involved in the interaction with the carboxy-terminal sequence of plexin-B1. Mutation of either the PDZ domain in LARG or the PDZ binding site in plexin-B1 eliminates the interaction. The interaction between plexin-B1 and LARG is specific for the PDZ domain of LARG and LARG does not interact with plexin-A1. A LARG-interaction defective mutant of the plexin-B1 receptor was created and was unable to stimulate RhoA activation. The data in this report suggest that LARG plays a critical role in plexin-B1 signaling to stimulate Rho activation and cytoskeletal reorganization.
Semaphorins comprise a family of soluble and membrane-associated proteins that were originally characterized in the nervous system and play a critical role in axon guidance. In vertebrates, the first identified semaphorin, Sema3A/collapsin-1, was characterized as an activity that causes growth cone collapse (1). Genetic investigation in Drosophila indicated that semaphorins function as repulsive cues in axon guidance (2). In addition, semaphorins also play important physiological functions in the immune response, cell migration, and tumor growth (3–5).
Neuropilin-1 (NP-1) was initially identified as a receptor for Sema3A (6). Later studies found that plexin-A1, in complex with NP-1, forms a functional receptor complex for Sema3A (7, 8). Plexins have a large intracellular region composed of two highly conserved domains that are required for semaphorin-induced signaling downstream of the receptor (9). Plexin-B1 is a receptor for semaphorin 4D (Sema4D) and neuropilins are not required for Sema4D binding to plexin-B1 (7, 8, 10).
Current evidence strongly implicates members of the Rho family of small GTPases in axon guidance (9, 11). The Rho family of small GTPases, which include Rho, Cdc42, and Rac, are key regulators of cell morphology and cytoskeletal structure (12). The activity of small GTPases are regulated by GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs). The Dbl family of GEFs stimulate nucleotide exchange on Rho family GTPases through a conserved Dbl homology (DH) domain (13). The small GTPase, Rac, has been implicated in semaphorin-mediated growth cone guidance (14). We and others have found that Rac directly interacts with the cytoplasmic domain of plexin-B1, indicating a direct role for Rac in plexin-B1 signaling (15–17). Recently, genetic studies showed that plexB inhibits Rac function in Drosophila (18). We have provided biochemical data to support this model and have shown that Rac also regulates the ligand binding function of plexin-B1 (19). Accumulating data suggests that attractive guidance cues activate Cdc42 and Rac to promote growth cone extension, whereas repulsive cues activate Rho to induce retraction (14). Recent studies have provided important new insights into how repulsive axon guidance receptors such as the EphA and ROBO receptors regulate the Rho family GTPases through associated GEFs and GAPs (20, 21). However, such findings have not been made with other guidance receptors such as DCC/Unc5 or plexins.
Genetic studies in Drosophila indicated that plexB directly interacts with Rho and that Rho activity is required for proper neuronal patterning (18). However, mammalian plexin-B1 does not interact directly with RhoA. Interestingly, crosslinking of a CD2-plexin-B1 chimeric receptor leads to stress fiber formation in Swiss3T3 cells, indicating that plexin-B1 does activate Rho downstream (15). Activation of Rho by plexin is consistent with the current model that semaphorins function as repulsive signals in growth cone guidance. However, the biochemical mechanism of RhoA activation by plexin-B1 is unknown.
In this report, we isolated leukemia-associated Rho GEF (LARG) and PSD-95/Dlg/ZO-1 homology (PDZ)-RhoGEF as plexin-B1-specific interacting proteins. LARG was initially identified as a mixed lineage leukemia fusion found in patients with primary acute myeloid leukemia (22). LARG is a multidomain protein that contains a Rho-specific GEF domain and a PDZ domain. We demonstrated that the PDZ domain of LARG directly interacts with the carboxy-terminal sequence of plexin-B1. LARG plays an important role in RhoA activation by plexin-B1 and Sema4D. Our results suggest a molecular mechanism by which Sema4D stimulated plexin-B1 signals via LARG to RhoA activation.
Materials and Methods
Plasmids.
pEBG-3X-HV-plexin-B1C and pcDNA3-HA-plexin-B1C were constructed by PCR amplification of the plexin-B1 cytoplasmic domain (amino acids 1512–2135). pEBG-3X-HV is a modified version of pEBG-3X containing the following multiple cloning site: GGATCCCATATGGATATCGCTAGCCCCGGGGTCGACACTAGTATCGATGCGGCCGCTGAATAG. pcDNA3-HA-plexin-A1C contains the cytoplasmic domain (amino acids 1347–1894). The mammalian expression constructs for AU1-LARG and AU1-PDZ-RhoGEF were described previously (23). Myc-Lin7 was provided by B. Margolis (University of Michigan). pcDNA3-VSV-plexin-B1 and -VSV-plexin-A1, pRK5- myc-Rho, Sema4D-SEAP have been described (13, 24).
Purification of Plexin-B1 Interacting Proteins.
HEK293 cells were transfected with the mammalian N-terminal GST vector pEBG-3X-HV-plexin-B1C by using Lipofectamine per the manufacturer's instructions (Invitrogen). Cells were lysed in buffer (20 mM Tris⋅Cl, pH 7.5/100 mM NaCl/1% Nonidet P-40/1 mM EDTA) and GST-plexin-B1C was purified.
One gram of brain tissue was dounce homogenized in 5 ml of buffer (PBS + 1% TX-100) per binding reaction. The samples were then centrifuged at 15,000 × g for 20 min. The soluble fraction was then incubated with 1 mg/ml of Sulfo-NHS-LC-Biotin (sulfo- n-hydroxysulfosuccinimide long-chain biotin; Pierce) for 2 h on ice. The lysate was then dialyzed overnight in PBS to remove unreacted biotin and centrifuged at 15,000 × g for 20 min, and the supernatant was precleared with glutathione-agarose beads for 2 h. The lysate was then incubated with purified GST-plexin-B1C or GST (5–10 μg) on beads. After incubation for 4 h, GST-plexin-B1C and GST were eluted, separated by SDS/PAGE, and Western blotted (WB) with streptavidin–horseradish peroxidase (HRP; Amersham Pharmacia).
When plexin-B1C interacting proteins were analyzed by silver stain the same purification procedure was followed as above; however, the lysate was not incubated with Sulfo-NHS-LC-biotin.
In-Gel Digestion.
The plexin-B1C-associated samples were resolved by SDS/PAGE and stained by SilverQuest (Invitrogen). All solutions were made in HPLC grade water. All plasticware used was purchased new and not autoclaved. Protein bands were excised and washed twice in water, treated with freshly made destaining solution (100 mM ammonium bicarbonate/50 mM sodium thiosulfate/15 mM potassium ferrocyanide) for 5 min with gentle shaking, and further washed three times with water for 5 min each. The gel slices were treated with 1 ml of 100 mM ammonium bicarbonate for 30 min, followed by 1 ml of 50% acetonitrile/50 mM ammonium bicarbonate for 30 min. The gel slices were reduced in 0.5 ml of 45 mM DTT in 100 mM ammonium bicarbonate at 60°C for 30 min, followed by alkylation in 45 mM iodoacetamide in 100 mM ammonium bircarbonate at room temperature for 30 min in the dark. The gel slices were then washed with 1 ml of 100 mM ammonium bicarbonate for 30 min and treated with 100 μl of 100% acetonitrile for 10 min, transferred into a new 0.5-ml Eppendorf tube, and dried by Speed Vac.
Sequencing grade trypsin (Promega) was diluted to 20 ng/μl in 25 mM ammonium bicarbonate and added (5–10 μl) to the dried gel slices to rehydrate them. The samples were incubated in a 37°C incubator with rotation for 12–24 h. To stop the trypsin digestion, formic acid was added (1 μl of formic acid for every 25 μl of digestion sample) and the gel slices were sonicated in a sonication water bath for 20 min. The aqueous solution was transferred to a new tube and the gel slices were extracted with 100 μl of 50% acetonitrile/50 mM ammonium bircarbonate and sonicated for 20 min. This new extraction solution was combined with the original digestion solution. The combined samples were dried to approximately 10–20 μl by Speed Vac. The digested peptides were purified by micro C18 Ziptip (Whatman). The samples were eluted in 3 μl of 50% acetonitrile/0.1% trifluoroacetic acid.
Mass Spectrometry Analysis.
The in-gel digested samples were spotted on to a 396-well Teflon plate for matrix-assisted laser desorption ionization (MALDI)-MS analysis. Dihydroxylbenzoic acid was used as a matrix. Mass spectrum (MS) and tandem MS–MS were collected on a Pulsar apparatus (Applied Biosystems). Data analysis was performed by either mascot (www.matrixscience.com) or prospector (http://prospector.ucsf.edu/) search programs.
Cell Culture and Immunoprecipitation.
HEK293 cells were maintained in DMEM (GIBCO) and supplemented with 10% FBS. Cells were cultured for 48 h posttransfection and harvested in 20 mM Hepes, pH 7.5/100 mM NaCl/1% Triton X-100/0.2% Nonidet P-40/1 mM MgCl2/1 mM DTT/0.1 mM phenylmethylsulfonyl fluoride/5 μg/ml aprotinin/5 μg/ml leupeptin. The supernatant was incubated with anti-VSV antibody (Boehringer Mannheim) or anti-HA antibody (Babco, Richmond, CA) for 1 h, after which 10 μl of a 50% slurry of protein-G Sepharose was added and incubated for another hour. The beads were washed and boiled in SDS-sample treatment buffer. Proteins were resolved by SDS/PAGE and Western blotted with anti-AU1 antibody (Covance, Berkeley, CA) or anti-myc9E10 antibody (Covance).
Yeast Two-Hybrid.
Plexin-B1C (amino acids 1543–2135), plexin-A1C (amino acids 1347–1894), plexin-B1C-HV4 (amino acids 1612–1910), and lamin were cloned into the plexAde bait vector (24). RacL61, smgGDS, and LARG-PDZ (residues 1–191) were cloned into the pVP16 target plasmid. These plasmids were transformed into the L40 yeast strain and selected for growth on synthetic complete (SC) media lacking leucine and tryptophan. Positive interactions were determined by growth on SC media lacking histidine, leucine, and tryptophan.
Rho Activation Assay.
HEK293 cells were transfected with myc-RhoA (100 ng), VSV-plexin-B1 (300 ng), and AU1-LARG (100 ng) for 24 h. Cells were starved in serum-free medium for 3 h, followed by stimulation with Sema4D (2 nM) for 1 h. Cells were lysed in RIPA buffer (50 mM Tris, pH 7.5/150 mM NaCl/50 mM NaF/1 mM EDTA/1 mM EGTA/0.1% SDS/1% Triton X-100/0.5% deoxycholate) containing 10 mM MgCl2. Lysates were cleared by centrifugation and the soluble fraction was incubated with 20 μg of GST-Rhotekin-RBD, precoupled to glutathione agarose beads for 45 min, and washed with wash buffer (50 mM Tris, pH 7.5/150 mM NaCl/1% Triton X-100). Proteins were eluted with SDS sample buffer and resolved by SDS/PAGE. Myc-RhoA in the pull-down and lysates was detected by anti-myc Western blot.
Results
Identification and Isolation of a Plexin-B1 Interacting Protein.
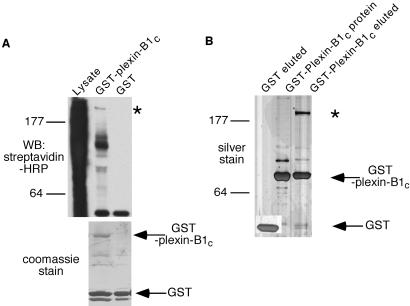
The intracellular domain of plexin has been established to play an essential role in transducing the signal downstream of the receptor (9, 25). It has been suggested that the intracellular domain of plexin has limited homology to the Ras GAP family and may function as a GAP to regulate small GTPase activity (16). However, direct biochemical data demonstrating that plexins possess GAP activity are lacking. To investigate the signal transduction mechanisms downstream of the plexin-B1 receptor, we searched for potential proteins that interact with plexin-B1. We expressed and purified the intracellular domain of plexin-B1 as a GST fusion protein (GST-B1C) from mammalian HEK293 cells and used it as an affinity matrix to isolate plexin-B1 binding proteins. The intracellular domain of plexin-B1 expressed and purified from Escherichia coli contained many impurities and degradation products and therefore was not suitable for this purpose. We prepared plexin-B1-specific antibodies and performed a Western blot of various mouse tissues, and determined that plexin-B1 was expressed most abundantly in mouse brain (data not shown). To this end, extracts from mouse brain were biotinylated with Sulfo-NHS-LC-Biotin and incubated with the GST-B1C matrix. After incubation, the matrix was washed and GST-B1C and proteins that co-purified were eluted with reduced glutathione. Bound proteins were visualized by Western blot with streptavidin-HRP. We observed several proteins that specifically co-purified with GST-plexin-B1C. These included two bands with apparent molecular weight of 120 and 200 kDa. The 120-kDa protein produced a stronger signal based on the streptavidin Western blot (Fig. 1A).
Fig 1.
Detection of plexin-B1 interacting proteins. (A) Detection of plexin-B1 interacting proteins from biotinylated mouse brain extracts. Biotinylated mouse brain lysates were subjected to affinity purification over either GST or GST-B1C affinity columns. Bound proteins were detected by streptavidin-HRP Western blot and eluted GST or GST-B1C was detected by Coomassie blue staining. An asterisk indicates the 200-kDa plexin-B1 interacting protein. (B) Detection of a 200-kDa plexin-B1 interacting protein by silver staining. Purification was performed essentially as described in A. Bound proteins were detected by silver staining. An asterisk indicates the 200-kDa plexin-B1 interacting protein.
To further confirm the presence of the plexin-B1 interacting proteins, we performed the identical purification as above; however, the brain extracts were not biotinylated. Bound proteins were detected by silver staining and we observed the same protein of 200 kDa that co-purified with GST-B1C (Fig. 1B). This protein was not present in the GST-B1C starting material or the GST control, which further suggests that it was from the mouse brain extracts. Surprisingly, the band at around 120 kDa, which was very strong in the streptavidin-HRP Western blot (Fig. 1A), was not detectable by silver staining. This could be due to the fact that the 120-kDa protein is an extremely good substrate for biotinylation, even though the protein level is below the limit of detection by silver staining. The above experiments showed that plexin-B1 specifically interacts with a 200-kDa protein in brain tissue.
Determination of the 200-kDa Plexin-B1 Interacting Band Containing both LARG and PDZ-RhoGEF.
To determine the identity of the 200-kDa plexin-B1 interacting protein, the proteins associated with GST-plexin-B1 were resolved by SDS/PAGE. The 200-kDa band detected by silver staining was excised and subjected to in-gel digestion by trypsin (see Materials and Methods). The digested peptides were analyzed by matrix-assisted laser desorption ionization (MALDI)-MS. The initial tryptic peptide fingerprint failed to determine the identity of the 200-kDa band (data not shown). However, several peptide peaks were uniquely present in the mass fingerprint of the 200-kDa band, but absent in the GST control. These unique mass peaks were analyzed by tandem MS–MS to obtain fragment ions of the peptides. A database search indicated that three of these peaks could be matched to the unique peptide sequences of GFPSILGPPR, VLDQVFYQR, and LQLLQEDYNR. These three peptides are perfect matches to LARG. MS–MS spectra of one of these peptides is shown in Fig. 2A. LARG was originally identified by Caligiuri's laboratory as a protein fused to mixed lineage leukemia in patients with acute myeloid leukemia (22). LARG contains multiple domains, including a PDZ, RGS, DH, and PH domain (Fig. 2B). In addition, MS–MS data also predicted a peptide with an amino acid sequence of DLIISEMQR; however, the signal for this peptide was much weaker than the LARG peptides. This sequence matches to PDZ-RhoGEF (Fig. 2B), which is also a Rho-specific GEF with similar domain structure as LARG (28). The identification of PDZ-RhoGEF was less definite because only one peptide was observed and the MS–MS spectrum was of low quality. The above data indicate that the 200-kDa band contains LARG and possibly PDZ-RhoGEF.
Fig 2.
Identification of the 200-kDa plexin-B1 interacting protein as LARG by mass spectrometry analysis. (A) MS–MS spectrum of the 1,040.60-Da peptide. MS–MS spectrum of the 1,040.60-Da peptide predicts the amino acid sequence of (R)GFPSILQPPR. The “(R)” is predicted based on the sequence of human LARG and the specificity of the trypsin digestion. The major Y ions are labeled. Y* indicates the Y ion with loss of an ammonium ion. Mass to charge ratio is denoted, m/z. (B) Domain structures of human LARG and PDZ-RhoGEF. LARG and PDZ-RhoGEF contain PDZ, regulator of G protein signaling (RGS), Dbl homology (DH), and pleckstrin homology (PH) domains as indicated. The three LARG peptides and the one PDZ-RhoGEF peptide identified by MS–MS spectra are indicated.
LARG Specifically Interacts with Plexin-B1.
To confirm the interaction between LARG and plexin-B1, and to test whether LARG interacts with other plexin family members, we analyzed the interaction of LARG with the cytoplasmic domain of plexin-B1 and plexin-A1 by coimmunoprecipitation in HEK293 cells. We observed that the cytoplasmic domain of plexin-B1 was able to coimmunoprecipitate LARG (Fig. 3A Left), and not that of plexin-A1 or plexin-A3 (data not shown). Furthermore, the full-length plexin-B1 receptor was also able to interact with LARG (Fig. 3A Right). Treatment with Sema4D did not significantly enhance the interaction of LARG with plexin-B1 (data not shown). The above observations show that LARG selectively interacts with plexin-B1.
Fig 3.
LARG and PDZ-RhoGEF interact with plexin-B1. (A) Full-length plexin-B1 and the cytoplasmic domain of plexin-B1 interact with LARG. HEK293 cells were transfected with the indicated plasmids. Immunoprecipitation with HA antibody (Left) or VSV antibody (Right) was performed. Coimmunoprecipitated LARG was detected by AU1 Western blot (Top). HA-A1C and HA-B1C are HA-tagged versions of the cytoplasmic domains of plexin-A1 and plexin-B1, respectively. VSV-B1 and VSV-A1 are full-length VSV-tagged versions of plexin-B1 and plexin-A1, respectively. (B) PDZ-RhoGEF interacts with plexin-B1 with lower affinity than with LARG. HEK293 cells were transfected with the indicated plasmids. Glutathione agarose was used to purify GST-B1C and bound AU1-LARG and AU1-PDZ-RhoGEF were detected by AU1 Western blot.
LARG belongs to a family of RhoGEFs that also includes the highly related PDZ-RhoGEF. Our MS data indicate that the 200-kDa plexin-B1 interacting band may also contain PDZ-RhoGEF. Hence, we tested and found that PDZ-RhoGEF also interacts with plexin-B1 (Fig. 3B). Interestingly, less PDZ-RhoGEF was found in the plexin-B1 complex than the LARG, indicating a lower affinity between PDZ-RhoGEF and plexin-B1 than that between LARG and plexin-B1. However, it is possible that the PDZ domain of PDZ-RhoGEF may be occupied by other cellular proteins and not completely accessible for plexin-B1 interaction.
The Plexin-B1 C Terminus Interacts with the PDZ Domain of LARG.
LARG contains a type I PDZ domain and is predicted to interact with the PDZ binding site consensus sequence X-S/T-X-φ located at the extreme C terminus of the target protein (26). Inspection of the plexin-B1 C terminus revealed the residues VTDL, which conforms to such a PDZ binding site (Fig. 4A). Interestingly, plexin-A1 contains a C-terminal sequence of QDMS that does not fit the typical PDZ recognition motif. These observations suggest that the PDZ domain of LARG may interact with the carboxyl-terminal sequence of plexin-B1. Hence we tested, using the yeast two-hybrid system, whether the PDZ domain of LARG mediates the interaction with plexin-B1. We detected a positive interaction between the PDZ domain alone of LARG (termed LARG-PDZ, amino acids 1–191) and the cytoplasmic domain of plexin-B1, but not with that of plexin-A1 (Fig. 4B).
Fig 4.
Plexin-B1 C terminus binds the PDZ domain of LARG. (A) Schematic representation of various plexin-B1 and LARG constructs used in HEK293 cell expression and yeast two-hybrid studies. HV4 corresponds to amino acids 1612–1910 of plexin-B1. Black boxes in plexin-B1 correspond to the two conserved regions in the cytoplasmic domain. The filled oval corresponds to the Cdc42/Rac interactive binding domain (17). (B) LARG-PDZ directly binds the C terminus of plexin-B1. Yeast two-hybrid assay was performed with the indicated plasmids. Transformed yeast were selected for on leucine and tryptophan-deficient media (SC-LW). Positive interactions were selected for by growth on histidine-, leucine-, and tryptophan-deficient media (SC-HLW). A1C and B1C represent the cytosolic domains of plexin-A1 and plexin-B1, respectively. LARG-PDZ denotes the PDZ-domain of LARG. B1C-HV4 indicates a truncated fragment of the intracellular domain of plexin-B1 lacking the carboxy-terminal sequence. Lamin and smgGDS are negative controls and RacL61 is a positive control. (C) Plexin-B1 does not interact with the Lin-7 type I PDZ domain. HEK293 cells were transfected as indicated and immunoprecipitation with HA antibody was performed. Coimmunoprecipitation of Lin-7 was detected by myc Western blot. No interaction between plexin-B1 and Lin-7 was observed (Left), whereas plexin-B1 interacted with LARG in the same experiment (Right). (D) The K77A mutation in the PDZ domain of LARG abolishes the interaction with plexin-B1. Yeast two-hybrid assay was performed as in B. (E) Mutation of K77A in full-length LARG abrogates interaction with the cytosolic domain of plexin-B1. HEK293 cells were transfected as indicated. Immunoprecipitation with HA antibody was performed. Coimmunoprecipitated LARG was detected by AU1 Western blot. (F) Masking of the plexin-B1 C-terminal PDZ-binding site abolishes the interaction with LARG. HEK293 cells were transfected as indicated. Immunoprecipitation of plexin was performed with VSV antibody and associated LARG was detected by AU1 Western blot.
Given that LARG contains a type I PDZ domain, we further tested whether the interaction with plexin-B1 was specific to LARG or if another type I PDZ domain, such as that found in Lin-7, could interact with plexin-B1 (27). Through coimmunoprecipitation studies we found that the interaction of plexin-B1 with LARG was in fact specific to the type I PDZ domain of LARG (Fig. 4C).
PDZ domains interact with the extreme C-termini of proteins containing PDZ-binding sites. This interaction is mediated by a conserved K/RXXXGLGF sequence in the PDZ domain as highlighted in the structure of the PSD-95 type I PDZ domain bound to the peptide corresponding to the C terminus of the NMDA receptor (28). In this structure a conserved arginine residue coordinates a water molecule, which in turn interacts with the extreme C terminus of the binding protein. We mutated the corresponding lysine 77 in LARG to an alanine and assayed the ability to bind plexin-B1. Mutation of lysine 77 to alanine significantly weakened the interaction with plexin-B1 as judged by coimmunoprecipitation and yeast two-hybrid studies (Fig. 4 D and E).
The observation that plexin-B1 contains a type I PDZ domain binding site suggested that the extreme C terminus of plexin-B1 may be responsible for the interaction with the PDZ domain of LARG. Therefore, we attempted to mask the PDZ binding site on plexin-B1 by introducing an HA tag (YPYDVPDYA) immediately following the VTDL sequence at the C terminus. Introduction of the HA tag disrupted the interaction of plexin-B1 with LARG and confirmed the role of the plexin-B1 PDZ binding site in mediating the interaction with the PDZ domain of LARG (Fig. 4F). Furthermore, LARG-PDZ did not interact with a C-terminal truncated form of the plexin-B1 intracellular domain, termed plexin-B1-HV4 (Fig. 4 A and B). These data demonstrate that the PDZ domain of LARG directly interacts with the carboxy terminal residues of plexin-B1. NP-1 also contains a type I PDZ-binding site at its C terminus. However, we found no interaction between LARG and NP-1 or the functional NP-1/plexin-A1 complex (data not shown).
LARG Mediates RhoA Activation by Sema4D and Plexin-B1.
Previous biochemical characterizations have shown that LARG GEF activity is specific for the small GTPase RhoA and not for Cdc42 or Rac (23, 29). In addition, it has been reported that clustering of the plexin-B1 receptor induces cytoskeletal changes indicative of Rho activation (15). Whether this involves the GEF activity of LARG is unknown. To measure the degree of Rho activation in cells, we used recombinantly expressed GST-Rhotekin RBD (Rho binding domain) to sequester GTP-bound Rho from the pool of total Rho (30). Using this assay, we examined RhoA activation in HEK293 cells (Fig. 5A). Treatment with Sema4D stimulated RhoA activation in cells expressing plexin-B1 and LARG. Coexpression of full-length B1 receptor alone had little effect on Rho activity in response to Sema4D stimulation. The Sema4D-induced RhoA activation depended on the C-terminal PDZ binding site of plexin-B1 because Sema4D treatment of plexin-B1-HA was unable to stimulate RhoA (Fig. 5A). These data indicate that the ability to interact with LARG is important for plexin-B1 to activate RhoA in response to Sema4D stimulation.
Fig 5.
Sema4D/plexin-B1 interaction stimulates RhoA through LARG binding. (A) Sema4D stimulates RhoA activation via plexin-B1 and LARG. GST-Rhotekin-RBD pull-down of GTP-RhoA was performed. GTP-RhoA was detected by myc Western blot (Top). Levels of plexin-B1 and RhoA were determined by Western blotting VSV and myc, respectively, in the lysate. (B) Model of plexin-B1 activation in regulation of Rho and Rac. Sema4D binding of plexin-B1 activates RhoA via LARG. The C terminus of plexin-B1 interacts with the PDZ domain of LARG, which in turn activates RhoA. Plexin-B1 also functions to sequester active Rac from downstream effectors and together with active RhoA promote cytoskeletal changes associated with growth cone turning/collapse.
Discussion
The signaling mechanisms involved in growth cone repulsion and collapse have remained elusive for some time. A growing body of evidence has suggested the involvement of the Rho family of GTPases in these processes; however, the molecular details have yet to be described. Recently, evidence from the EphA/EphA receptor growth cone repulsion signaling pathway has shown the involvement of a Rho GEF called ephexin, which complexes with the EphA receptor to promote Rho activation and Rac and Cdc42 inhibition (20). A similar mechanism occurs downstream of the Robo receptor, whereupon stimulation by the Slit ligand, the Rho family GAP, srGAP, associates with the receptor and down-regulates Cdc42 activity (21). It appears that regulators, including GEFs and GAPs, of the Rho family GTPases may play a general role in axon guidance. Rho regulators may also directly couple to other axon guidance receptors to regulate growth cone morphology. However, there have been no reports to date that describe the signaling events that link semaphorin/plexin receptor signaling to downstream events such as Rho activation.
We have identified LARG and PDZ-RhoGEF as plexin-B1-specific interacting proteins. LARG has previously been shown to specifically activate RhoA but not Rac or Cdc42. Furthermore, evidence is presented that LARG plays a role in RhoA activation in response to Sema4D stimulation in transfected cells. These data are consistent with the function of semaphorins as repulsive signals in axon guidance. We would like to propose that plexin-B1 stimulates Rho activation via LARG (Fig. 5B). LARG acts as the molecular link between activation of the plexin-B1 receptor and Rho. The PDZ domain of LARG directly interacts with the C-terminal residues of plexin-B1. Sema4D interacts with the plexin-B1 receptor and activates the receptor presumably by clustering. This permits activation of the GEF activity of LARG. Therefore, LARG functions as a key signal transducer between the plexin-B1 receptor and RhoA activation. Both genetic data from Drosophila and biochemical data from cell culture indicate that plexin-B1 inhibits Rac possibly via a direct interaction (18, 19). Hence, the model also predicts that plexin-B1 inhibits Rac by sequestering Rac from its downstream effectors.
Our model provides a molecular mechanism of how plexin-B1 regulates the function of RhoA and Rac. Although we have not investigated the function of LARG in axon guidance directly, the biochemical functions of plexin-B1 is completely consistent with the repulsive function of semaphorins in axon guidance. While this paper was in preparation, Swiercz et al. (31) reported that PDZ-RhoGEF functions in plexin-B1-mediated growth cone collapse in developing hippocampal neurons. Their observations are consistent with our data that plexin-B1 also interacts with PDZ-RhoGEF. The interaction between plexin-B1 and LARG appears to not be directly stimulated by Sema4D. However, plexin-B1 may regulate LARG function by recruiting the LARG to the plasma membrane where activation of RhoA occurs. It is also possible that the N-terminal domain of LARG has an inhibitory effect on the C-terminal GEF domain. Binding of plexin-B1 to the N-terminal PDZ domain may activate the RhoGEF activity by relieving the inhibition. Alternatively, plexin-B1 may cluster LARG to form a functional complex. Further studies are needed to elucidate the molecular basis of how LARG is activated by Sema4D and plexin-B1.
LARG and PDZ-RhoGEF share high sequence homology and a similar domain structure. Both have been implicated in signal transduction of trimeric G protein-coupled receptor signaling (23, 32–34). LARG has also been shown to directly interact with the insulin-like growth factor (IGF) receptor (26). The PDZ domain of LARG interacts with the C-terminal residues of the IGF receptor. We observed that LARG protein levels are significantly higher than plexin-B1 in brain tissues (data not shown). These observations indicate that LARG can couple to several different cell surface receptors to regulate cytoskeletal structure. We also observed an interaction between plexin-B1 and PDZ-RhoGEF and found that the interaction is weaker than that of plexin-B1 and LARG. Therefore, the plexin-B1 receptor may also regulate different PDZ containing RhoGEFs.
All plexin family members share high sequence similarities in their intracellular domains and possibly function in a similar manner to regulate Rho family GTPases. The three members of the plexin-B subfamily contain a conserved PDZ binding motif and are predicted to interact with LARG. In contrast, other plexin family members do not contain a typical C-terminal PDZ recognition sequences and we consistently failed to detect an interaction between LARG and plexin-A1. However, we would like to speculate that other plexin family members may also modulate the Rho family GTPases through different GEFs or GAPs. They may also directly interact with various RhoGEFs to induce Rho activation and growth cone repulsion; however, they may use different modes of protein–protein interaction to accomplish this. It is worth noting that searches of the human genome have predicted a large family of RhoGEFs far exceeding the numbers of Rho family GTPases. Interestingly, these putative RhoGEFs contain various protein-protein interaction domains. Therefore, in response to wide range of extracellular and intracellular signals, the RhoGEF family may serve as specific mediators to regulate the Rho family GTPases.
Acknowledgments
We thank Z. He and B. Margolis for plasmids and Tianquin Zhu for technical assistance. This work is supported by grants from the National Institutes of Health and the Walther Cancer Institute, and a MacArthur Fellowship (to K.L.G.). J.A. is supported by Pharmocological Sciences Training Program Grant GMO7767 from the National Institute of General Medical Sciences.
Abbreviations
GAP, GTPase activating protein
GEF, guanine nucleotide exchange factor
LARG, leukemia-associated Rho GEF
NP-1, neuropilin-1
PDZ, PSD-95/Dlg/ZO-1 homology
Sema4D, semaphorin 4D
References
- 1.Luo Y., Raible, D. & Raper, J. A. (1993) Cell 75, 217-227. [DOI] [PubMed] [Google Scholar]
- 2.Kolodkin A. L., Levengood, D. V., Rowe, E. G., Tai, Y. T., Giger, R. J. & Ginty, D. D. (1997) Cell 90, 753-762. [DOI] [PubMed] [Google Scholar]
- 3.Christensen C. R., Klingelhofer, J., Tarabykina, S., Hulgaard, E. F., Kramerov, D. & Lukanidin, E. (1998) Cancer Res. 58, 1238-1244. [PubMed] [Google Scholar]
- 4.Hall K. T., Boumsell, L., Schultze, J. L., Boussiotis, V. A., Dorfman, D. M., Cardoso, A. A., Bensussan, A., Nadler, L. M. & Freeman, G. J. (1996) Proc. Natl. Acad. Sci. USA 93, 11780-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao H. Q., Soker, S., Feiner, L., Alonso, J. L., Raper, J. A. & Klagsbrun, M. (1999) J. Cell Biol. 146, 233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Z. & Tessier-Lavigne, M. (1997) Cell 90, 739-751. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T., Fournier, A., Nakamura, F., Wang, L. H., Murakami, Y., Kalb, R. G., Fujisawa, H. & Strittmatter, S. M. (1999) Cell 99, 59-69. [DOI] [PubMed] [Google Scholar]
- 8.Tamagnone L., Artigiani, S., Chen, H., He, Z., Ming, G. I., Song, H., Chedotal, A., Winberg, M. L., Goodman, C. S., Poo, M., et al. (1999) Cell 99, 71-80. [DOI] [PubMed] [Google Scholar]
- 9.Patel B. N. & Van Vactor, D. L. (2002) Curr. Opin. Cell Biol. 14, 221-229. [DOI] [PubMed] [Google Scholar]
- 10.Rohm B., Ottemeyer, A., Lohrum, M. & Puschel, A. W. (2000) Mech. Dev. 93, 95-104. [DOI] [PubMed] [Google Scholar]
- 11.Dickson B. J. (2001) Curr. Opin. Neurobiol. 11, 103-110. [DOI] [PubMed] [Google Scholar]
- 12.Hall A. (1998) Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- 13.Cerione R. A. & Zheng, Y. (1996) Curr. Opin. Cell Biol. 8, 216-222. [DOI] [PubMed] [Google Scholar]
- 14.Liu B. P. & Strittmatter, S. M. (2001) Curr. Opin. Cell Biol. 13, 619-626. [DOI] [PubMed] [Google Scholar]
- 15.Driessens M. H., Hu, H., Nobes, C. D., Self, A., Jordens, I., Goodman, C. S. & Hall, A. (2001) Curr. Biol. 11, 339-344. [DOI] [PubMed] [Google Scholar]
- 16.Rohm B., Rahim, B., Kleiber, B., Hovatta, I. & Puschel, A. W. (2000) FEBS Lett. 486, 68-72. [DOI] [PubMed] [Google Scholar]
- 17.Vikis H. G., Li, W., He, Z. & Guan, K. L. (2000) Proc. Natl. Acad. Sci. USA 97, 12457-12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H., Marton, T. F. & Goodman, C. S. (2001) Neuron 32, 39-51. [DOI] [PubMed] [Google Scholar]
- 19.Vikis H. G., Li, W. & Guan, K. L. (2002) Genes Dev. 16, 836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamah S. M., Lin, M. Z., Goldberg, J. L., Estrach, S., Sahin, M., Hu, L., Bazalakova, M., Neve, R. L., Corfas, G., Debant, A. & Greenberg, M. E. (2001) Cell 105, 233-244. [DOI] [PubMed] [Google Scholar]
- 21.Wong K., Ren, X. R., Huang, Y. Z., Xie, Y., Liu, G., Saito, H., Tang, H., Wen, L., Brady-Kalnay, S. M., Mei, L., et al. (2001) Cell 107, 209-221. [DOI] [PubMed] [Google Scholar]
- 22.Kourlas P. J., Strout, M. P., Becknell, B., Veronese, M. L., Croce, C. M., Theil, K. S., Krahe, R., Ruutu, T., Knuutila, S., Bloomfield, C. D. & Caligiuri, M. A. (2000) Proc. Natl. Acad. Sci. USA 97, 2145-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuhara S., Chikumi, H. & Gutkind, J. S. (2000) FEBS Lett. 485, 183-188. [DOI] [PubMed] [Google Scholar]
- 24.Vojtek A. B. & Hollenberg, S. M. (1995) Methods Enzymol. 255, 331-342. [DOI] [PubMed] [Google Scholar]
- 25.He Z., Wang, K. C., Koprivica, V., Ming, G. & Song, H. J., (2002) Sci. STKE http: //stke.science.mag.org/cgi/content/full/sigtrans;2002/119/re1. [DOI] [PubMed]
- 26.Taya S., Inagaki, N., Sengiku, H., Makino, H., Iwamatsu, A., Urakawa, I., Nagao, K., Kataoka, S. & Kaibuchi, K. (2001) J. Cell Biol. 155, 809-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamberov E., Makarova, O., Roh, M., Liu, A., Karnak, D., Straight, S. & Margolis, B. (2000) J. Biol. Chem. 275, 11425-11431. [DOI] [PubMed] [Google Scholar]
- 28.Doyle D. A., Lee, A., Lewis, J., Kim, E., Sheng, M. & MacKinnon, R. (1996) Cell 85, 1067-1076. [DOI] [PubMed] [Google Scholar]
- 29.Reuther G. W., Lambert, Q. T., Booden, M. A., Wennerberg, K., Becknell, B., Marcucci, G., Sondek, J., Caligiuri, M. A. & Der, C. J. (2001) J. Biol. Chem. 276, 27145-27151. [DOI] [PubMed] [Google Scholar]
- 30.Ren X. D. & Schwartz, M. A. (2000) Methods Enzymol. 325, 264-272. [DOI] [PubMed] [Google Scholar]
- 31.Swiercz J. M., Kuner, R., Behrens, J. & Offermanns, S. (2002) Neuron 35, 51-63. [DOI] [PubMed] [Google Scholar]
- 32.Fukuhara S., Murga, C., Zohar, M., Igishi, T. & Gutkind, J. S. (1999) J. Biol. Chem. 274, 5868-5879. [DOI] [PubMed] [Google Scholar]
- 33.Booden M. A., Siderovski, D. P. & Der, C. J. (2002) Mol. Cell. Biol. 22, 4053-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chikumi H., Vazquez-Prado, J., Servitja, J. M., Miyazaki, H. & Gutkind, J. S. (2002) J. Biol. Chem. 277, 27130-27134. [DOI] [PubMed] [Google Scholar]