Abstract
This article provides information concerning a novel research subject recruitment registry developed at Vanderbilt University. Project goals were (1) to provide a mechanism for lay individuals to self-enter information conveying interest in volunteering for clinical research and (2) provide tools for researchers to select and contact potential volunteers based on study-specific inclusion criteria. The registry was built and offered as an institutional resource to all university scientists conducting institutional review board–approved research. The authors present (1) a model for redesigning workflow associated with subject registration, volunteer retrieval, and subject contact; (2) details of a Web-based software application used as a focal point in designing workflow for our system; (3) descriptive statistics for volunteer and researcher use of the system during the first 32 months of operation; (4) cost estimates for the project; and (5) a set of recommendations for other medical centers wishing to adopt similar methodology.
Subject recruitment is critical to the success of clinical research, both for scientific return and financial viability. Research studies that fail to meet recruitment goals provide minimal scientific return and may have a negative financial impact on the institution. The National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) requires investigators of clinical studies involving more than 150 subjects to regularly report recruitment milestone data.1 Multicenter trials reimburse institutions for investigator effort and study-associated costs on a per-patient basis. There is substantial up-front institutional investment in the initiation of each of these protocols (institutional review board [IRB] review, contract negotiations, administration). Inadequate recruiting may increase study costs, delay time to completion, and possibly invalidate a trial due to insufficient study power. For industry studies, recruitment problems translate into potential revenue losses associated with delays in bringing a new drug to market. This has been estimated to cost up to one million dollars per day.2,3 Thus, even modest improvements in the subject recruitment process may pay large dividends in accelerating the bench-to-bedside cycle of new drug therapies or medical devices. Despite these facts, the importance of subject recruitment in clinical research is often underestimated in academic medical centers, and institutional resources are rarely made available to facilitate subject recruitment.
A significant number of people remain committed to clinical research and actively seek to participate in clinical studies, but often have trouble finding relevant studies. Unsolicited requests from research volunteers are common events. Institutional telephone operators have a difficult time directing individuals to appropriate research opportunities, resulting in frustration of potential volunteers and delay in enrolling or loss of potential research subjects. The difficulty in identifying subjects for proposed and ongoing clinical research trials is heightened in the genomic era in which large numbers of subjects are needed to explore complex gene interactions.
A survey of commercial recruitment software and services resulted in no products capable of meeting our needs within the initial operating budget. Consequently, we sought to develop an effective subject recruitment model that would accommodate both volunteers and researchers. The model included redesigning the workflow associated with subject registration, volunteer information retrieval, and subject contact. This article reports our experience in developing a Web-based software application for a large-scale research subject recruitment registry.
Design Objectives and System Description
Primary project objectives were to (1) provide methods for potential subjects to register for current and future research studies, (2) provide efficient methods for researchers to review and contact potential volunteers, and (3) ensure compliance with regulations dealing with protection of confidential information during all phases of the project. In addition to redesigning workflow processes associated with capturing and disseminating subject information, we recognized early in the project that a software application could be used to automate much of the work. We first describe software development methods and then describe personnel and workflow methodology.
Software Design Requirements and Architecture
We designed a software solution to meet the following objectives:
Web-based architecture
Encrypted data exchange and firewall protection against intruders
Intuitive interface allowing potential volunteers a method to self-enter personal information concerning demographics and health-related interests
Record confirmation procedures allowing registry managers to quickly confirm self-entered information records, with automated e-mail confirmation postings to registrants during the approval process
Autonomous search and contact tools for researchers using the registry for subject recruitment purposes
Transparent usage log providing date-stamped documentation of viewing and electronic contact history
We chose a Web-based software architecture to maximize end-user accessibility and minimize end-user hardware and software operating system requirements. We built the software application using PHP and mySQL open-source programming resources based on software stability, institutional infrastructure support, and low implementation costs. PHP is a popular programming language used for building dynamic data-driven Web sites and is strongly supported by the open-source community.4 The open-source mySQL database engine is similarly stable and widely used for Web database applications.5 Web software resides on a secure production-level Apache Web server located behind university firewalls. Database software resides on a separate secure server where routine backup procedures are conducted daily. All Web-based data transmission is encrypted at the 128-bit level. Researcher access to personal subject information is accessible only through an intranet connection using institutional-level user authentication and application-level security role registration. Access is limited to researchers who submit a data confidentiality agreement and proof of active IRB-approved research project(s).
Personnel and Workflow Methodology
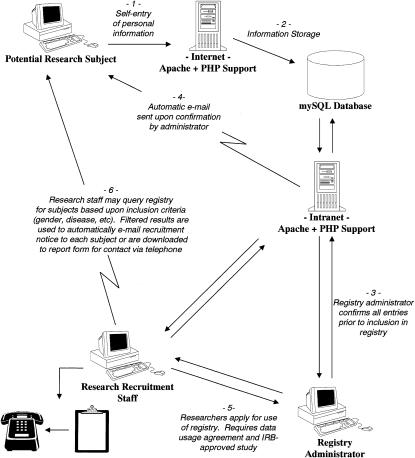
▶ illustrates basic workflow methodology developed for the recruitment registry process.
Step 1: Potential research subjects use a secure Internet connection to self-enter personal information indicating a willingness to volunteer for clinical research studies.
Step 2: Before their personal information is entered in the system database, registrants are required to approve plans for confidentiality and data usage.
Step 3: A registry administrator reviews each new submission and confirms or denies inclusion in the database before information is made available to researchers. The registry administrator may deny registry confirmation to persons posting obvious nonsensical information.
Step 4: The confirmation process includes an automated customized electronic mail posting sent to each subject acknowledging receipt.
Step 5: Researchers wishing to use the registry for recruitment must apply to the registry administrator. This process includes showing evidence of an active IRB-approved study and signing a data usage form agreeing to treat information as strictly confidential and to use the registry only for identification of potential subjects for participation in active IRB-approved studies.
Step 6: Researchers access the system using a secure, password-protected intranet connection and complete a form-driven survey that returns only those subjects meeting investigator-designated search criteria such as age, gender, and disease of interest. Once selection criteria are specified, potential subjects can be contacted by telephone, by individual e-mail contact, or by sending an e-mail posting containing an IRB-approved advertisement to all identified research subjects.
Figure 1.
Registration process diagram using central software application.
Initial Registry Seeding
A central issue in developing the recruitment registry was seeding the database with a critical mass of potential subjects prior to granting access to researchers. We employed passive advertising by providing information and links to the registry site from two prominent Vanderbilt University patient-oriented research sites: the General Clinical Research Center (www.mc.vanderbilt.edu/gcrc/) and the Clinical Trials Center (www.mc.vanderbilt.edu/ctc/). Additionally, we sent an IRB-approved e-mail advertisement for the registry to all e-mail addresses, approximately 14,000, within the Vanderbilt community (university and medical center). This single e-mail advertisement directed to the university community proved to be an effective tool, resulting in nearly 800 entry submissions within a two-week period (October 4 through October 18, 2002). Overall, we registered approximately 2,900 volunteer entries during the first 32 months of operation for the Volunteer for Vanderbilt Research Program.
Development of Alternative Submission Method
After successfully deploying the Web-based registry software, we developed a paper brochure for collection of information from potential subjects wishing to submit information via nonelectronic means. Brochures have been given to clinical managers throughout Vanderbilt University Medical Center outpatient units for distribution to clinic patients and visitors. Upon completion of the paper form, subjects sign confidentiality and data use agreement and are asked to return the completed form to Vanderbilt University. Upon return receipt to Vanderbilt, subject data are entered into the Web-based registry by administrative staff. Registration using the paper method has proven important in allowing inclusion via nonelectronic means. However, the number of registrants by paper form constitutes less than 1% of total subjects in the registry.
Institutional Review Board Approval
Data entry methods (Web site and volunteer brochure) were reviewed and approved by the Vanderbilt IRB. Data captured by these processes is included in ▶. The IRB review was helpful in auditing features that allowed subjects to edit or remove their personal information and refined the language concerning proxy registration of minors by a parent or legal guardian. The IRB review also ensured process compliance with HIPAA regulations dealing with protection and disclosure of confidential health information. The Web data entry screen and the brochure were designed to serve as both an information-gathering tool and consent document for potential research volunteers (see www.volunteer.mc.vanderbilt.edu/ to review specific language).
Table 1.
Data Requested from Potential Recruitment Volunteers
| Required Information | Optional Information Requested |
|---|---|
| First name* | Race |
| Last name* | Disease of interest no.1† |
| Street address* | Disease of interest no.2† |
| City* | Disease of interest no.3† |
| State* | Phone (night)* |
| Zip code* | E-mail* |
| Phone (day)* | Do you use tobacco? (yes/no) |
| Gender | Regular medications? (yes/no) |
| Date of birth | Have you participated in research before? (yes/no) |
| Height (feet/inches) | |
| Weight (lb) | |
| Are you “a normal volunteer”? (yes/no) |
Specific language can be found at www.volunteer.mc.vanderbilt.edu/.
Completed with parent or guardian information if user is submitting data for children younger than age 18.
Registrants may choose up to three diseases of interest from the following choices: addictive behavior, aging, allergies, arthritis, asthma, attention-deficit/hyperactivity disorder, blood disorders, brain, spinal cord and nervous system, breathing/lung, cancer, children's health, cholesterol, diabetes, digestive system, genetics, headaches, hearing/ear, heart and cardiovascular, hypertension/high blood pressure, infectious disease/immune system, kidney and urinary tract, liver disorders, men's health, mental health, muscle and bone, nutrition/metabolism, other, pain control, skin, sleep disorders, vision/eye, voice/speech disorders, weight control, women's health.
Status Report
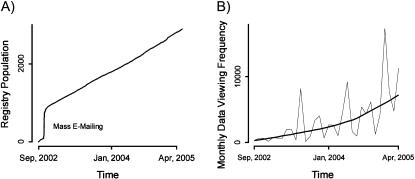
The Volunteer for Vanderbilt Research Program has proven successful in attracting potential subject response during the first 32 months of operation. ▶ shows registry population as a function of time. Clearly, the mass e-mailing to Vanderbilt University faculty, staff, and students succeeded in seeding the database with many potential volunteers. Discounting this effect, the registry grew by approximately 65 new entries per month with no marketing effort, relying only on passive links placed in the Vanderbilt University Web site.
Figure 2.
(A) Registry population versus time. Note the initial Vanderbilt Medical Center and campus e-mailing resulted in approximately 800 registrants. Since this time, the registry has averaged approximately 65 entries per month based largely on passive Web site advertisement. (B) Monthly data viewing frequency versus time. Viewing information was reported for each event in which an individual researcher was presented contact information for an individual subject registrant. Although cyclical, the general trend shows progressive use of the registry by researchers actively recruiting subjects for clinical studies.
Demographic Information Reporting
▶ provides descriptive statistics for 2,907 subjects registered during the period between August 22, 2002, and April 29, 2005. Not all questions were required fields for inclusion in the registry, and the questionnaire has evolved over time. Because not all questions were answered by all registry volunteers, we report the total number of respondents answering each question and percentages based on total respondents in each category.
Table 2.
Research Subject Responses to Information Requests
| Geographic location (total respondents = 2,907) | |
| Tennessee | 2,511 (86.4%) |
| Kentucky | 82 (2.8%) |
| Other states (39) | 314 (10.8%) |
| Gender (total respondents = 2,907) | |
| Male | 890 (30.6%) |
| Female | 2,017 (69.4%) |
| Race (total respondents = 2,786) | |
| White | 2,389 (85.8%) |
| African American | 226 (8.1%) |
| Asian | 55 (2.0%) |
| Hispanic | 46 (1.7%) |
| Other | 70 (2.5%) |
| Age (total respondents = 2,907) | |
| Mean ± SD | 34.4 ± 12.7 yr |
| BMI (total respondents = 2,893) | |
| Mean ± SD | 27.1 ± 7.3 |
| Inclusion of e-mail address (total respondents = 2,907) | |
| Yes | 2,705 (93.1%) |
| No | 202 (6.9%) |
| Healthy volunteer status (total respondents = 2,907) | |
| Yes | 2,172 (74.7%) |
| No | 735 (25.2%) |
| Regular medication status (total respondents = 2,723) | |
| Yes | 1,429 (52.5%) |
| No | 1,294 (47.5%) |
| Regular tobacco use (total respondents = 2,776) | |
| Yes | 557 (20.1%) |
| No | 2,219 (79.9%) |
| Twin status (total respondents = 253) | |
| Yes | 6 (2.4%) |
| No | 247 (97.6%) |
BMI = Body Mass Index.
We received registry submission information from 41 states. Not surprisingly, most respondents reported their home state as Tennessee (86.4%) or other surrounding states. Female respondents outnumber males by a factor of 2.3 (2,017 vs. 890 respondents). Most respondents (96%) answered the optional race category question. Registrants included an overrepresentation of white respondents (86% vs. 77%) and an underrepresentation of African-American or black respondents (8% vs. 16%) compared to United States Census 2000 data for Middle Tennessee (Davidson, Dickson, Robertson, Rutherford, Sumner, Trousdale, Williamson, Wilson counties).6 Other racial categories were similar to Census 2000 demographic data.
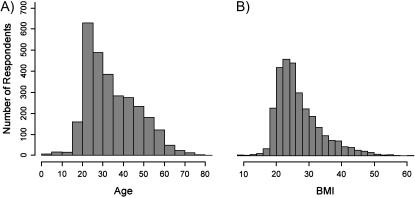
Subjects were asked to provide date of birth, weight, and height information during the submission process. These data are later used to compute age and subject body mass index (BMI) for use in filtering potential subjects based on specific study inclusion data. ▶ shows histogram data for respondent age and BMI. Age (34.4 ± 12.7 years: mean ± SD; median = 31.5 years) data are slightly skewed and may reflect a trend for subject volunteers to be younger than the general population. According to U.S. 2000 Census data, the median age for Tennessee residents was 35.9 years.6 Registry BMI data were also slightly skewed (27.1 ± 7.3 kg/m2: mean ± SD; median = 25.2 kg/m2). A recent survey indicates 58% of adult Tennesseans maintain BMI values greater than 25, which is higher than the 52.3% calculated from subjects in our registry.7 Healthy volunteer bias is a known phenomenon and must be considered during any subject recruitment process,8 but our data indicate that this may be an especially important consideration with registries populated largely through the Internet.
Figure 3.
Histogram representation of (A) age and (B) body mass index (BMI) data collected from subject response data. Age and BMI data are slightly skewed, indicating a potential for selection bias toward young, healthy volunteers.
Most respondents (93%) included an e-mail address when completing the Web-based survey. Most registrants (75%) also responded “yes” when asked whether they wished to be considered as a healthy volunteer. This number may be deceptively high because the language in our original entry form did not clearly identify subjects with medical conditions when asking the question. Our intent was to identify either healthy volunteers or individuals with a medical illness. Instead, we received entries from individuals who listed both medical conditions and a desire to participate as a healthy volunteer. Given this confusion, we changed the wording in the data entry form to make a clearer distinction.
Over half (53%) of respondents answering an optional question concerning regular medications answered “yes.” Approximately 20% of respondents answered “yes” to an optional question concerning tobacco usage, slightly less than the estimated 24.4% of individuals aged 18 and older in Tennessee believed to be current smokers.9 A question concerning twin status was added after initial deployment of the registry. Approximately 2% of those answering the question indicated twin status, a number similar to Tennessee twin prevalence estimates of 2% to 3%.10
Disease Reporting
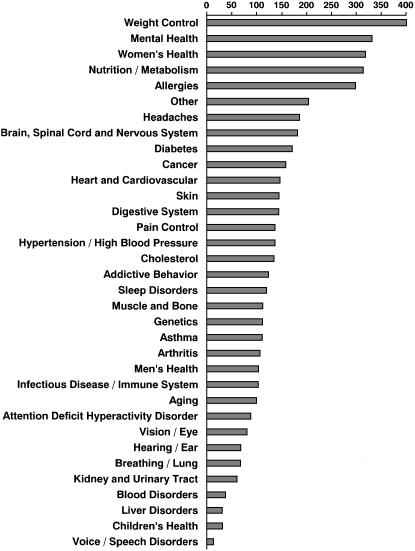
The registration process allowed individuals the option of choosing up to three diseases of interest from the list shown in ▶. The limit of three choices was made based on convenience, and these data fields were optional in the registration process. However, at least one choice was typically selected and the three-choice limit was not perceived as a limiting factor in registry operation or subject filtering criteria. Of 2,907 respondents, 68% of individuals indicated at least one disease of interest. The disease category frequency of occurrence distribution is shown in ▶. Combining data from all registrants resulted in the following five most frequently reported categories: weight control, mental health, women's health, nutrition and metabolism, and allergies. We perceive these choices to indicate general interest in health areas by subjects rather than being a reflection of medical specialties within our institution.
Figure 4.
Disease of interest. Of 2,907 respondents, 918 (32%) individuals volunteered no disease of interest, whereas 1,989 (68%) individuals indicated at least one disease of interest. Of those individuals who volunteered disease(s) of interest, each individual may have chosen up to three categories of interest. Disease categories are shown in order of frequency of selection.
Researcher Use of Volunteer Registry
Researcher subject selection software tools are intuitive and may be used autonomously by registered investigators and staff. End users narrow the field of registrants for contact by submitting filtering options based on common study inclusion and exclusion criteria (gender, race, healthy status, tobacco usage, medication status, age limits, BMI limits, and diseases of interest). Contact information is displayed so subjects can be contacted by surface mail, telephone, or e-mail. The application provides researchers with the option to simultaneously send an IRB-approved e-mail posting to all filtered registrants giving study details and contact information, thereby requesting potential subjects to contact researchers if interested in the study.
A decision was made early in the design process to offer the volunteer registry application as an institutional resource rather than attempting to charge researchers a fee for usage. As a result of this decision, we did not build mechanisms to track the actual number of subjects enrolled into trials as a result of the registry. We did, however, log registry utilization access history and counted a viewing event whenever an individual researcher gained access to an individual subject's contact information. ▶B shows monthly subject data viewing event counts for a total of 70 research users. Although cyclical, the general trend shows progressive use of the registry by scientists actively recruiting subjects for clinical studies.
We recently administered an anonymous survey to researcher end users to gauge perception and effectiveness of the registry. A total of 28 research personnel responded to the survey request and indicated that they had used the registry. Overall perception ranking among respondents was 7.5 ± 2.1 on a scale of 0 (worst) to 10 (best). Approximately 89% reported they had recruited subjects using the registry. This group reported an estimated average of 17 subjects recruited (minimum = 2, maximum = 100, total = 434) and indicated that approximately 51% of those recruited were recruited as normal volunteers. A majority (68%) indicated that the registry had saved them time in the recruitment process. The average time savings reported per recruited subject was 51 ± 33 minutes (minimum = 15 minutes per subject, maximum = 120 minutes per subject).
In addition to the actual recruitment process, the registry has proven useful to investigators preparing grant applications involving the recruitment of large cohorts of patients with given characteristics. Grant applications are strengthened by providing accurate estimates of the patient population readily available for recruitment. Furthermore, grant reviewers are reassured that the study population is in place to successfully complete clinical research studies.
Discussion
Registry Use and Relevance
The subject recruitment registry has proven a successful method for collecting information on potential research volunteers. Although rudimentary, our recent anonymous survey to researcher end users indicated a generally good perception ranking for the registry and also that the tool was effectively being used to recruit subjects into clinical research trials. Voluntary submission of information by the general public has exceeded expectations. Advertising to the Vanderbilt community was a very effective method to populate the registry, but may have resulted in a relatively higher percentage of healthy, educated volunteers. The high enrollment of women was not entirely unexpected, as females tend to have a favorable impression of clinical research.11 We hypothesize that easily accessible registries, such as the one described, may help improve recruitment of women in clinical trials. However, we may need to devise additional targeting strategies to improve recruitment of minorities and underserved populations, a significant and multifactorial problem due in part to limited access to care and technology, societal issues, and possibly questions of trust.11,12
The new recruitment approach has several advantages over traditional methods. Subjects in the registry are, in effect, self-selected toward participating in research and, therefore, are more likely to enroll in studies when contacted by researchers. Researcher tools are available to filter subjects based on study-specific criteria such as age, race, gender, disease interest, BMI, height, and weight. Limiting initial contact data to subjects identified as wishing to participate in trials and meeting rudimentary inclusion criteria significantly increases the odds of participation. Furthermore, sending an e-mail posting (with IRB-approved study information content) to a group of potential candidates is a very effective screening tool when compared to other recruitment contact methods.
Project Cost of Development and Ownership
This project evolved over a period of several months and was preceded by other recruitment registries developed and maintained at Vanderbilt University. We were fortunate to secure shared access to centralized medical center Web and database servers, thereby reducing dedicated computer hardware costs for this project. By making the decision to use open-source programming tools for software development, we also eliminated software purchase and licensing costs. One programmer dedicated approximately 200 hours to develop and refine the software application and information workflow processes. Although initial application development time was significant, programming support for project maintenance has proven minimal in scope. Ongoing administrative support includes (1) confirming individual registrant information prior to official inclusion in registry, (2) distribution and data entry for paper brochures, (3) interaction with researchers wishing to gain access to registry search and contact tools, and (4) periodic correspondence with the IRB for ongoing review and other regulatory issues. We estimate approximately one to two hours per week are needed to maintain the registry. Design and printing costs associated with the paper brochure have been approximately $5,000 to date and were provided by the Vanderbilt University Office of Medical Communications.
Recommendations for Other Centers
During the first 32 months of operation, we received numerous requests from other institutions wishing to develop similar methods to assist with research subject recruitment. Before committing resources, institutions often require a plan to recover implementation costs (such as charging investigators for individual project use of a recruitment registry). At Vanderbilt, we elected to design an informatics solution with low development and maintenance costs so that we could offer the service as an institutional resource to anyone conducting IRB-approved research within the university. We track data-viewing history for reasons of privacy and confidentiality, but do not track the number of enrolled subjects for reasons of cost sharing. This decision greatly reduced the complexity of software and workflow processes and the maintenance cost of operating the registry has proven very minimal.
We propose the following recommendations to other academic centers wishing to develop similar methods.
Develop information flow and exchange methodology first and then design a software solution to fit this model if commercial software is not possible.
Identify and use institutional resources to defray hardware and software costs. Partner with a general clinical research center if available.
Consult IRB and HIPAA experts early in the project development stage.
Ensure authentication and data security procedures are in place at all stages of project development.
Develop clear policies for registry use by investigators.
Balance the need to keep public registrant data entry as simple as possible with the goal of collecting sufficient information to allow researchers to prescreen subjects most likely to qualify for participation.
Make the system as autonomous and self-sustaining as possible.
Instruct university clinical personnel and operators to encourage potential volunteers who inquire about research studies to use the registry.
Conclusions
The Volunteer for Vanderbilt Research Program has proven successful in fulfilling initial goals of (1) providing a means for individuals to self-enter personal information related to their willingness to participate in clinical research studies and (2) providing a mechanism for researchers to select and contact potential volunteers based on study-specific inclusion criteria. Implementation time proved significant, but ongoing maintenance costs are very low and provide a much-needed service freely offered to all researchers conducting IRB-approved studies at Vanderbilt University. The electronic subject recruitment registry will not replace traditional recruitment methods and was not designed as an all-encompassing research enterprise software package capable of handling all phases of clinical research projects. However, the workflow methodology and informatics application have proven to be an effective complement to the recruiting process at our institution. Among future plans for improving the registry, we will consider active advertising campaigns directed toward specific patient populations. We will also strive to integrate the system into other Vanderbilt informatics–based systems in efforts to streamline the study recruitment process.
This work was supported by NCRR grant 5M01-RR00095.
The authors gratefully acknowledge Jerry Zhao, Vanderbilt University Medical Center Webmaster, for assistance with computing resources. They also appreciate the assistance of Joel Lee, Associate Vice Chancellor, Vanderbilt University Medical Center Communications, for help in publishing information regarding the registry.
References
- 1.National Heart, Lung, and Blood Institute. Terms and conditions for accrual of research subjects in research. Available from: http://www.nhlbi.nih.gov/funding/policies/terms.htm.
- 2.Marks RG, Conlon M, Ruberg SJ. Paradigm shifts in clinical trials enabled by information technology. Stat Med. 2001;20:2683–96. [DOI] [PubMed] [Google Scholar]
- 3.Marks L, Power E. Using technology to address recruitment issues in the clinical trial process. Trends Biotechnol. 2002;20:105–9. [DOI] [PubMed] [Google Scholar]
- 4.PHP Hypertext Preprocessor. Available from: http://www.php.net/.
- 5.mySQL Database Engine. Available from: http://www.mysql.com/.
- 6.Census 2000. Washington, DC: United States Census Bureau, 2000.
- 7.Tennessee Health Status Report 2001–2002. Tennessee Department of Health; 2003. Nashville, TN.
- 8.Ganguli M, Lytle ME, Reynolds MD, Dodge HH. Random versus volunteer selection for a community-based study. J Gerontol A Biol Sci Med Sci. 1998;53:M39–46. [DOI] [PubMed] [Google Scholar]
- 9.Cancer prevention and early detection—facts and figures 2003. Atlanta, GA: American Cancer Society, 2003.
- 10.Martin JA, Park MM. Trends in twin and triplet births: 1980–97. Natl Vital Stat Rep. 1999;47:1–16. [PubMed] [Google Scholar]
- 11.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–63. [DOI] [PubMed] [Google Scholar]
- 12.Chang BL, Bakken S, Brown SS, Houston TK, Kreps GL, Kukafka R, et al. Bridging the digital divide: reaching vulnerable populations. J Am Med Inform Assoc. 2004;11:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]