Abstract
The human TOP3α gene encoding DNA topoisomerase IIIα (hTop3α) has two potential start codons for the synthesis of proteins 1,001 and 976 aa residues in length. The sequence of the N-terminal region of the 1,001-residue form resembles signal peptide sequences for mitochondrial import, and fluorescence microscopy shows that the addition of as few as the first 34 aa of the 1,001-residue form of hTop3α to a green fluorescent protein can direct the chimeric protein to mitochondria. Biochemical analyses of subcellular fractions of HeLa cells further demonstrate that a distinctive fraction of hTop3α is present inside mitochondria, as evidenced by its resistance to proteinase K. This fraction constitutes several percent of the enzyme in the nuclear fraction, suggesting that the distribution of the mitochondrial and nuclear forms of hTop3α is roughly in proportion to the DNA contents of these cellular compartments. The presence of a type IA DNA topoisomerase in the mitochondria of other eukaryotes is supported by an examination of the amino acid sequences of mouse and Drosophila DNA topoisomerase IIIα and Schizosaccharomyces pombe DNA topoisomerase III. Given the presence of at least one type IA DNA topoisomerase in all forms of life examined to date, the finding of a type IA enzyme in mitochondria further supports the notion of a key role of such enzymes in DNA transactions.
It is well known that nearly all cellular transactions of DNA involve one or more DNA topoisomerases (1–3). In human and mouse, the presence of six DNA topoisomerases belonging to the three subfamilies IA (DNA topoisomerases IIIα and IIIβ), IB (DNA topoisomerase I and mitochondrial DNA topoisomerase I), and IIA (DNA topoisomerases IIα and IIβ) is well known (4). In addition, mammalian orthologues of the yeast SPO11 gene product, which shares sequence homology with the A-subunit of type IIB DNA topoisomerases and is involved in the formation of double-stranded DNA breaks during meiotic recombination (5, 6), have been identified (7).
In mammalian cells, mitochondrial DNA (mtDNA) represents the only genetic material outside the nucleus. A typical mammalian cell has about a thousand mitochondria, each containing multiple copies of mtDNA in the form of a covalently closed ring 16–18 kb in length (8). Given the multiple roles of DNA topoisomerases in DNA metabolism, it has long been thought that they are present in the mitochondrial matrix where mtDNA resides. Especially for a ring-shaped mtDNA, separation of the intertwined parental DNA strands during replication would seem impossible in the absence of a DNA topoisomerase. Indeed, biochemical studies of purified mitochondria from a variety of sources have implicated the presence of a type IB DNA topoisomerase with properties different from those of the nuclear enzyme DNA topoisomerase I (9–12). The presence of a distinctive type IB DNA topoisomerase in mitochondria has received strong support by the identification of a human chromosomal gene encoding a mitochondria-targeting type IB DNA topoisomerase (13).
Several studies have also implicated the presence of a type IIA DNA topoisomerase in the mitochondria of a number of organisms. The strongest evidence came from studies of the protozoan parasites Crithidia fasciculata and Trypanosoma brucei. The single mitochondrion of these organisms, termed the kinetoplast, contains a unique mtDNA with thousands of DNA rings topologically linked into a planar network (14). The presence of a type IIA DNA topoisomerase in the kinetoplast was initially suggested by immunolocalization experiments (15), and recent RNA interference experiments have indicated that inhibition of DNA topoisomerase II leads to shrinkage and loss of the kinetoplast DNA network and cessation of parasite growth (16). The presence of a type IIA DNA topoisomerase in the slime mold Dictyostelium discoideum was suggested by the cloning of a gene that encodes a 1,282-aa protein that shares sequence similarities with the known type IIA DNA topoisomerases and possesses a mitochondria-targeting signal at its N terminus (17). Partial purification of DNA topoisomerase activities from subcellular fractions has also implicated the presence of the type IIA DNA topoisomerases in the mitochondria of various organisms and tissues, including Plasmodium falciparum (18) and bovine heart (19).
Conspicuously missing in the literature are reports of any type IA DNA topoisomerase in mitochondria. The type IA DNA topoisomerases are among the most conserved proteins in nature, and their presence in all organisms is supported by extensive biochemical and genomic sequence data (3, 4). This universal presence suggests that the type IA DNA topoisomerases play an indispensable role in one or more fundamental processes involving DNA, plausibly in the removal of double Holliday junctions (4). Thus, it seemed plausible that a type IA DNA topoisomerase would be present in mitochondria. We report here experiments addressing this possibility, and our finding that human DNA topoisomerase IIIα (hTop3α) is localized to mitochondria as well as the nucleus. In contrast, a closely related type IA enzyme, human DNA topoisomerase IIIβ (hTop3β), is likely a nuclear entity absent in the mitochondria.
Materials and Methods
Construction of Plasmids Expressing hTop3α and hTop3α Fragments Fused to a Green Fluorescent Protein.
The entire human TOP3α ORF was amplified by the PCR, using pGEM-hTOP3 (20) as the template and a pair of primers 5′-AAGTAACTCGAGGCCACCATGATCTTTCCTGTCGCCCG-3′ (the forward primer) and 5′-CAATTCCGCGGTCTGTTCTGAGGACAAAAGG-3′ (the reverse primer). The underlined hexamer sequences indicate a XhoI and SacII restriction site that were placed in the primers to facilitate subsequent cloning of the PCR product. Several nucleotides immediately preceding the ATG initiation codon (in bold in the forward primer) were chosen to provide a preferred sequence for the initiation of translation (21). The 3-kb PCR products were digested with XhoI and SacII, and inserted in between the corresponding sites in a vector pEGFP N1 (CLONTECH). The resulting plasmid, phTOP3α-enhanced green fluorescent protein (EGFP), expresses a fusion protein hTop3α(1–1001)-EGFP with a green fluorescent protein EGFP linked to the C-terminal end of human DNA topoisomerase IIIα; because of the particular cloning strategy used, 10 extra amino acid residues were also introduced in between the two component polypeptides. All other plasmids expressing various fragments of hTop3α fused to EGFP were similarly constructed.
HeLa Cell Culture and Transfection.
HeLa cells were cultured in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% (vol/vol) FBS (HyClone), 2 mM L-glutamine (Life Technologies), 100 units/ml penicillin, and 100 μg/ml streptomycin (Life Technologies). Cells were incubated at 37°C in a humidified chamber supplemented with 5% CO2. Transfection of various htop3α-EGFP fusion constructs into the cells was performed with a commercial kit (Qiagen, Chatsworth, CA).
Confocal Microscopy.
Twenty-four to forty-eight hours after transfection, HeLa cells grown on coverslips of microscope slides were placed in fresh medium containing 500 nM of a dye MitoTracker Red (Molecular Probes) that specifically stains mitochondria, and incubated for another 40 min. Cells were subsequently fixed with 4% paraformaldehyde at room temperature for 10 min, and then treated with ice-cold methanol for 2 min. After washing four times with PBS, nuclei were stained with 4,6-diamino-2-phenylindole (1 μg/ml). Coverslips were then mounted onto slides with 0.64 g/ml 1,4-diazabicyclo-octane and 22% glycerol in PBS, and visualized in a digital confocal microscope (Zeiss Axioplan 2). Images of cells were captured by using a CCD camera with appropriate filters.
Subcellular Fractionation.
The nuclear, mitochondrial, and cytosolic fractions were obtained from a total of 18 confluent 150 × 25 mm tissue culture dishes of HeLa cells, as described (22). Unless stated otherwise, all operations were performed on ice. Cells were washed in ice-cold PBS, scraped from the dishes, resuspended in 20 ml of a mitochondria isolation buffer (MIB) consisted of 0.3 M sucrose, 1 mM EGTA, 5 mM Mops, 5 mM KH2PO4, and 0.1% BSA (pH 7.4), and subjected to 60 strokes in a Dounce homogenizer. Disrupted cells were centrifuged twice at 2,600 × g for 7 min to pellet unlysed cells and nuclei. The supernatant fraction (S1) was kept on ice. The pellets were resuspended in 20 ml of a buffer containing 10 mM KCl, 10 mM MgCl2, 10 mM Tris⋅HCl, pH 7.4, and 10 mM DTT, and subjected to an additional 50 strokes of homogenization. Pellets were again collected by centrifugation as before, and washed with 20 ml of MIB. Centrifugation was repeated once more, and the pellets were resuspended in a total volume of 8 ml of MIB to give the “nuclear fraction.” The supernatant fraction S1 was centrifuged at 15,000 × g for 10 min to pellet mitochondria, and the supernatant was saved as the cytosolic fraction. The pelleted crude mitochondria fraction was washed with 20 ml of MIB, and pelleted once more before resuspending in 3 ml of MIB containing 12.5% Percoll (Sigma). This fraction was layered on top of a Percoll step-gradient consisting of 3.5 ml each of 25% and 40% Percoll in MIB. The Percoll gradient was centrifuged at 30,000 × g for 15 min in an SW40 rotor (Beckman), and ≈1 ml of purified mitochondria were collected from the region between the 12.5% and 25% Percoll layers (23). This mitochondrial fraction was then diluted 4-fold in MIB, and centrifuged at 15,000 × g for 10 min to remove Percoll. The pellet was resuspended in 1 ml of MIB; centrifugation was repeated once more, and the pellet was resuspended in 200 μl of MIB. This “purified mitochondrial fraction” was checked for cytochrome c oxidase activity (24), and was found to contain intact mitochondria. Fresh preparations were used without delay in the experiments described below.
Proteinase K Sensitivity Assays.
The nuclear, mitochondrial, and cytosolic fractions, all in MIB, were subjected to proteinase K treatment. For each subcellular fraction, a set of three tubes, each containing 50 μl of a sample, were placed on ice. To each of the triplets, 50 μl of 0.6 M mannitol and 10 mM Tris⋅HCl, pH 7.4, were added to the first tube, and 50 μl of the same buffer containing 1 mg/ml proteinase K were added to each of the second and third tubes. After mixing, 1 μl of Triton X-100 (1% final concentration) was mixed into the third tube sample to disrupt mitochondrial membranes. All mixtures were kept on ice for 30 min before the addition of 2 μl of 100 mM freshly prepared PMSF to inhibit proteinase K. Samples were then placed in a boiling-water bath for 10 min, and 25 μl of a SDS-gel-loading buffer (315 mM Tris⋅HCl, pH 6.8/5% SDS/700 mM 2-mercaptoethanol/0.05% bromophenol blue/50% glycerol) were added to each tube. Electrophoresis of the samples was performed in SDS-polyacrylamide (8 or 12%) gels.
Immunoblotting Analyses.
After electrophoresis, proteins were transferred electrophoretically to nitrocellulose membrane (Schleicher & Schuell), and the gel blots were separately or sequentially probed with the following antibodies: rabbit anti-human DNA topoisomerase IIIα and IIIβ (kindly provided by T.-S. Hsieh, Duke University), rabbit anti-Tom20 and anti-cytochrome c (Santa Cruz Biotechnology, 1:500 dilution), mouse anti-proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology, 1:2,000 dilution), mouse anti-mitochondrial hsp70 (Affinity BioReagents, Golden, CO; 1:2,000 dilution), and rabbit anti-GFP (CLONTECH; 1:800 dilution). The Cruz Marker (Santa Cruz Biotechnology) and MagicMark (Invitrogen) were used as protein molecular mass standards. Peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibodies (Santa Cruz Biotechnology) were used at a dilution of 1:10,000. The protein bands were finally visualized by chemiluminescence, using reagents purchased from Pierce.
Results
Human TOP3α Gene Seems to Encode a Mitochondrial and a Nuclear Form of DNA Topoisomerase IIIα.
As described in the introduction, we reasoned that if a type IA DNA topoisomerase is required for the proper processing of intracellular DNA, then one or more of such enzymes should be present in mitochondria as well as the nucleus. We therefore searched for mitochondria-targeting signals in a number of type IA DNA topoisomerases of known sequences.
Of particular interest is the amino acid sequence of mammalian DNA topoisomerase IIIα. When the human TOP3α gene was identified in 1996 (20), two potential in-frame ATG starts separated by 24 codons were noted; initiation of translation at the first ATG would yield a 1,001-residue protein, to be referred to as hTop3α(1–1001), and initiation at the second would yield a 976-residue protein hTop3α(26–1001). Because the sequence context at the second ATG appeared to be more favorable for initiation of translation, it was thought that the major product of human TOP3α was probably the 976-residue form (20). The codons spanning the two in-frame ATGs exhibit several features reminiscent of those encoding a mitochondria-targeting signal (Fig. 1). These codons translate into a peptide abundant in positively charged amino acids, arginine in particular. Secondary structure prediction of this N-terminal peptide (25, 26) suggested the formation of an amphipathic α-helix, and analysis of the hTop3α(1–1001) amino acid sequence by an algorithm for predicting mitochondria-targeting proteins (27) indicated a high probability (83%) of mitochondrial localization. In contrast, human topoisomerase IIIβ, a protein highly homologous to IIIα but lacking the N-terminal 25 residues of the latter, only showed a 30% probability of mitochondria import.
Fig 1.
Human Top3α(1–1001) has a putative mitochondria-targeting sequence at its N terminus. The N-terminal 35 aa residues of hTop3α(1–1001) are shown. The two plausible initiation codons are marked in bold fonts, and the underlined sequence represents an amphipathic α-helical region predicted by the program PHDSEC (25, 26). Positively charged amino acids are marked by plus signs, and the arrow bisecting the RAFS region marks the most likely mitochondrial endopeptidase cleavage site.
Fusion of N-terminal Fragments of hTop3α(1–1001) to a Green Fluorescent Protein Yields Products That Localize to Mitochondria.
To test further the presence of a mitochondrial localization signal at the N terminus of hTop3α(1–1001), two plasmids expressing fusion proteins joining residues 1–261 and 1–655 of hTop3α(1–1001) to the N terminus of a green fluorescent protein EGFP were constructed. These fragments of hTop3α(1–1001) were expected to form distinct structural domains based on the known crystal structures of the type IA DNA topoisomerases (28, 29), and they were thus selected for fusion to EGFP.
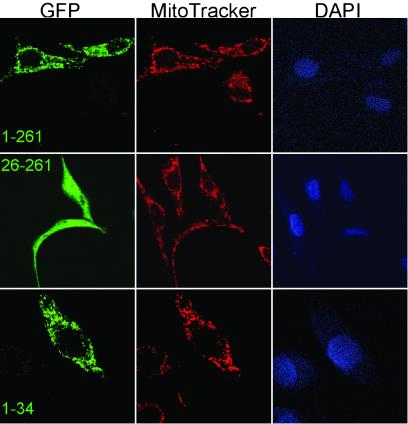
The two hTop3α constructs and a control expressing EGFP itself were transfected into HeLa cells, and the emission of green fluorescence in various subcellular compartments was examined in a confocal fluorescence microscope. Cells transfected with the control plasmid exhibited a uniform presence of green fluorescence throughout the entire cell, as reported by many others (data not shown). In contrast, cells expressing the two fusion proteins hTop3α (1–261)-EGFP and hTop3α (1–655)-EGFP exhibited a punctated EGFP-fluorescence pattern that largely coincided with the staining pattern of the mitochondria-specific dye Mito-Tracker (Fig. 2 Top and data not shown). The fusion protein hTop3α (26–261)-EGFP showed a pattern similar to EGFP itself, and the larger N-terminal deletion construct hTop3α (26–655)-EGFP showed a uniform rather than punctated cytosolic distribution (Fig. 2 Middle and data not shown).
Fig 2.
Subcellular localization of N-terminal fragments of hTop3α fused to EGFP. Fluorescence images of HeLa cells, transiently transfected with the respective construct indicated in the leftmost image of each row, are depicted. The three images of each row show the patterns of the green EGFP fluorescence (Left), the mitochondria-specific dye MitoTracker Red (Center), and the DNA-specific reagent 4,6-diamino-2-phenylindole (DAPI) that prominently stains the nuclei (Right).
The results above provide strong evidence for the presence of an N-terminal mitochondrial localization signal in hTop3α(1–1001). This notion was further supported by the subcellular localization patterns of the fragments hTop3α (1–34), hTop3α (1–63), and hTop3α (26–63), all fused to EGFP. Together, all experiments with various fusion proteins indicate that the first 34 aa residues of hTop3α(1–1001) are sufficient to direct a fusion protein to mitochondria (Fig. 2 Bottom and results not shown), and none of the fusion proteins lacking the first 25 aa residues of hTop3α(1–1001) exhibited mitochondrial localization.
Cleavage of the Mitochondria-Targeting Signal Peptide of hTop3α(1–1001).
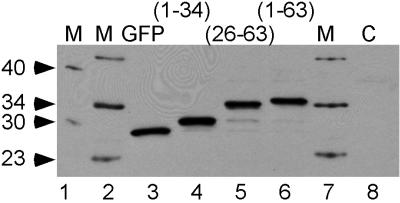
For the nuclear encoded mitochondria proteins with an N-terminal mitochondria-targeting signal, the signal peptide is usually cleaved off the precursor by mitochondrial endopeptidases immediately after import of the proteins into mitochondria (30). To test whether the putative N-terminal mitochondria-targeting signal peptide of hTop3α(1–34)-EGFP and hTop3α(1–63)-EGFP is clipped off after their import, we examined the sizes of these fusion proteins, and the sizes of hTop3α(26–63)-EGFP and EGFP itself, by SDS-gel electrophoresis of proteins recovered from transiently transfected HeLa cells. The protein bands after gel electrophoresis were transferred to a nitrocellulose membrane, and probed with anti-GFP antibodies. As shown in Fig. 3, protein extracted from untransfected HeLa cells gave no immunoreactive band (lane 8), and each of the samples extracted from the same cells expressing EGFP, hTop3α(1–34)-EGFP, hTop3α(26–63)-EGFP, or hTop3α(1–63)-EGFP displayed one major protein band (lanes 3–6). Relative to EGFP, the apparent molecular mass of the three fusion proteins hTop3α(1–34)-EGFP, hTop3α(26–63)-EGFP, and hTop3α(1–63)-EGFP was found to increase by 2.6, 5.3, and 5.8 kDa, respectively. On the other hand, the calculated molecular masses of the hTop3α peptides hTop3α(1–34), hTop3α(26–63), and hTop3α(1–63), plus that contributed by the decameric linker peptide in these fusion constructs, are 5.2, 5.3, and 8.3 kDa, respectively. Thus, for the two fusion proteins containing the putative mitochondria-targeting signal peptide, hTop3α(1–34)-EGFP and hTop3α(1–63)-EGFP, their molecular masses were apparently reduced by about 2.5–2.6 kDa from those of the intact fusion proteins. This finding in turn suggested that the pair had been subjected to proteolytic cleavage around residue 22. The peptide sequence around this region contains a motif Arg-X-X-Ser (residue19–22). Such a motif has been found in several mitochondria-targeting signals, and cleavage is known to occur at its center after mitochondrial import of the proteins (31, 32). Thus cleavage of hTop3α(1–1001) localized to mitochondria is likely to occur between amino acid residues 20 and 21 (see Fig. 1).
Fig 3.
Cleavage of N-terminal fragments of hTop3α(1–1001) fused to EGFP on mitochondrial import. Whole-cell lysates from HeLa cells transfected with constructs expressing EGFP, hTop3α (1–34)-EGFP, hTop3α (26–63)-EGFP, and hTop3α (1–63)-EGFP (lanes 3–6) were analyzed by electrophoresis in a SDS-polyacrylamide gel, and probed with anti-GFP antibodies. Lanes 1, 2, and 7 contained molecular-mass standards; the known molecular masses of the bands (in kilodaltons) are indicated in the left-hand margin. Lane 8 contained a control lysate from untransfected HeLa cells.
Full-Length hTop3α(1–1001)-EGFP Fusion Protein Is Primarily Localized to the Nucleus.
The results above show that when various N-terminal fragments of hTop3α(1–1001) are fused to EGFP, the fusion proteins are exclusively localized to the mitochondria. Thus, synthesis of these fusion proteins most likely starts predominantly from the first rather than the second ATG codon of the TOP3α ORF. This inference is reasonable because the particular expression constructs used in these experiments had been engineered for efficient initiation of translation at the first ATG codon of the fusion proteins (see Materials and Methods).
In contrast, the majority of HeLa cells transfected with a similar construct that expressed intact hTop3α(1–1001) fused to EGFP displayed a predominantly nuclear pattern of green fluorescence (data not shown). The major difference between the expected fusion protein product hTop3α(1–1001)-EGFP and the other fusion protein products containing the mitochondria-targeting signal, such as hTop3α(1–655)-EGFP, is most likely the presence of a nuclear localization signal in the C-terminal region of hTop3α(1–1001). Analysis of the amino acid sequence of hTop3α by the PSORT program (33) suggests that the regions spanning residues 656–672 and 965–983 are likely to specify nuclear localization. Indeed, the presence of at least one nuclear localization signal within the C-terminal 349 aa of hTop3α was supported by the finding that a fusion protein joining hTop3α(653–1001) to EGFP is predominantly localized to the nucleus (data not shown).
Our results with the hTop3α-EGFP fusion proteins therefore indicate that those proteins possessing a mitochondrial but no nuclear targeting signal are predominantly localized to the mitochondria, and those proteins possessing a mitochondrial as well as a nuclear localization signal are predominantly localized to the nucleus. To confirm this finding further, two additional plasmids were constructed for the expression of two derivatives of hTop3α(1–1001)-EGFP. In one of the pairs, the first ATG codon was changed to ATC, a codon for isoleucine; in the second, both the second and third ATG (codons 26 and 28) were changed to ATC. In both instances, the fusion protein localized predominantly to the nucleus (data not shown). Thus, even when translation is expected to initiate exclusively from the first ATG to yield a protein with a mitochondria-targeting signal, localization of the fusion protein was primarily in the nucleus.
Curiously, in the experiments above, a few percent of transfected HeLa cells expressing hTop3α(1–1001)-EGFP, or its derivative in which the second and third methionine were replaced by isoleucine, the green fluorescence pattern coincided with the mitochondria-specific dye Mito-Tracker (data not shown). None of the cells expressing the other derivative, in which the first ATG codon of hTOP3α was changed to ATC and therefore the protein product was not expected to possess a mitochondria-targeting signal, was observed to show mitochondrial localization. The significance of these observations is not clear, and it is plausible that nuclear transport of proteins might be defective in transfected cells that exhibited exclusive mitochondrial localization of hTop3α(1–1001)-EGFP. This type of uneven partition of a protein in the mitochondria and nuclei of different populations of transfected cells was observed for human DNA ligase III (34).
A Significant Fraction of Endogenous Human DNA Topoisomerase IIIα Is Present Inside Purified Mitochondria, as Evidenced by Proteinase K Sensitivity Assays.
Whereas studies of various hTop3α-EGFP fusion proteins clearly indicate the presence of a mitochondria-targeting signal at the N terminus of hTop3α(1–1001), this information alone could not answer the question whether a significant fraction of endogenous DNA topoisomerase IIIα would be localized to the mitochondria. Two issues contributed to this uncertainty. First, in the endogenous human TOP3a gene, the ATG start for the putative 1,001-residue protein is embedded in a rather poor sequence context for translational initiation, and therefore the 1,001-residue form of the enzyme is likely to be a small fraction of the gene products. Second, even for this small fraction, the presence of both the nuclear and mitochondrial localization signals may result in a predominant nuclear localization of the protein, as suggested by our results with hTop3α(1–1001)-EGFP.
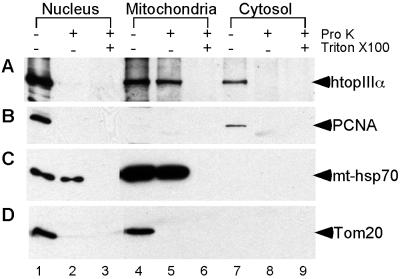
To test whether the presence of a mitochondria-targeting signal peptide in hTop3α(1–1001) effects an authentic localization of DNA topoisomerase IIIα to mitochondria, subcellular fractionation of HeLa cell extracts was performed. Mitochondria were separated from the nuclear and cytosolic fractions by differential centrifugation, and further purified by sedimentation through a Percoll step-gradient. The mitochondrial preparation was found to be enriched for the known mitochondrial proteins mt-hsp70 (compare lanes 1, 4, and 7 of Fig. 4) and cytochrome c (data not shown). Furthermore, the nuclear protein PCNA was absent in the purified mitochondria fraction (compare lanes 1, 4, and 7 of Fig. 4), suggesting a rather low level of nuclear contamination in our mitochondria preparation. The nuclear fraction was contaminated with mitochondria, however, as indicated by the presence of a significant portion of mt-hsp70 in this fraction (compare lanes 1 and 4 of Fig. 4).
Fig 4.
Presence of endogenous human DNA topoisomerase IIIα in both the nucleus and mitochondria. HeLa cells were collected and separated into nuclear (lanes 1–3), mitochondrial (lanes 4–6), and cytosolic fractions (lanes 7–9), as described in Materials and Methods. For each subcellular fraction, the samples were incubated for 30 min on ice in the absence of proteinase K (lanes 1, 4, and 7), in the presence of proteinase K (lanes 2, 5, and 8), or in the presence of both proteinase K and Triton X-100 (lanes 3, 6, and 9), as indicated on top of the figure. (The plus sign indicates the presence and the minus sign the absence of the reagent specified in the rightmost column; pro K, proteinase K.) The treated samples were then subjected to SDS/PAGE and the resolved protein bands were immunoblotted by using antibodies against human Top3α (A), PCNA (B), mt-hsp70 (C), and Tom20 (D). In each case, only one prominent band was detected, and its apparent molecular mass agreed with that expected of the target of the antibodies used (marked in the right-hand margin).
The bulk of hTop3α was detected in the nuclear fraction, but a significant amount of the protein was also found in the purified mitochondrial and cytosolic fractions (compare lanes 1, 4, and 7 of Fig. 4). To confirm that a significant fraction of hTop3α is present inside mitochondria rather than adventitiously associated with the outer surface of the organelle, proteinase K sensitivity tests were performed. Proteinase K is a nonspecific serine protease that can traverse nuclear pores but not mitochondrial membranes. Because of these properties, proteinase K treatment of purified mitochondria has often been used to assess the mitochondrial localization of a protein (22, 35–37). As shown in Fig. 4, hTop3α in the mitochondrial fraction and the same protein in the nuclear and cytosolic fractions differed markedly in their proteinase K sensitivity. In the nuclear and cytosolic fractions, hTop3α was readily digested by proteinase K (Fig. 4, lanes 1, 2, 7, and 8); in contrast, hTop3α in the mitochondrial fraction was largely resistant to the same treatment (Fig. 4, lanes 4 and 5). Upon the addition of a detergent Triton X-100 known to disrupt mitochondrial membranes, however, hTop3α in the mitochondrial fraction was rendered proteinase K-sensitive (Fig. 4, compare lanes 5 and 6). In the same set of experiments, the proteinase K sensitivity of the nuclear protein PCNA, the mitochondrial protein mt-hsp70, and a third protein Tom20, a major receptor of the mitochondrial precurser protein transport system that binds to the outer membrane through its N-terminal domain (38), was also examined. PCNA and Tom20, in either the nuclear or the cytosolic fraction, were readily digested (Fig. 4, lanes 1, 2, 4, and 5). In contrast, mt-hsp70, whether found in the purified mitochondria or in the mitochondria-contaminated nuclear fraction, was resistant to proteinase K digestion unless the detergent Triton-X 100 was added (Fig. 4, lanes 1–3 and 4–6).
The fraction of DNA topoisomerase IIIα inside mitochondria was estimated from the intensity of the hTop3α band of the purified mitochondria fraction and that of serial dilutions of the nuclear fraction, by using the mitochondrial protein mt-hsp70 as a marker to correct for the presence of some of the mitochondria in the nuclear fraction. We estimated that the mitochondrial fraction of hTop3α constituted about 5% of the total hTop3α in HeLa cells (data not shown).
Taken together, the subcellular localization studies of various hTop3α-EGFP fusion proteins and proteinase K sensitivity tests of endogenous hTop3α in various cellular fractions show that this enzyme is present inside both the nucleus and mitochondria, and that the first 20 of the 1,001-residue enzyme is most likely clipped off after its transport into mitochondria. In contrast, probing the gel blots shown in Fig. 4 with antibodies against human DNA topoisomerase IIIβ indicated that no proteinase K-resistant form of hTop3β was detectable in the purified mitochondria fraction (data not shown). The latter finding is consistent with a lack of a readily identifiable mitochondria-targeting signal in the amino acid sequence of hTop3β.
Discussion
The results presented above indicate that human DNA topoisomerase IIIα, but not IIIβ, is present in both the nucleus and mitochondria. Mitochondrial import of hTopIIIα requires initiation of translation from the first ATG codon of the TOP3α ORF to give a 1,001-residue protein hTopIIIα(1−1001); initiation from a second ATG downstream yields a 976-residue protein hTopIIIα(26−1001) lacking a mitochondria-targeting signal. The presence of codons for a mitochondria-targeting signal in between two tandem ATG codons at the beginning of human TOP3α is reminiscent of several eukaryotic genes encoding proteins designated for the mitochondria and another cellular compartment (34, 35, 39). In the case of human TOP3α, the two potential start codons are located within the same exon, and thus differential initiation or splicing of premRNA is not expected to play a role in the regulation of the relative amounts of the larger and smaller forms. As noted (20), the sequence context of the second ATG appears to be a more preferred site for translation initiation than the first, and thus the major form of human DNA topoisomerase IIIα is probably the smaller form lacking the mitochondria-targeting signal, in accordance with the “leaky scanning mechanism” of translation (40).
It is likely, however, that the subcellular distribution of hTopIIIα is regulated both translationally and posttranslationally, and even the larger form with a mitochondria-targeting signal localizes to both the nucleus and mitochondria. Such dual localization of a protein possessing both nuclear and mitochondria-targeting sequences was recently reported for several proteins, including the human RNA helicase MDDX28 (41) and the yeast protein Nfs1p (42). Another interesting example is the yeast repair enzyme Apn1p that apparently possesses a mitochondrial as well as a nuclear localization sequence; in this case, targeting of the enzyme to mitochondria is enhanced by another protein that interferes with nuclear transport of Apn1p (43).
The precise role of hTop3α in mitochondria is yet to be elucidated. On the basis of studies in Escherichia coli and the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, the type IA DNA topoisomerases are most likely involved in chromosome segregation, especially in the resolution of chromosomes that had undergone recombinational repair (4, 44–48). In animals, the possibility of recombinational repair of mtDNA remains uncertain (reviewed in ref. 49; see also refs. 50 and 51). However, at least for a ring-shaped mtDNA, one or more DNA topoisomerases would also be essential for the separation of parental DNA strands during replication. Although strong evidence exists for the presence of a type IB DNA topoisomerase (13), the type IB enzymes are known to act on a double-stranded DNA segment (3), and thus are unlikely to permit the removal of the last few parental strand intertwines (4). It is thus plausible that the mitochondrial form of hTop3α may participate in the resolution of mtDNA rings in their final stage of replication as well as the resolution of intermediates of recombinational repair (4). Whereas our results demonstrating the presence of hTop3α in mitochondria are suggestive of a functional role, genetic experiments are needed to confirm this conjecture. In the mouse model, targeted gene disruption showed that embryos lacking DNA topoisomerase IIIα die shortly after implantation (52), but the underlining causes of this lethality is presently unclear.
We suspect that a type IA DNA topoisomerase is likely to be present in the mitochondria of most if not all eukaryotes, especially in those possessing ring-shaped mitochondrial DNA. Analyses of the amino acid sequences of several other type IA DNA topoisomerases by the same algorithm used for hTop3α (27) yielded fairly high scores for the probability of importing these proteins into mitochondria: 0.69 and 0.92, respectively, for S. cerevisiae and S. pombe DNA topoisomerase III, and 0.88 and 0.98, respectively, for Drosophila and mouse DNA topoisomerase IIIα. In comparison, the computed scores are 0.28 for E. coli DNA topoisomerase III, and 0.24, 0.57, and 0.31, respectively, for Drosophila, mouse and human DNA topoisomerase IIIβ. For the type IB and type IIA DNA topoisomerases of these organisms, the scores are typically lower than 0.2 with two exceptions. The human mitochondrial DNA topoisomerase I has a high score of 0.98, as expected for its known mitochondrial localization. Surprisingly, S. pombe DNA topoisomerase I has a high score of 0.99, in contrast to a low score of 0.01 for S. cerevisiae DNA topoisomerase I. The budding and fission yeast are known to have only one type IB DNA topoisomerase, and the very different scores for the two type IB enzymes raises the interesting question whether S. pombe but not S. cerevisiae DNA topoisomerase I might be localized to both the nucleus and mitochondria.
Several previous reports have been published on the localization of hTop3α to nuclear compartments (53–56). Predominant localization of the protein to the nucleolus of peripheral blood lymphocytes after phytohemagglutinin activation (53), and to the promyelocytic leukemia protein nuclear bodies by interaction with the Bloom syndrome protein BLM (54, 56), a member of the RecQ helicase family, were seen. Mitochondrial localization of the protein was not observed in previous studies, presumably because of the low amounts of the protein in the organelle. However, the total amount of mtDNA is only a few percent of the nuclear DNA, and thus the low amount of the protein in mitochondria is likely significant.
Studies in yeast and mammalian cells have provided strong evidence for physical and functional interaction between type IA DNA topoisomerases and helicases of the RecQ family (45–47, 54–58). In mammalian cells, Top3α has been suggested to interact with the BLM helicase. Examination of the amino acid sequences of known mammalian RecQ helicases revealed no apparent mitochondria-targeting signal peptides (results not shown). Although several mitochondrial helicases have been identified (41, 59, 60), the plausible association of hTop3α with any one of these, or a presently unknown mitochondrial DNA helicase, is yet to be studied.
Recent findings on the relation between mutations in mtDNA and degenerative diseases, aging, and cancer, and the role of mitochondria in the regulation of apoptosis, have led to a renewal of interest in the study of mitochondria (see, for examples, the reviews in refs. 61 and 62). Whereas the presence of one or more DNA topoisomerases in mitochondria has been implicated for many decades, little information has been available on the precise roles of these enzymes in mitochondria. The identification of a nuclear gene encoding a mitochondrial type IB DNA topoisomerase (13), and the results reported here demonstrating the presence of a mitochondria-targeting signal at the N terminus of a type IA DNA topoisomerase, have now made it possible to dissect genetically the functions of these enzymes in mitochondria.
Acknowledgments
We thank Drs. Tina Wilson Sali and Tao-shih Hsieh (Duke University) for the generous gifts of anti-hTop3α and hTop3β antibodies, and Dr. Xiaoqi Liu for technical advice on fluorescence microscopy. This work was supported by National Institutes of Health Grants GM24544 and CA47958.
Abbreviations
mt, mitochondrial
hTop3α, human DNA topoisomerase IIIα
hTop3β, human DNA topoisomerase IIIβ
EGFP, enhanced green fluorescent protein
MIB, mitochondria isolation buffer
PCNA, proliferating cell nuclear antigen
References
- 1.Wang J. C. (1996) Annu. Rev. Biochem. 65, 635-692. [DOI] [PubMed] [Google Scholar]
- 2.Nitiss J. L. (1998) Biochim. Biophys. Acta 1400, 63-81. [DOI] [PubMed] [Google Scholar]
- 3.Champoux J. J. (2001) Annu. Rev. Biochem. 70, 369-413. [DOI] [PubMed] [Google Scholar]
- 4.Wang J. C. (2002) Nat. Rev. Mol. Cell. Biol. 3, 430-440. [DOI] [PubMed] [Google Scholar]
- 5.Bergerat A., de Massy, B., Gadelle, D., Varoutas, P. C., Nicolas, A. & Forterre, P. (1997) Nature (London) 386, 414-417. [DOI] [PubMed] [Google Scholar]
- 6.Keeney S., Giroux, C. N. & Kleckner, N. (1997) Cell 88, 375-384. [DOI] [PubMed] [Google Scholar]
- 7.Keeney S. (2001) Curr. Top. Dev. Biol. 52, 1-53. [DOI] [PubMed] [Google Scholar]
- 8.Shadel G. S. & Clayton, D. A. (1997) Annu. Rev. Biochem. 66, 409-435. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus G. M., Henrich, J. P., Kelly, W. G., Schmitz, S. A. & Castora, F. J. (1987) Biochemistry 26, 6195-6203. [DOI] [PubMed] [Google Scholar]
- 10.Kosovsky M. J. & Soslau, G. (1991) Biochim. Biophys. Acta 1078, 56-62. [DOI] [PubMed] [Google Scholar]
- 11.Topcu Z. & Castora, F. J. (1995) Biochim. Biophys. Acta 1264, 377-387. [DOI] [PubMed] [Google Scholar]
- 12.Tua A., Wang, J., Kulpa, V. & Wernette, C. M. (1997) Biochimie 79, 341-350. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Barcelo, J. M., Lee, B., Kohlhagen, G., Zimonjic, D. B., Popescu, N. C. & Pommier, Y. (2001) Proc. Natl. Acad. Sci. USA 98, 10608-10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro T. A. & Englund, P. T. (1995) Annu. Rev. Microbiol. 49, 117-143. [DOI] [PubMed] [Google Scholar]
- 15.Melendy T., Sheline, C. & Ray, D. S. (1988) Cell 55, 1083-1088. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z. & Englund, P. T. (2001) EMBO J. 20, 4674-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komori K., Kuroe, K., Yanagisawa, K. & Tanaka, Y. (1997) Biochim. Biophys. Acta 1352, 63-72. [DOI] [PubMed] [Google Scholar]
- 18.Chavalitshewinkoon-Petmitr P., Worasing, R. & Wilairat, P. (2001) Southeast Asian J. Trop. Med. Public Health 32, 733-738. [PubMed] [Google Scholar]
- 19.Low R. L. (2002) Methods Mol. Biol. 197, 317-329. [DOI] [PubMed] [Google Scholar]
- 20.Hanai R., Caron, P. R. & Wang, J. C. (1996) Proc. Natl. Acad. Sci. USA 93, 3653-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozak M. (1987) Nucleic Acids Res. 15, 8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffey G. & Campbell, C. (2000) Nucleic Acids Res. 28, 3793-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajapakse N., Shimizu, K., Payne, M. & Busija, D. (2001) Brain Res. Protoc. 8, 176-183. [DOI] [PubMed] [Google Scholar]
- 24.Storrie B. & Madden, E. A. (1990) Methods Enzymol. 182, 203-225. [DOI] [PubMed] [Google Scholar]
- 25.Rost B. & Sander, C. (1993) J. Mol. Biol. 232, 584-599. [DOI] [PubMed] [Google Scholar]
- 26.Rost B. & Sander, C. (1994) Proteins 19, 55-72. [DOI] [PubMed] [Google Scholar]
- 27.Claros M. G. & Vincens, P. (1996) Eur. J. Biochem. 241, 779-786. [DOI] [PubMed] [Google Scholar]
- 28.Lima C. D., Wang, J. C. & Mondragon, A. (1994) Nature (London) 367, 138-146. [DOI] [PubMed] [Google Scholar]
- 29.Mondragon A. & DiGate, R. (1999) Struct. Fold. Des. 7, 1373-1383. [DOI] [PubMed] [Google Scholar]
- 30.Neupert W. (1997) Annu. Rev. Biochem. 66, 863-917. [DOI] [PubMed] [Google Scholar]
- 31.Gavel Y. & von Heijne, G. (1990) Protein Eng. 4, 33-37. [DOI] [PubMed] [Google Scholar]
- 32.Schneider G., Sjoling, S., Wallin, E., Wrede, P., Glaser, E. & von Heijne, G. (1998) Proteins 30, 49-60. [PubMed] [Google Scholar]
- 33.Nakai K. & Horton, P. (1999) Trends Biochem. Sci. 24, 34-36. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmipathy U. & Campbell, C. (1999) Mol. Cell. Biol. 19, 3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer M., Rainey, M., Pullen, T. & Stirling, C. J. (1999) Curr. Biol. 9, 1085-1094. [DOI] [PubMed] [Google Scholar]
- 36.Scheller K., Sekeris, C. E., Krohne, G., Hock, R., Hansen, I. A. & Scheer, U. (2000) Eur. J. Cell Biol. 79, 299-307. [DOI] [PubMed] [Google Scholar]
- 37.Kanaji S., Iwahashi, J., Kida, Y., Sakaguchi, M. & Mihara, K. (2000) J. Cell Biol. 151, 277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider H., Sollner, T., Dietmeier, K., Eckerskorn, C., Lottspeich, F., Trulzsch, B., Neupert, W. & Pfanner, N. (1991) Science 254, 1659-1662. [DOI] [PubMed] [Google Scholar]
- 39.Slusher L. B., Gillman, E. C., Martin, N. C. & Hopper, A. K. (1991) Proc. Natl. Acad. Sci. USA 88, 9789-9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozak M. (1999) Gene 234, 187-208. [DOI] [PubMed] [Google Scholar]
- 41.Valgardsdottir R., Brede, G., Eide, L. G., Frengen, E. & Prydz, H. (2001) J. Biol. Chem. 276, 32056-32063. [DOI] [PubMed] [Google Scholar]
- 42.Nakai Y., Nakai, M., Hayashi, H. & Kagamiyama, H. (2001) J. Biol. Chem. 276, 8314-8320. [DOI] [PubMed] [Google Scholar]
- 43.Vongsamphanh R., Fortier, P. K. & Ramotar, D. (2001) Mol. Cell. Biol. 21, 1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallis J. W., Chrebet, G., Brodsky, G., Rolfe, M. & Rothstein, R. (1989) Cell 58, 409-419. [DOI] [PubMed] [Google Scholar]
- 45.Gangloff S., McDonald, J. P., Bendixen, C., Arthur, L. & Rothstein, R. (1994) Mol. Cell. Biol. 14, 8391-8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodwin A., Wang, S. W., Toda, T., Norbury, C. & Hickson, I. D. (1999) Nucleic Acids Res. 27, 4050-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maftahi M., Han, C. S., Langston, L. D., Hope, J. C., Zigouras, N. & Freyer, G. A. (1999) Nucleic Acids Res. 27, 4715-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Q., Pongpech, P. & DiGate, R. J. (2001) Proc. Natl. Acad. Sci. USA 98, 9766-9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croteau D. L., Stierum, R. H. & Bohr, V. A. (1999) Mutat. Res. 434, 137-148. [DOI] [PubMed] [Google Scholar]
- 50.Thyagarajan B., Padua, R. A. & Campbell, C. (1996) J. Biol. Chem. 271, 27536-27543. [DOI] [PubMed] [Google Scholar]
- 51.Ladoukakis E. D. & Zouros, E. (2001) Mol. Biol. Evol. 18, 1168-1175. [DOI] [PubMed] [Google Scholar]
- 52.Li W. & Wang, J. C. (1998) Proc. Natl. Acad. Sci. USA 95, 1010-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin C. W., Darzynkiewicz, Z., Li, X., Traganos, F., Bedner, E. & Tse-Dinh, Y. C. (2000) Exp. Cell Res. 256, 225-236. [DOI] [PubMed] [Google Scholar]
- 54.Johnson F. B., Lombard, D. B., Neff, N. F., Mastrangelo, M. A., Dewolf, W., Ellis, N. A., Marciniak, R. A., Yin, Y., Jaenisch, R. & Guarente, L. (2000) Cancer Res. 60, 1162-1167. [PubMed] [Google Scholar]
- 55.Wu L., Davies, S. L., North, P. S., Goulaouic, H., Riou, J. F., Turley, H., Gatter, K. C. & Hickson, I. D. (2000) J. Biol. Chem. 275, 9636-9644. [DOI] [PubMed] [Google Scholar]
- 56.Hu P., Beresten, S. F., van Brabant, A. J., Ye, T. Z., Pandolfi, P. P., Johnson, F. B., Guarente, L. & Ellis, N. A. (2001) Hum. Mol. Genet. 10, 1287-1298. [DOI] [PubMed] [Google Scholar]
- 57.Bennett R. J., Noirot-Gros, M. F. & Wang, J. C. (2000) J. Biol. Chem. 275, 26898-26905. [DOI] [PubMed] [Google Scholar]
- 58.Bennett R. J. & Wang, J. C. (2001) Proc. Natl. Acad. Sci. USA 98, 11108-11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C. M., Sedman, J., Neupert, W. & Stuart, R. A. (1999) J. Biol. Chem. 274, 20937-20942. [DOI] [PubMed] [Google Scholar]
- 60.Sedman T., Kuusk, S., Kivi, S. & Sedman, J. (2000) Mol. Cell. Biol. 20, 1816-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallace D. C. (1999) Science 283, 1482-1488. [DOI] [PubMed] [Google Scholar]
- 62.Olson M. & Kornbluth, S. (2001) Curr. Mol. Med. 1, 91-122. [DOI] [PubMed] [Google Scholar]