Abstract
Standardized extract from the leaves of the Ginkgo biloba tree, labeled EGb761, has been used in clinical trials for its beneficial effects on brain functions, particularly in connection with age-related dementias and Alzheimer's disease (AD). Substantial experimental evidence indicates that EGb761 protects against neuronal damage from a variety of insults, but its cellular and molecular mechanisms remain unknown. Using a neuroblastoma cell line stably expressing an AD-associated double mutation, we report that EGb761 inhibits formation of amyloid-β (Aβ) fibrils, which are the diagnostic, and possibly causative, feature of AD. The decreased Aβ fibrillogenesis in the presence of EGb761 was observed both in the conditioned medium of this Aβ-secreting cell line and in solution in vitro. In the cells, EGb761 significantly attenuated mitochondrion-initiated apoptosis and decreased the activity of caspase 3, a key enzyme in the apoptosis cell-signaling cascade. These results suggest that (i) neuronal damage in AD might be due to two factors: a direct Aβ toxicity and the apoptosis initiated by the mitochondria; and (ii) multiple cellular and molecular neuroprotective mechanisms, including attenuation of apoptosis and direct inhibition of Aβ aggregation, underlie the neuroprotective effects of EGb761.
Extracts of Ginkgo biloba leaves have been marketed as therapeutic dietary supplements to counteract a variety of neurological disorders, including Alzheimer's disease (AD) (1). Substantial experimental evidence indicates that Ginkgo extracts have neuroprotective effects in animals and humans (2); however, the cellular and molecular mechanisms remain unclear (3). There is much to be learned about the pathophysiology of the disease and, importantly, about possible novel therapeutic approaches from analyzing the pharmacological mechanisms of this traditional remedy.
Autosomal dominant forms of AD appear to be caused by deposition of insoluble amyloid β (Aβ), a proteolytic fragment of 40–42 amino acid residues derived from amyloid precursor protein (APP), in the brain (4, 5). Mutations in APP and presenilins (PS1, PS2), associated with familial AD, increase the production of the total Aβ or the more amyloidogenic form Aβ42 (6). Accumulating experimental evidence suggests links between deposition of Aβ, oxidative stress, and apoptosis associated with AD (7) and aging (8). The Aβ peptide has been shown to induce apoptosis in neurons, which may contribute to the neuronal degeneration in AD (9). Brain tissue from AD patients contains deposits of oxidized Aβ (10) and activated caspase-3, a cysteine protease that mediates mitochondrion-initiated apoptosis (11). Other members of the caspase family have been found to mediate apoptosis resulting from Aβ cytotoxicity (12). These findings suggest potential pharmacological targets that can be used in strategies aimed at slowing the progression of neuronal loss in AD. The goal of the present work was to study the protective mechanisms of the standardized extract EGb761 against Aβ-induced apoptosis by using well-established methods of cell and molecular biology and biochemistry. We report that the mouse neuroblastoma cell line expressing double-mutated human APP and PS1 exhibits Aβ aggregation and activation of caspase 3. Both the Aβ aggregation and the caspase-3 activity were inhibited by treatment with EGb761. Furthermore, EGb761 interacted directly with Aβ and had a profound inhibitory effect on the formation of Aβ fibrils. These results suggest that EGb761 provides a combination of antioxidive, antiamyloidogenic, and antiapoptotic effects, which can all be potentially used in the treatment and/or prevention of AD.
Materials and Methods
Reagents.
The standardized Ginkgo biloba leaf extract EGb761 used in the clinical trials (2) was from Schwabe Pharmaceuticals (Karlsruhe, Germany). The main active components are flavone glycosides (24%) and terpene lactones (6%), which include the ginkgolides and the bilobalide (1). The ginkgolides A, B, C, and J and the bilobalide B were isolated as described (13). Aβ1–40 was purchased from Sigma or W. M. Keck Biotechnology (New Haven, CT). Antibodies to Aβ17–24 (4G8, which is specific for Aβ and APP), and Aβ1–17 (6E10, which recognizes Aβ and also APPsα), were from Signet Laboratories (Dedham, MA). Antibody to Aβ1–5 (3D6) was provided by Athena Neurosciences (San Diego). The caspase-3 activity assay kit was from Biomol (Plymouth Meeting, PA). Other chemicals were from Sigma.
Cell Lines.
The N2a neuroblastoma control cells [wild type (wt)] or the N2a cell line stably expressing Swedish mutant APP695 and the exon-9 deletion mutant PS1 (swe/Δ9) were maintained, as described (14, 15), in the medium containing 50% DMEM and 50% Opti-MEM, supplemented with 5% FBS, 200 μg/ml of G418, and other antibiotics (GIBCO). The expression of the transgenes was induced by the addition of 1 μM butyric acid (sodium salt) for 12 h in a 1% serum medium.
Fibrillogenesis of Purified Aβ by Using an in Vitro Fluorescence Assay.
Aβ was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol and sonicated in a bath sonicator to produce a concentrated stock (4.6 mM) of the monomeric peptide (16). The peptide was diluted with PBS to 46 μM and incubated at room temperature for 96 h. To measure the EGb761 effects, the peptide was mixed with EGb761 (100 μg/ml) or its components bilobalide B, ginkgolides A, B, C or J (29 μg/ml). Aliquots were transferred into the measuring solution (5 mM thioflavin T/50 mM glycine-NaOH buffer, pH 8.5). Fluorescence was measured within 5 s in a Hitachi F-2000 spectrofluorometer with the excitation and emission wavelengths of 435 and 485 nm, respectively (17).
Electron and Immunoelectron Microscopy.
An aqueous suspension of aggregated Aβ was incubated on formvar-coated nickel grids for 20 min and negatively stained with 1% phosphotungstic acid (adjusted to pH 7.0). For immunolabeling of Aβ fibrils in cell culture, the conditioned media were collected and centrifuged at 12,000 × g for 20 min. The pellets were resuspended in 20 μl of water and incubated on the grids for 20 min. The grids were washed (150 mM NaCl/5 mM Tris⋅HCl, pH 7.4), blocked with BSA, incubated with an anti-Aβ antibody (4G8, 1:300 dilution), and rinsed. Goat anti-mouse IgG antibody conjugated with gold particles (diluted 1:40, Electron Microscopy Sciences, Fort Washington, PA) was applied, followed by rinsing and fixing with 2% glutaraldehyde. Samples were negatively stained with phosphotungstic acid and examined by using a Zeiss 109-T transmission electron microscope (Leo Electron Microscopy, Thornwood, NY).
Immunoblotting of Aβ Aggregation in Solution and Cell Culture Media.
Purified Aβ (40 μM) was incubated either alone or in the presence of EGb761 (100 μg/ml) for 24 h or 8 days at room temperature. Proteins were separated by SDS/PAGE on a ready-made 4–20% gradient acrylamide gel (Bio-Rad). A control lane contained freshly prepared Aβ peptide. The Aβ species were identified by immunoblotting by using the antibody 6E10 and the standard Western blotting protocol. For detecting Aβ species in culture medium, transgene expression in the control (wt) or mutant cells (swe/Δ9), preincubated with or without EGb761 for 48 h, was induced with butyric acid overnight. The media were then collected and immunoprecipitated with the antibody 3D6, followed by Western blotting with the antibody 6E10.
Apoptosis Assay by Mitochondrial Staining.
The integrity of the mitochondria was tested with an ApoAlert staining kit (CLONTECH), which uses a proprietary dye mixture called Mitosensor, whose fluorescence exhibits spectral shift in apoptotic cells. In healthy cells, the dye is taken up in the mitochondria, where it forms aggregates that fluoresce bright red. In apoptotic cells, a shift in the mitochondrial membrane potential prevents the dye from accumulating in the mitochondria. It remains as monomers in the cytoplasm, where it fluoresces green. The control (wt) and mutant cells (swe/Δ9) were cultured on coverslips in 6-well plates and grown to approximately 60% confluency in 5% CO2 at 37°C. The cells were treated with EGb761 (100 μg/ml) or vitamin E (25 μM) 48 h before induction of expression. The Aβ expression was induced by addition of 1 μM butyric acid. After 12 h, the cells were gently washed with serum-free medium and incubated with the Mitosensor dye for 20 min at 37°C. The stained coverslips were inverted, mounted onto glass slides, and examined with a fluorescence microscope (Olympus BX60, Tokyo). The images were captured by a camera and processed with PHOTOSHOP 5.5 (Adobe Systems, Mountain View, CA).
Analysis of Cell Death.
Cell death was quantified by the trypan blue exclusion method. Cells were grown in a 96-well plate to a density of about 2,000 cell/well, untreated or treated with EGb761 for 48 h, washed with PBS, and stained with 0.1% trypan blue for 15 min. After another wash, 0.1 M NaOH was added, and the dye uptake by the cells was quantified at 562 nm in a microtiter plate reader (BioTek, Winooski, VT).
Caspase-3 Activity Assay.
A quantitative enzymatic activity assay was carried out according to instructions of the Biomol colorimetric assay kit manufacturer. After drug treatment, cells were washed with ice-cold PBS, lysed, centrifuged, and analyzed for total protein by the Bradford assay. Samples containing 50 μg of total protein were assayed for caspase-3 activity with Ac-DEVD as a caspase-3-specific substrate. Absorbance was measured at 405 nm in a plate reader. For immunoblotting, equal amounts of total protein from cell extract were subjected to SDS/PAGE and probed with an antibody specific for cleaved caspase 3 (PharMingen). Caspase-3 immunoreactivity was detected with a chemiluminescence kit (Amersham Pharmacia). The blot was stripped and reblotted with an antiactin antibody to prove that all of the lanes were loaded with the same amount of protein.
Results
EGb761 Prevents Aβ Aggregation in Vitro.
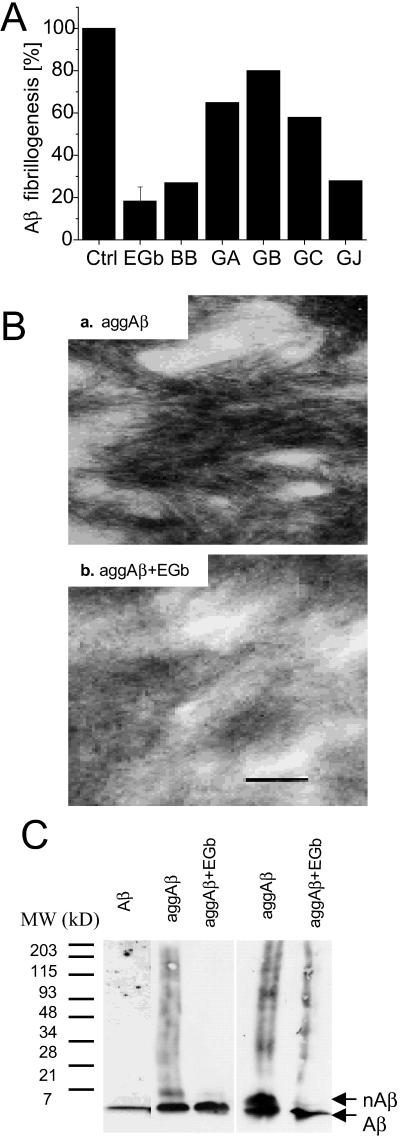
To determine whether EGb761 affects Aβ fibrillogenesis, we first measured in vitro aggregation of Aβ in the absence and presence of EGb761 by the thioflavin T method (17). The data in Fig. 1A show that EGb761 directly prevents Aβ fibrillogenesis. In all controls (buffer, EGb761 alone, and 46 μM albumin as an irrelevant protein), thioflavin T showed low constant fluorescence for the duration of each experiment (data not shown). Assuming that the fluorescence intensity is proportional to the extent of Aβ aggregation, the results indicate that 100 μg/ml of EGb761 inhibits aggregation by 82 ± 6% (average ± SEM, n = 4, P = 0.0014 by the unpaired two-tailed t test). The inhibition by EGb761 appears to be thermodynamic rather than kinetic, because it could not be overcome by very long incubations (up to 1 mo).
Fig 1.
In vitro Aβ fibrillogenesis in the presence or absence of EGb761. (A) Thioflavin T fluorescence assay. Aβ (46 μM) was incubated in the absence (control) or presence of 100 μg/ml of EGb761 (EGb), or 29 μg/ml of bilobalide (BB) or ginkgolides A, B, C, and J (GA, GB, GC, and GJ, respectively) for 96 h at room temperature. The whole extract EGb761 was tested in four independent experiments, the difference was statistically significant: P = 0.0014 by the unpaired two-tailed t test. The individual components were only tested once, therefore no variability or statistical significance is given. (B) Electron microscopy. Aβ peptide was incubated overnight either alone (a) or with 100 μg/ml of EGb761 (b) and examined by electron microscopy. (Bar = 100 nm.) The results were qualitatively reproduced in three independent experiments. (C) Immunoblotting of Aβ species using the antibody 6E10. Lane 1, freshly prepared Aβ; lanes 2 and 3, Aβ incubated without or with EGb761 for 24 h, respectively; lanes 4 and 5, Aβ incubated without or with EGb761 for 8 days, respectively. Arrows indicate Aβ monomers (Aβ) or oligomers (nAβ).
In an attempt to determine ID50, the concentration required for a 50% inhibition of Aβ aggregation, assays were performed with 0, 1, 4, 10, 20, and 100 μg/ml of EGb761. Significant inhibition was observed only at the highest concentration, indicating that the ID50 value is between 20 and 100 μg/ml.
Several individual constituents of EGb761 were tested for their ability to inhibit Aβ aggregation in vitro by the same assay. Each component was used at an arbitrary concentration of 29 μg/ml, which is higher than that expected in the whole extract (maximum 6 μg/ml in 100 μg/ml of EGb761). The higher concentrations were used because in these experiments, the individual components cannot exhibit synergism, which is often postulated in the action of the whole EGb761. Inhibition of aggregation comparable to that obtained with the whole extract was observed with bilobalide (73%) and ginkgolide J (72%). Ginkgolides A, B, and C had much smaller effects: 35, 20, and 42%, respectively.
To confirm the thioflavin T data by an alternative method, Aβ fibrillogenesis was assessed by electron microscopy (Fig. 1B) and Western blotting (Fig. 1C). When freshly prepared Aβ peptide was incubated with or without EGb761 as described above, a significant difference was observed between the two preparations. Aβ incubated with EGb761 exhibited electron-dense disordered aggregates (Fig. 1Bb) but not the characteristic fibrils that were observed in the absence of EGb761 (Fig. 1Ba). These results were confirmed by Western blotting with the antibody 6E10 (Fig. 1C). The aggregated Aβ was present in the form of oligomers (nAβ), presumably dimers or trimers, according to the molecular-weight standards. More dimers were observed after longer incubation times (Fig. 1C, lane 4 vs. 2). EGb761 consistently prevented Aβ dimer formation even at the long incubation time (Fig. 1C, lane 4 vs. 5).
EGb761 Prevents Aβ Aggregation in the Medium of Aβ-Producing Cells.
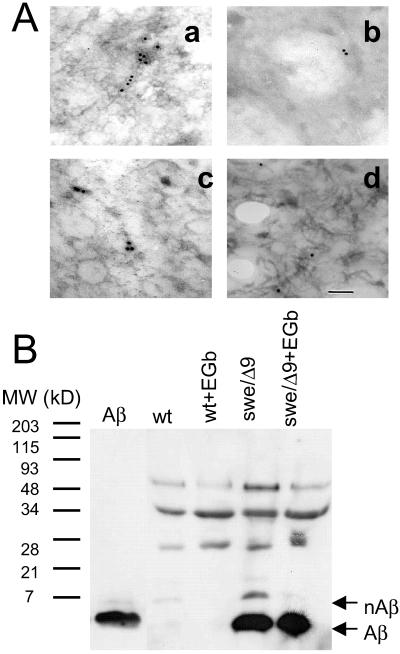
To further confirm the antifibrillogenic activity of EGb761 in a cell system, immunoelectron microscopy was used to search for Aβ fibrils in the media of the N2a wt and swe/Δ9 cells. Culture medium of the swe/Δ9 cells probed only with the secondary antibody showed a little gold deposition due to a low level of nonspecific binding (data not shown). As a positive control, purified Aβ was incubated for 48 h as described above and incubated with the monoclonal antibody 4G8 and a gold-conjugated secondary antibody. Fig. 2Aa shows that gold-labeled fibrils were present in this preparation. Similar immunoreactive fibrils were observed in the medium recovered from swe/Δ9 cells (Fig. 2Ac) but not in the medium from N2a wt cells (Fig. 2Ab), which showed a level of gold labeling comparable to the negative control. Importantly, labeled fibrils were not detected in swe/Δ9 cells treated with EGb761 (Fig. 2Ad), indicating that EGb761 prevented Aβ fibril formation in the cell culture. Similar results were obtained with three independent preparations.
Fig 2.
Aβ fibrillogenesis in mutant neuroblastoma cells in the presence or absence of EGb761. (A) Immunoelectron microscopy of Aβ fibrils in culture media. (a) In vitro aggregated Aβ immunolabeled with the antibody 4G8; (b) the wt cell culture medium collected and immunolabeled with 4G8; (c) the swe/Δ9 cell culture medium collected and probed with 4G8; (d) the culture medium collected from swe/Δ9 cells treated with EGb761 for 48 h and probed with 4G8. a–d represent areas of several grids in three separate experiments. (B) Immunoblotting of Aβ species from the culture media of neuroblastoma cells: purified Aβ (lane 1); wt untreated (wt, lane 2) or treated with 100 μg/ml of EGb761 for 48 h (wt + EGb, lane 3); mutant cell line untreated (swe/Δ9, lane 4) or treated with 100 μg/ml of EGb761 for 48 h (swe/Δ9 + EGb, lane 5). Arrows indicate Aβ monomer (Aβ) or aggregated oligomers (nAβ). The blot represents three independent experiments.
Immunochemical labeling of Aβ (Fig. 2B) further supported this finding. Cell culture media from the N2a and swe/Δ9 cells, untreated or treated with EGb761, were collected and immunoprecipitated with the antibody 3D6, followed by Western blotting with the antibody 6E10. There was no detectable Aβ immunoreactivity in the medium from the N2a cells (Fig. 2B, lanes 2 and 3 vs. 1). In contrast, an aggregated Aβ band (nAβ), at a molecular mass between 7 and 21 kDa (Fig. 2B, lane 4), presumably dimers or higher oligomers, was detected in the culture medium of the swe/Δ9 mutant cells. Most obviously, the Aβ oligomer band (nAβ) was absent in the medium recovered from cells treated with EGb761 (Fig. 2B, lane 5), demonstrating that EGb761 inhibited Aβ fibril formation. EGb761 had no effect on Aβ production, nor did it affect the levels of APPsα, a product of α secretase, or total cellular APP (data not shown). Hence, the EGb761-induced attenuation of Aβ fibrillogenesis is not because of an overall inhibition of Aβ production.
EGb761 Attenuates Mitochondrion-Sensitive Neurotoxicity and Caspase-3 Activity in Neuroblastoma Cells.
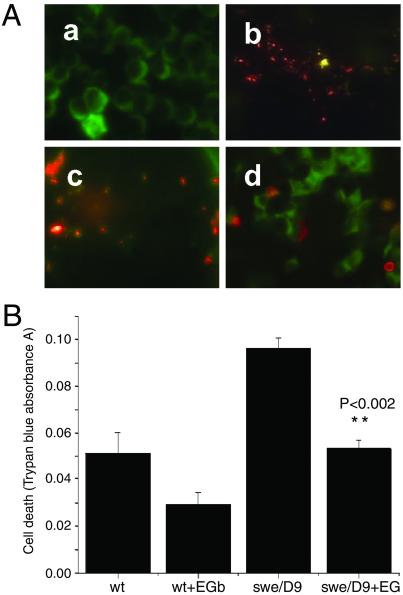
To provide a possible link between endogenous Aβ production and apoptosis activation, cell viability in Aβ-producing neuroblastoma cells expressing mutated APP and PS1 was first measured by Mitosensor. Fig. 3A demonstrates that the swe/Δ9 cells fluoresced green after induction of transgene expression, indicating mitochondrion-initiated apoptosis (Fig. 3Aa), whereas N2a wt cells showed red fluorescence indicative of healthy mitochondria (Fig. 3Ab). The apoptosis in swe/Δ9 cells was attenuated by treatment with EGb761 for 48 h before induction of expression (Fig. 3Ac). As a comparison, vitamin E, a known antioxidant, attenuated apoptosis to a lesser degree (Fig. 3Ad). Cell viability measured by trypan blue exclusion confirmed this observation quantitatively (Fig. 3B). Both N2a wt and mutant (swe/Δ9) exhibited vulnerability after stimulation with butyric acid to induce transgene expression. However, cell death in the mutant was 2-fold higher than that in the wt, and in both cases EGb761 attenuated it. In the N2a wt cells, the EGb761 attenuation was not statistically significant (P = 0.098, n = 6), but in the mutant it was (P = 0.0015, n = 6).
Fig 3.
Mitochondrion-sensitive Aβ cytotoxicity in the mutant cells untreated or treated with EGb761. (A) Representative fluorescence staining for apoptosis in the swe/Δ9 cells with and without drug treatments. Mitochondrial integrity was probed by using Mitosensor (see Materials and Methods). Mitochondria of healthy cells exhibit red fluorescence, cells undergoing apoptosis exhibit green fluorescence. (a) Mutant cells (swe/Δ9) stimulated with 1 μM butyric acid for 12 h to express the transgene; (b) unstimulated wt N2a cells (wt); (c) swe/Δ9 cells pretreated with EGb761 for 48 h; (d) swe/Δ9 cells pretreated with vitamin E (VE) for 48 h. Similar results were obtained in three experiments. (B) Cell death assay by using quantitative trypan blue exclusion. wt or mutant (swe/Δ9) neuroblastoma cells were treated with 100 μg/ml of EGb761 for 48 h and stimulated with 1 μM butyric acid to express the transgene. Results are mean ± SEM (n = 6). EGb761 had a significant effect on cell death in the mutant cell line (P = 0.0015), but not the wt (P = 0.098).
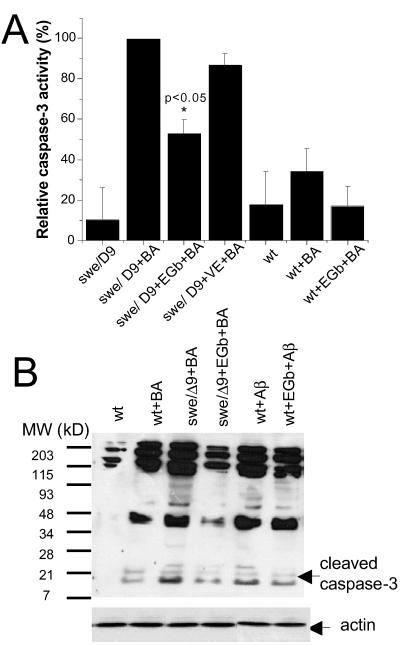
Changes in the mitochondrial membrane potential lead to the release of cytochrome c into the cytoplasm, caspase activation, and apoptosis (18). Previous studies have indicated that mitochondrion-initiated apoptosis is mediated by caspase 3 (19, 20). To determine whether caspase 3 was activated in the double mutant, the enzymatic activity in the swe/Δ9 cells and the wt was measured by both a quantitative colorimetric caspase-3 activity assay (Fig. 4A) and Western blotting with an antibody specific for activated caspase 3 (Fig. 4B). Caspase 3 was activated in both cell types after stimulation with 1 μM butyric acid but much less so in wt (Fig. 4A, wt vs. wt + BA, P > 0.05, n = 4; swe/Δ9 vs. swe/Δ9 +BA, P < 0.005, n = 4, Fig. 4B, lanes 2 vs. 3). We demonstrated unambiguously that the internally activated caspase 3 in the mutant cells was significantly inhibited by pretreatment of the cells with EGb761 (swe/Δ9+BA vs. swe/Δ9 + EGb + BA, P < 0.05, n = 4, Fig. 4B lane 3 vs. 4). The inhibition of caspase 3 activity by EGb761 in the wt stimulated with butyric acid was not significant (wt + BA vs. wt + EGb + BA, P > 0.05, n = 4). Vitamin E, a known antioxidant, did not show significant inhibition in the mutant (Fig. 4A, swe/Δ9 + BA vs. swe/Δ9 + VE + BA, P > 0.05, n = 5). To support the notion that caspase-3 activation is due to expression of Aβ in the mutant cells, purified Aβ was added to the culture medium of control N2a cells. The cleaved 17-kDa caspase 3 was observed in this preparation (Fig. 4B, lane 5). Further, Aβ-induced activation of caspase 3 was attenuated by the treatment of the cells with EGb761 (Fig. 4B, lane 6). Immunoblotting of the samples with antiactin (Fig. 4B Lower) proves that equal amounts of protein were loaded in each lane. These results strongly suggest that the apoptosis in the cultured swe/Δ9 cells is mediated by caspase 3 and the protective action of EGb761 may be carried out, at least in part, by inhibition of caspase-3 activation.
Fig 4.
Caspase-3 activity in neuroblastoma cells in the presence or absence of EGb761. (A) Caspase-3 enzymatic activity assay in the N2a cells (wt), and the swe/Δ9 (swe/Δ9) cells alone, or treated with EGb761 for 48 h. Data are expressed as percentage of the maximum caspase-3 activity in swe/Δ9 cells, which, in average, was equivalent to 1.6 pmol/mg of protein/min. *, statistical significance (P < 0.05, n = 4) by unpaired t test. (B) Representative Western blots of caspase-3 activation in the control and the mutant cells. Lane 1: unstimulated N2a cells; lane 2: N2a cells stimulated with 1 μM butyric acid for 12 h; lane 3: mutant cells stimulated with butyric acid for 12 h; lane 4: mutant cells treated with 100 μg/ml of EGb761 for 48 h before stimulation with butyric acid; lane 5: N2a wt cells treated with 0.1 μM Aβ for 12 h; lane 6: N2a cells treated with 100 μg/ml of EGb761 for 48 h before treatment with 0.1 μM Aβ for 12 h. Arrows indicate cleaved (activated) caspase 3 at about 17 kD. The lower blot is an immunoblot of actin indicating that same amount of proteins were loaded in each lane. Results are representative of two independent experiments.
Discussion
On the basis of numerous pharmacological studies with animals, and more recently with humans, it has been proposed that EGb761 ameliorates neurodegeneration associated with aging (1, 2). This study was designed to investigate the effects of the Ginkgo biloba extract EGb761 at the cellular and molecular levels by using well-established methods of cell and molecular biology and biochemistry. Our results demonstrate that EGb761 directly inhibits amyloid fibril formation in solution and in the cell culture medium. We further demonstrate that EGb761 prevents amyloid neurotoxicity and inhibits caspase-3 activity in cell culture.
Aβ fibril formation is believed to be the main determinant in the pathogenesis of AD, although it is not clear how Aβ fibrils exert their cytotoxic effects. There are reports that Aβ fibrils permeabilize lipid membranes (21) and induce calcium currents (22) and other electrophysiological changes (23) in neurons. Thus, inhibition of cerebral Aβ aggregation is an important goal in AD therapy. Several compounds have been reported to have such an effect: melatonin (24), some nonsteroidal antiinflammatory drugs (25), sulfated mono- and disaccharides (26), synthetic peptides (27, 28), and cerebrospinal-fluid proteins such as thransthyretin (29). Because of the known relationship between oxidative stress and AD and because of the established antioxidative properties of Ginkgo biloba, it has been proposed that EGb761 exerts its neuroprotective effects mostly as an intracellular antioxidant (1). However, results presented herein suggest that the antiamyloidogenic property of EGb761 could be a consequence of its direct interaction with Aβ. The preventative effect of EGb761 on in vitro formation of amyloid fibrils was recently (while our work was in progress) reported by two other laboratories (30, 31). Our results confirm these observations and demonstrate the effect in a cellular system, namely, in the culture medium of an Aβ-producing cell line.
The EGb761 extract is a relatively complex mixture of compounds. We attempted to address this issue by testing several individual constituents of the terpenoid fraction of EGb761. In vitro, bilobalide and ginkgolide J seem to be the most active terpenoids in EGb761 (Fig. 1A), consistent with the observed effect of EGb761 constituents on caspase-3 activation in differentiated PC12 cells (32). Ramassamy et al. (30) tested just one terpenoid—ginkgolide B—and found it did not inhibit Aβ fibrillogenesis, which is confirmed in the present work (Fig. 1A). Instead, they suggest that the active components of EGb761 are in the flavonoid fraction, which has not been included in the present study.
A variety of effects of Aβ on cells have been reported (33, 34), including induction of cytotoxicity as well as apoptotic neuronal death. In most of these studies, exogenous Aβ was added to the culture medium at much higher concentrations than physiological. In the present study, a stably transfected Aβ-producing neuroblastoma cell line (swe/Δ9) was used (14), acting as the source of endogenous Aβ. We found that caspase-3 was activated in these cells after the expression of the transgene. The present work demonstrates constitutively increased caspase-3 activity in the N2a cells expressing mutated human APP and PS1. Apart from the implications for understanding Aβ cytotoxicity, this finding is an important contribution to the characterization of this cell line.
Butyric acid has been known to induce cytotoxicity and apoptosis in cells (35). Therefore, its use as an inducer of transgene expression in our work deserves some discussion. First, concentrations of butyric acid used to induce apoptosis are usually in the millimolar range, whereas we used 1 μM. Second, although the wt cells did exhibit apoptosis when stimulated with butyric acid (Fig. 4B, lane 2), it was much less than the mutant cells (Fig. 4B, lane 3). The only significant difference between the two cell lines is the production of the amyloidogenic Aβ peptide. Consequently, the increased apoptosis and caspase-3 activation in the swe/Δ9 cells must be because of the presence of the endogenous Aβ. When the control cells were incubated with exogenous Aβ, caspase 3 was also activated. Further, when the mutant cells were incubated with a caspase-3 inhibitor before the butyric acid stimulation, the activation of caspase 3 was blocked (data not shown). The contribution of butyric acid to the activation of caspase 3 in our system was not negligible. But, interestingly enough, EGb761 inhibited caspase-3 activity in all cases, irrespective of the activation mechanism, as demonstrated in Fig. 4A. This observation suggests that, in addition to the inhibition of Aβ fibrillogenesis, EGb761 may act on the intracellular signaling pathways.
The two best-studied pathways of caspase activation are the cell-surface–death-receptor pathway, i.e., Fas-mediated apoptosis, and the mitochondrion-initiated pathway (36). The present data show that Aβ expression activates the key element of the mitochondrion-initiated pathway. Although other recent studies have suggested that caspases 2 and 12 mediate Aβ-induced cell death in several neuronal populations and in experimental animals (28, 37), our data implicate another member of the apoptosis-signaling cascade, caspase 3. It is possible that the AD pathophysiology at the cellular level involves both direct Aβ toxicity and mitochondrial sensitization to the initiation of apoptosis. The ability to attenuate the intrinsic caspase-3 activation in the AD-associated mutant cells by EGb761 suggests a potential role for this herbal extract in AD therapy.
In summary, the results reported herein indicate that EGb761 exerts a combination of antioxidative, antiamyloidogenic, and antiapoptotic effects. Our conclusion is consistent with the recent result of a genome-wide monitoring of the biochemical effects of herbal remedies in mice, in which the neuromodulatory actions of Ginkgo biloba were pinpointed to many proteins that are significantly overtranscribed in the hippocampus and cortex (38). It is of a particular interest that the apoptosis-related genes were either up- or down-regulated in PC12 cells treated with EGb761 (32).
Acknowledgments
We thank Dr. Sabine Heinhorst for helpful discussion, Ann Curry for technical assistance with electron microscopy, Slobodanka D. Manceva for Aβ sample preparation, and Jay Russell for immunoblotting. J.P.B. was a participant in the University of Southern Mississippi's Summer Undergraduate Research Program. This work was supported by a National Institutes of Health/National Center for Complementary and Alternative Medicine Grant AT00293–01A2 (to Y.L.) and, in part, by a grant from the Alzheimer's Association (to H.X.).
Abbreviations
AD, Alzheimer's disease
Aβ, amyloid β
APP, amyloid precursor protein
PS, presinilin
wt, wild type
References
- 1.DeFeudis F. V. & Drieu, K. (2000) Curr. Drug Targets 1, 25-58. [DOI] [PubMed] [Google Scholar]
- 2.Christen Y. (2001) Adv. Ginkgo biloba Extr. Res. 8, 1-12. [Google Scholar]
- 3.Luo Y. (2001) J. Alz. Dis. 3, 401-407. [DOI] [PubMed] [Google Scholar]
- 4.Gandy S., Caporaso, G., Buxbaum, J., Frangione, B. & Greengard, P. (1994) Neurobiol. Aging 15, 253-256. [DOI] [PubMed] [Google Scholar]
- 5.Sisodia S. S. (1999) J. Clin. Invest. 104, 1169-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price D. L. & Sisodia, S. S. (1998) Annu. Rev Neurosci. 21, 479-505. [DOI] [PubMed] [Google Scholar]
- 7.Marx J. (2001) Science 293, 2192-2194. [DOI] [PubMed] [Google Scholar]
- 8.Kokoszka J. E., Coskun, P., Esposito, L. A. & Wallace, D. C. (2001) Proc. Natl. Acad. Sci. USA 98, 2278-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yankner B. A. (1996) Neuron 16, 921-932. [DOI] [PubMed] [Google Scholar]
- 10.Head E., Garzon-Rodriguez, W., Johnson, J. K., Lott, I. T., Cotman, C. W. & Glabe, C. (2001) Neurobiol. Dis. 8, 792-806. [DOI] [PubMed] [Google Scholar]
- 11.Stadelmann C., Deckwerth, T. L., Srinivasan, A., Bancher, C., Bruck, W., Jellinger, K. & Lassmann, H. (1999) Am. J. Pathol. 155, 1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa T., Zhu, H., Morishima, N., Li, E., Xu, J., Yankner, B. A. & Yuan, J. (2000) Nature (London) 403, 98-103. [DOI] [PubMed] [Google Scholar]
- 13.Ganzera M., Zhao, J. & Khan, I. A. (2001) Chem. Pharm. Bull. (Tokyo) 49, 1170-1173. [DOI] [PubMed] [Google Scholar]
- 14.Thinakaran G., Borchelt, D. R., Lee, M. K., Slunt, H. H., Spitzer, L., Kim, G., Ratovitsky, T., Davenport, F., Nordstedt, C., Seeger, M., et al. (1996) Neuron 17, 181-190. [DOI] [PubMed] [Google Scholar]
- 15.Xu H., Sweeney, D., Wang, R., Thinakaran, G., Lo, A. C., Sisodia, S. S., Greengard, P. & Gandy, S. (1997) Proc. Natl. Acad. Sci. USA 94, 3748-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder S. W., Ladror, U. S., Wade, W. S., Wang, G. T., Barrett, L. W., Matayoshi, E. D., Huffaker, H. J., Krafft, G. A. & Holzman, T. F. (1994) Biophys. J. 67, 1216-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeVine H. & Scholten, J. D. (1999) Methods Enzymol. 309, 467-476. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. (1996) Cell 86, 147-157. [DOI] [PubMed] [Google Scholar]
- 19.Green D. R. & Reed, J. C. (1998) Science 281, 1309-1312. [DOI] [PubMed] [Google Scholar]
- 20.Thornberry N. A. & Lazebnik, Y. (1998) Science 281, 1312-1316. [DOI] [PubMed] [Google Scholar]
- 21.Arispe N., Pollard, H. B. & Rojas, E. (1993) Proc. Natl. Acad. Sci. USA 90, 10573-10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson K. L., Butler, L. & Ingram, V. M. (1997) Brain Res. 744, 7-14. [DOI] [PubMed] [Google Scholar]
- 23.Hartley D. M., Walsh, D. M., Ye, C. P., Diehl, T., Vasquez, S., Vassilev, P. M., Teplow, D. B. & Selkoe, D. J. (1999) J. Neurosci. 19, 8876-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappolla M., Bozner, P., Soto, C., Shao, H., Robakis, N. K., Zagorski, M., Frangione, B. & Ghiso, J. (1998) J. Biol. Chem. 273, 7185-7188. [DOI] [PubMed] [Google Scholar]
- 25.Weggen S., Eriksen, J. L., Das, P., Sagi, S. A., Wang, R., Pietrzik, C. U., Findlay, K. A., Smith, T. E., Murphy, M. P., Bulter, T., et al. (2001) Nature (London) 414, 212-216. [DOI] [PubMed] [Google Scholar]
- 26.Fraser P. E., Darabie, A. A. & McLaurin, J. A. (2001) J. Biol. Chem. 276, 6412-6419. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard B. J., Konopka, G., Russell, M. & Ingram, V. M. (1997) Brain Res. 776, 40-50. [DOI] [PubMed] [Google Scholar]
- 28.Soto C., Sigurdsson, E. M., Morelli, L., Kumar, R. A., Castano, E. M. & Frangione, B. (1998) Nat. Med. 4, 822-826. [DOI] [PubMed] [Google Scholar]
- 29.Tsuzuki K., Fukatsu, R., Yamaguchi, H., Tateno, M., Imai, K., Fujii, N. & Yamauchi, T. (2000) Neurosci. Lett. 281, 171-174. [DOI] [PubMed] [Google Scholar]
- 30.Ramassamy C., Christen, Y. & Poirier, J. (2001) Adv. Ginkgo biloba Extr. Res. 8, 71-89. [Google Scholar]
- 31.Yao Z., Drieu, K. & Papadopoulos, V. (2001) Brain Res. 889, 181-190. [DOI] [PubMed] [Google Scholar]
- 32.Smith, J. V., Burdick, A. J., Golik, P., Khan, I., Wallace, D. & Luo, Y. (2002) Cell. Mol. Biol., in press. [PubMed]
- 33.Mattson M. P., Partin, J. & Begley, J. G. (1998) Brain Res. 807, 167-176. [DOI] [PubMed] [Google Scholar]
- 34.Yankner B. A., Duffy, L. K. & Kirschner, D. A. (1990) Science 250, 279-282. [DOI] [PubMed] [Google Scholar]
- 35.Kurita-Ochiai T., Ochiai, K. & Fukushima, K. (2001) Clin. Diagn. Lab. Immunol. 8, 325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budihardjo I., Oliver, H., Lutter, M., Luo, X. & Wang, X. (1999) Annu. Rev. Cell Dev. Biol. 15, 269-290. [DOI] [PubMed] [Google Scholar]
- 37.Troy C. M., Rabacchi, S. A., Friedman, W. J., Frappier, T. F., Brown, K. & Shelanski, M. L. (2000) J. Neurosci. 20, 1386-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe C. M., Wolffram, S., Ader, P., Rimbach, G., Packer, L., Maguire, J. J., Schultz, P. G. & Gohil, K. (2001) Proc. Natl. Acad. Sci. USA 98, 6577-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]