Abstract
Conformationally altered proteins and protein fragments derived from the extracellular matrix and hemostatic system may function as naturally occurring angiogenesis inhibitors. One example of such a protein is cleaved high molecular weight kininogen (HKa). HKa inhibits angiogenesis by inducing apoptosis of proliferating endothelial cells, effects mediated largely by HKa domain 5. However, the mechanisms underlying the antiangiogenic activity of HKa have not been characterized, and its binding site on proliferating endothelial cells has not been defined. Here, we report that the induction of endothelial cell apoptosis by HKa, as well as the antiangiogenic activity of HKa in the chick chorioallantoic membrane, was inhibited completely by antitropomyosin monoclonal antibody TM-311. TM-311 also blocked the high-affinity Zn2+-dependent binding of HKa to both purified tropomyosin and proliferating endothelial cells. Confocal microscopic analysis of endothelial cells stained with monoclonal antibody TM-311, as well as biotin labeling of cell surface proteins on intact endothelial cells, revealed that tropomyosin exposure was enhanced on the surface of proliferating cells. These studies demonstrate that the antiangiogenic effects of HKa depend on high-affinity binding to endothelial cell tropomyosin.
Angiogenesis plays a central role in tumor progression (1, 2). This process is stimulated by growth factors such as vascular endothelial growth factor (3, 4) and basic fibroblast growth factor (bFGF; ref. 5) and inhibited by conformationally altered proteins or protein fragments often derived from the extracellular matrix (6) or hemostatic system (7). Although tumors remain dormant when the influences of pro- and antiangiogenic factors are balanced (8), triggering of an “angiogenic switch” may lead to a net increase in angiogenesis and tumor progression (9, 10).
We recently reported that the two-chain form of human high molecular weight kininogen (HKa) inhibits angiogenesis by selectively inducing apoptosis of proliferating endothelial cells (11). These effects are mediated largely by kininogen domain 5 (11), particularly regions within the C terminus of this domain that mediate the binding of HKa to endothelial cells (12). However, the endothelial-binding site for HKa through which these effects are mediated has not been defined, and our previous studies failed to demonstrate an essential role for any of the known endothelial cell receptors for single-chain kininogen (HK) or HKa including the urokinase receptor (13), the receptor for the globular heads of C1q (14, 15), and cytokeratin 1 (16). Here, we report that the antiangiogenic activity of HKa depends on a high-affinity binding interaction with tropomyosin exposed on the surface of proliferating endothelial cells.
Materials and Methods
Materials.
Two-chain HKa was purchased from Enzyme Research Laboratories (Bloomington, IN). Recombinant bFGF and vascular endothelial growth factor were from Becton-Dickinson Biosciences (Franklin Lakes, NJ). The antitropomyosin monoclonal antibody (mAb) TM-311, raised against chicken gizzard tropomyosin, was obtained as ascites from Sigma and purified by using protein G-Sepharose. Affinity-purified rabbit antibodies that block the binding of HKa to domains 2 and 3 of the urokinase receptor have been described (13). A rabbit antibody that blocks HK binding to cytokeratin 1 was a gift of Alvin Schmaier (16), and an mAb that blocks binding of HK to the endothelial cell receptor for the globular heads of C1q was a kind gift of Berhane Geebreheweit (14). N-hydroxysuccinimide (sulfo)-LC-biotin and bis(sulfosuccinimidyl) suberate were from Pierce. Control antibodies against O6-methylguanine DNA methyltransferase and nuclear factor κB (NF-κB) were from Kamiya Biomedical (Seattle) and Santa Cruz Biotechnology, respectively.
Cloning and Expression of HKa Domain 5 (HKa D5).
Recombinant HKa D5 was produced as a calmodulin-binding protein (CBP) conjugate in Escherichia coli. Briefly, domain 5 cDNA was PCR-amplified from a full-length HK cDNA by using primers 5′-CGGGATCCGTAAGTCCACCCCACACTTC-3′ and 5′-CGAATTCTCAGCTTGCCAAATGCTC-3′. The purified PCR product was digested with BamHI and EcoRI and ligated into pCAL-n (Stratagene). The vector was transformed into BL21(DE3) cells, and subclones were grown and induced with 1 mM isopropyl β-D-thiogalactoside. SDS/PAGE revealed that the majority of CBP–HKa D5 was in inclusion bodies. To purify these, the pellet from a 500-ml bacterial culture was lysed, homogenized in 4% tergitol, and centrifuged at 10,000 × g for 45 min. The purified inclusion bodies were sonicated in 7 M guanidine HCl, and the denatured protein was clarified by centrifugation and then added to 1,000 ml of 50 mM bicine, pH 8.8, containing 150 mM NaCl. The refolded CBP–HKa D5 was purified by chromatography on HiTrap SP (Amersham Pharmacia) and then digested with α-thrombin (2.5 μg/mg CBP–D5). Free HKa D5 was purified by using Mono S.
Cell Culture.
Human umbilical vein endothelial cells were isolated and cultured as described (11). MDA-MB-231 breast carcinoma cells were obtained from the American Type Culture Collection.
Endothelial Cell Proliferation Assays.
The effect of HKa on endothelial cells in the absence or presence of mAb TM-311 was assessed initially by using a proliferation assay (11). Relative numbers of cells remaining in each well of a 96-well microplate after incubation for 48 h in the absence or presence of HKa were determined by using the AQueous cell-proliferation assay (Promega). Results are presented as the percent inhibition of bFGF-induced endothelial proliferation, which reflects HKa-induced endothelial cell apoptosis (11). Although bFGF was used in most studies, identical results were obtained by using vascular endothelial growth factor.
Assessment of Endothelial Cell Apoptosis.
The effect of TM-311 on HKa or HKa D5-induced endothelial apoptosis was determined by using several methods. First, staining of control or HKa-exposed endothelial cells by using 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI, Molecular Probes) was used to highlight apoptosis-associated changes in nuclear morphology (11). Second, apoptosis was assessed by terminal deoxynucleotidyltransferase-mediated dUTP end-labeling staining using the Apo-Direct kit (PharMingen). Cells were counterstained with propidium iodide to define all nuclei, and the percentage of apoptotic (terminal deoxynucleotidyltransferase-mediated dUTP end labeling-positive) nuclei was determined by manual counting. Finally, to assess endothelial apoptosis by endonucleolytic cleavage of DNA, endothelial cell DNA was isolated and separated by using 0.8% agarose gel electrophoresis. Gels were stained with ethidium bromide, and DNA was visualized by using UV light (11).
Exposure of Tropomyosin on the Cell Surface.
Two approaches were used to detect exposure of tropomyosin on endothelial cells. First, confluent or proliferating endothelial cells cultured in Lab-Tek chambers (Nunc) were fixed by exposure to 3.7% paraformaldehyde, blocked by using 10% donkey serum, and then incubated with either mAb TM-311 or nonimmune murine IgG1. Bound antibody was detected by using rhodamine-conjugated donkey anti-mouse IgG, and stained cells were examined by using a Bio-Rad MRC 600 laser scanning confocal microscope. For confocal imaging, control stains were set to a black background, and positive samples were viewed at the same laser intensity, aperture, gain, and black-level settings. One-micrometer optical slices were taken for each sample, beginning at the coverslip and ending at the apical surface. Projections were acquired by using CONFOCAL ASSISTANT IMAGING 4.02 software.
To assess further the exposure of tropomyosin on the surface of confluent or proliferating endothelial cells, unfixed cells were labeled by using N-hydroxysuccinimide (sulfo)-LC-biotin as described (17). After labeling, cell extracts were prepared in a buffer containing 0.1 M Tris⋅HCl (pH 7.4), 1% Triton X-100, and protease inhibitors, and equal amounts of protein from confluent and subconfluent cultures were immunoprecipitated with mAb TM-311. Precipitated proteins were separated by 10% SDS/PAGE (18) and transferred to a poly(vinylidene difluoride) membrane. Biotinylated proteins were detected by incubating the membrane with streptavidin-peroxidase and chemiluminescence reagent (Super Signal, Pierce) before exposure to Kodak XL blue autoradiographic film. Parallel studies were performed by using control antibodies against two known intracellular proteins, O6-methylguanine DNA methyltransferase and NF-κB.
Cross-Linking of HKa to Endothelial Cells.
To determine whether HKa interacted with a specific protein on proliferating endothelial cells, we determined whether it could be cross-linked to such a protein. Biotin-labeled HKa was incubated with proliferating or confluent endothelial cells for 30 min at 37°C. Cells then were washed and exposed to the bifunctional, membrane-impermeable cross-linker bis(sulfosuccinimidyl) suberate for 15 min at room temperature (17). Detergent extracts were prepared, and 40 μg of cell protein from proliferating and confluent cultures were separated by 7.5% SDS/PAGE. Proteins were transferred to poly(vinylidene difluoride), and biotinylated proteins were detected by using streptavidin-peroxidase and chemiluminescence. To assess the specificity of the cross-linking procedure, studies were performed also in the presence of a 50-fold molar excess of unlabeled HKa and with MDA-MB-231 breast carcinoma cells.
Binding of HKa to Endothelial Cells and Purified Tropomyosin.
Binding of HKa to endothelial cells was measured as described (12). Briefly, human umbilical vein endothelial cells (3 × 104 cells per ml) were cultured in 96-well microplates and then washed and incubated with increasing concentrations of biotin-HKa for 2 h at 4°C. After brief washing, cells were incubated sequentially with a 1:750 dilution of streptavidin peroxidase and the peroxidase substrate turbo-TMB (Pierce) before measurement of A490. Binding was measured in the presence (to determine total binding) and absence (to determine nonspecific binding) of 10 μM Zn2+, and specific binding was defined as the difference between total and nonspecific binding (19). The dissociation constant (Kd) was determined by fitting the saturation isotherm by nonlinear regression with PRISM software (GraphPad, San Diego).
In selected experiments, the ability of mAb TM-311 to inhibit the binding of HKa to cells was assessed by incubating biotin-HKa with endothelial cells in the presence of increasing concentrations of antibody. The concentration of TM-311 that inhibited HKa binding by 50% (IC50) was determined from plots of bound HKa versus the log of the TM-311 concentration.
To measure the binding of HKa to purified tropomyosin, 96-well microplates were coated with 20 μg/ml of chicken gizzard tropomyosin (Sigma) or BSA as a control. Wells then were blocked by incubation with PBS containing 5% nonfat milk and incubated with increasing concentrations of HKa (0.01–20 nM) for 2 h. Specifically bound HKa was quantitated as described for cell-binding assays (19) and by assessing the ability of a 100-fold molar excess of unlabeled HKa to compete with biotin-HKa for binding.
In selected experiments, the ability of mAb TM-311 and recombinant HKa D5 to inhibit the binding of biotin-HKa to tropomyosin was assessed. These studies allowed determination of the Kd for binding of HKa D5 to tropomyosin using the equation Kd(D5) = IC50/(1 + [HKa])/Kd(HKa), where IC50 is the concentration of HKa D5 that inhibited HKa binding by 50%, and Kd(HKa) is the Kd for binding of HKa to tropomyosin.
Chick Chorioallantoic Membrane (CAM) Assay.
We used the CAM assay to assess the role of tropomyosin in the antiangiogenic activity of HKa in vivo (20). Three-day-old fertilized White Leghorn chicken eggs were cracked in sterile Petri dishes. Embryos then were cultured at 37°C under 4% CO2 until day 7, at which time a 3.0-mm filter disk containing 30 ng of bFGF (positive control), 30 ng of bFGF and 10 μg HKa, or 30 ng of bFGF, 10 μg of HKa, and 20 μg of TM-311 was placed on the CAM. Each experimental condition was tested in at least six eggs. On day 10, embryos were photographed by using a SPOT digital camera. Angiogenesis was quantitated by counting the number of neovessels in direct contact with the filter disk.
Results
mAb TM-311 Blocks the Antiendothelial Cell Activity of HKa.
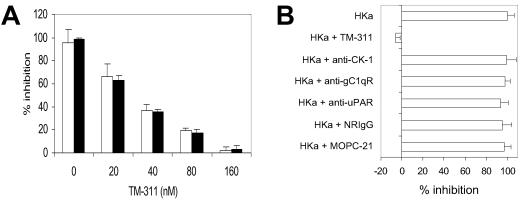
The rationale for assessing the role of tropomyosin in the antiangiogenic activity of HKa was based on preliminary studies aimed at defining the structure of Zn2+-bound HKa D5∥ that suggested structural homology between HKa D5 and endostatin, a Zn2+-binding, antiangiogenic polypeptide comprised of the NC domain of collagen XVIII (21, 22). In light of prior studies in which we could not demonstrate an essential role for previously reported endothelial cell HK or HKa-binding sites in mediating the antiangiogenic activity of HKa (11) and a recent report suggesting that the antiangiogenic activity of endostatin was mediated through binding to tropomyosin (23), we questioned whether tropomyosin might function also in such a capacity for HKa. To address this hypothesis, we determined whether mAb TM-311 affected the ability of HKa to block growth factor-induced endothelial proliferation (11). TM-311 blocked the inhibition of bFGF and vascular endothelial growth factor-induced endothelial cell proliferation by HKa and HKa D5 in a concentration-dependent manner (Fig. 1A), whereas antibodies that block the binding of HKa to the urokinase receptor (13), the receptor for the globular heads of C1q (14, 15), or cytokeratin 1 (ref. 16; Fig. 1B) did not.
Fig 1.
(A) Inhibition of bFGF (10 ng/ml)-induced endothelial cell proliferation by 20 nM HKa (open bars) and 60 nM HKa D5 (black bars) is prevented by increasing concentrations of mAb TM-311. Proliferation was measured as described in Materials and Methods. (B) Effect of mAb TM-311 and antibodies against other endothelial cell HK or HKa-binding proteins on the inhibition of endothelial cell proliferation caused by 20 nM HKa. Proliferation was measured in response to 10 ng/ml bFGF. All antibodies were used at a concentration of 300 nM. The control antibody for polyclonal antiurokinase receptor (uPAR) and cytokeratin 1 (CK-1) antibodies was normal rabbit IgG (NRIgG), and that for mAb TM-311 and mAbs against the receptor for the globular heads of C1q (gC1qR) was MOPC-21.
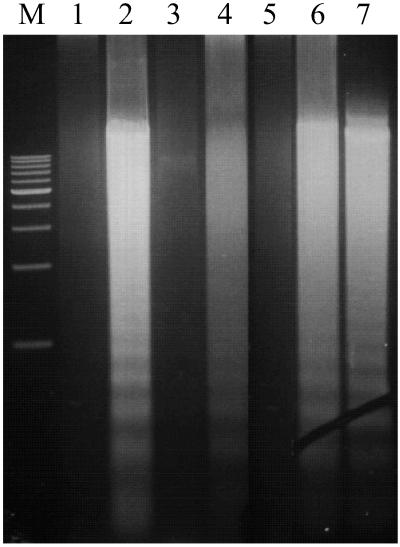
These effects reflected inhibition of HKa-induced endothelial cell apoptosis by TM-311. TM-311 prevented the endonucleolytic fragmentation of DNA (Fig. 2) as well as the characteristic apoptotic changes in nuclear morphology and the increase in terminal deoxynucleotidyltransferase-mediated dUTP end labeling-positive cells after exposure of proliferating endothelial cells to HKa or HKa D5 (data not shown; ref. 11). No such effect of nonimmune murine IgG (MOPC-21) was observed.
Fig 2.
Effect of mAb TM-311 on HKa-induced endothelial cell apoptosis assessed by endonucleolytic cleavage of DNA. Endothelial cell DNA was isolated from cells cultured for 12 h in the presence of mAb TM-311 alone (lane 1), 20 nM HKa (lane 2), 20 nM HKa + 60 nM TM-311 (lane 3), 50 nM HKa D5 (lane 4), 50 nM HKa D5 + 150 nM mAb TM-311 (lane 5), 2 μM 2-methoxyestradiol (lane 6), or 2 μM 2-methoxyestradiol + 6 μM mAb TM-311 (lane 7) and analyzed as described in Materials and Methods. M, markers.
To determine the specificity of these effects, we assessed the ability of mAb TM-311 to inhibit endothelial cell apoptosis induced by 2-methoxyestradiol (24). However, even when used at a concentration of 6 μM, mAb TM-311 did not inhibit 2 μM 2-methoxyestradiol-induced endothelial apoptosis (Fig. 2, lanes 6 versus 7).
Tropomyosin Is Present on the Surface of Proliferating Endothelial Cells.
The results described above suggested an essential role for tropomyosin in mediating HKa-induced endothelial cell apoptosis. However, tropomyosin is a cytoskeletal protein, and exposure of tropomyosin on the endothelial surface has not been reported. Indeed, in only one prior study was a single isoform of tropomyosin, human tropomyosin (hTM)5, shown to be externalized by colonic epithelial cells and a colon carcinoma cell line (25). Although a recent report suggested that the antiangiogenic activity of endostatin requires interaction with tropomyosin, it was hypothesized that internalization of endostatin was necessary for this to occur (23).
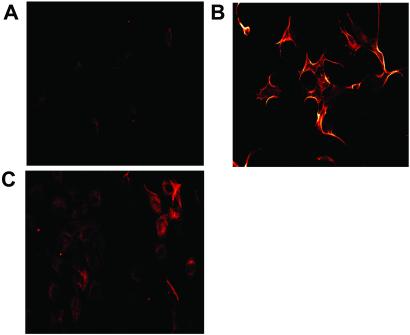
To assess whether tropomyosin is expressed on the endothelial surface and to address the selectivity of HKa for proliferating endothelial cells (11), we first used confocal scanning laser microscopy. Proliferating cells stained specifically with TM-311 (Fig. 3 A versus B) and more prominent surface staining of these cells compared with confluent cells was observed (Fig. 3 B versus C). The staining pattern of proliferating cells was unchanged after permeabilization by exposure to 0.2% Triton X-100 (data not shown), although an intracellular pool of tropomyosin was more evident in the confluent cells (Fig. 3C).
Fig 3.
Confocal laser scanning microscopic analysis of proliferating and confluent endothelial cells. (A) Proliferating endothelial cells stained with MOPC-21. (B) Proliferating endothelial cells stained with TM-311. (C) Confluent endothelial cells stained with TM-311. Cells were permeabilized by exposure to 0.1% Triton X-100 before staining.
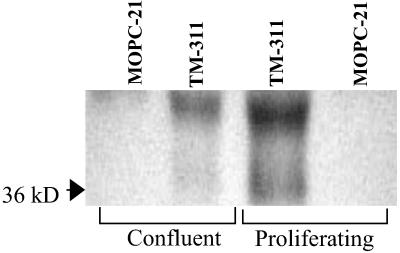
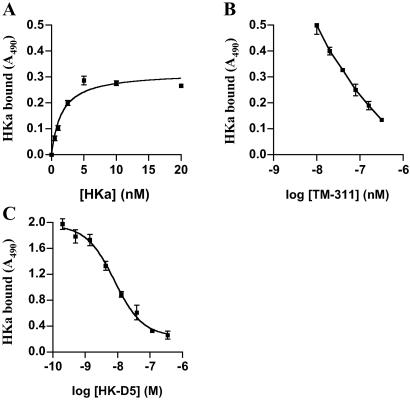
To confirm these results, surface proteins on proliferating or confluent endothelial cells were labeled with biotin, and detergent extracts of the labeled cells were immunoprecipitated with mAb TM-311. TM-311 precipitated proteins of ≈39 and ≈36 kDa, consistent with the expected molecular mass of tropomyosin (26, 27), from both endothelial cell cultures (Fig. 4). However, these proteins were precipitated in markedly greater amounts from proliferating cells (Fig. 4). Control antibodies against O6-methylguanine DNA methyltransferase and NF-κB successfully precipitated their target antigens from surface-labeled endothelial cells, although, as expected, the precipitated proteins were not labeled.
Fig 4.
Immunoprecipitation of endothelial tropomyosin by mAb TM-311. Cell surface proteins on proliferating and confluent human umbilical vein endothelial cells were labeled with N-hydroxysuccinimide (sulfo)-LC-biotin. Equal amounts of protein from detergent extracts of each culture were immunoprecipitated by using TM-311 or MOPC-21. Immunoprecipitated proteins were separated by using 10% SDS/PAGE, transferred to poly(vinylidene difluoride), and detected by using streptavidin-peroxidase and chemiluminescence.
Characterization of the Binding of HKa to Endothelial Cells and Tropomyosin.
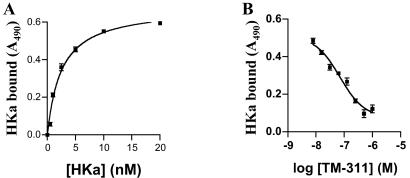
To explore further the possibility that HKa might bind to tropomyosin on endothelial cells, we used several approaches. First, we determined whether mAb TM-311 blocked the binding of HKa to subconfluent endothelial cells. Biotin-HKa bound specifically to these cells in a Zn2+-dependent manner, with a Kd of ≈2.5 nM (Fig. 5A). MAb TM-311 but not MOPC-21 blocked most of the specific binding of HKa to cells (Fig. 5B).
Fig 5.
(A) Specific binding of HKa to proliferating endothelial cells. Cells were cultured as in proliferation assays and incubated with increasing concentrations of biotin-HKa in the absence or presence of 10 μM Zn2+. Cell-bound biotin-HKa was detected by using streptavidin peroxidase and the peroxidase substrate turbo-TMB. The curve was fit by nonlinear regression, yielding a Kd of ≈2.5 nM. (B) Inhibition of the binding of biotin-HKa to proliferating endothelial cells by mAb TM-311. Biotin-HKa (20 nM) was incubated with endothelial cells in the presence of 10 μM Zn2+ and increasing concentrations of mAb TM-311. Cell-bound biotin-HKa was determined as described for A.
Next, we analyzed the binding of HKa to purified tropomyosin immobilized in 96-well microplates. HKa bound with similar affinity (Kd ≈ 1.6 nM) and in a Zn2+-dependent manner to purified tropomyosin (Fig. 6A). MAb TM-311 (Fig. 6B) as well as HKa D5 (Fig. 6C) blocked binding, confirming that HKa D5 binds with high affinity to tropomyosin (estimated Kd ≈ 2.1 nM). HK also bound to tropomyosin but with lower affinity than HKa, because it was a less effective competitor for HKa binding to tropomyosin than HKa D5 or HKa itself.
Fig 6.
(A) Specific binding of HKa to purified tropomyosin. Ninety-six-well plates were coated with tropomyosin and then incubated with increasing concentrations of biotin-HKa in the absence or presence of 10 μM Zn2+. Bound ligand was determined by using streptavidin-peroxidase and turbo-TMB, and the curve was fit by nonlinear regression, yielding a Kd of ≈1.6 nM. (B) Inhibition of the binding of 20 nM biotin-HKa to purified tropomyosin by mAb TM-311. (C) Inhibition of the binding of biotin-HKa (10 nM) to purified tropomyosin by HKa D5. The IC50 for inhibition of HKa binding by HKa D5 was ≈8.1 nM.
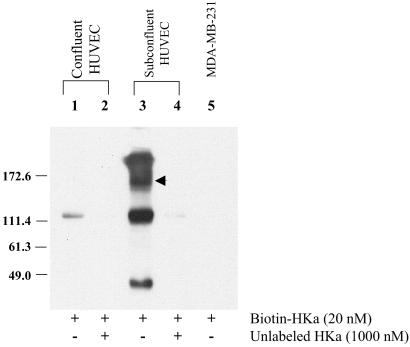
Finally, we determined whether biotinylated HKa could be cross-linked to an endothelial cell surface protein of similar size as tropomyosin. When incubated with confluent endothelial cells before exposure to the membrane-impermeable cross-linker bis(sulfosuccinimidyl) suberate, biotin-HKa was barely detectable in cell extracts (Fig. 7, lane 1). However, HKa was detected in both an uncomplexed form (Mr ≈110) and within a broad, high molecular weight complex (Fig. 7, lane 3) in extracts of proliferating cells. A complex between HKa and tropomyosin should exhibit an Mr of ≈140–150, and a discrete band of this size was observed within the broad band (Fig. 7, arrow). The specificity of this interaction is supported by the observations that complex formation was prevented by excess unlabeled HKa (Fig. 7, lane 4) and that high molecular mass complexes were not observed when studies were performed by using MDA-MB-231 breast carcinoma cells (Fig. 7, lane 5). Moreover, blotting of the immunoprecipitate with TM-311 revealed an immunoreactive band at ≈140 kDa, demonstrating incorporation of tropomyosin into the high molecular mass complex. The presence of HKa within complexes >150 kDa may reflect association of the HKa–tropomyosin complex with actin or other tropomyosin-binding proteins or complexes between HKa and other endothelial-binding proteins.
Fig 7.
Cross-linking of HKa to endothelial cell surface proteins. Biotin-HKa was incubated with confluent (lanes 1 and 2) or proliferating (lanes 3 and 4) endothelial cells in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of a 20-fold molar excess of unlabeled HKa before cross-linking by using bis(sulfosuccinimidyl) suberate. Biotin-HKa was incubated also with MDA-MB-231 breast carcinoma cells under identical conditions (lane 5). Detergent extracts were separated by SDS/PAGE and then transferred to poly(vinylidene difluoride) and detected by chemiluminescence. The arrowhead denotes a prominent band of ≈140–150 kDa, the expected size of an HKa-tropomyosin complex. HUVEC, human umbilical vein endothelial cells.
TM-311 Blocks the Antiangiogenic Effects of HKa in Vivo.
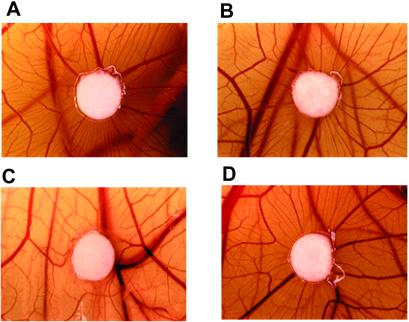
We next used the CAM assay to assess the functional role of tropomyosin in an in vivo angiogenesis model. HKa inhibited CAM angiogenesis induced by bFGF-containing filter discs by ≈85% as determined by vessel counts (Fig. 8 A versus C). Similar results were obtained by using HKa D5 (data not shown; ref. 28). However, the antiangiogenic effect of HKa was blocked by TM-311 (Fig. 8 C versus D), whereas nonimmune murine IgG was without effect (data not shown). No effect of mAb TM-311 alone or in the presence of bFGF on vessel formation was observed. These studies demonstrate that tropomyosin mediates the antiangiogenic activity of HKa in vivo as well as in vitro.
Fig 8.
Effect of HKa and/or TM-311 on in vivo angiogenesis in the CAM. Angiogenesis is represented by the fine neovessels contacting and radiating from the filter disk in a spoke-like pattern. The effects of bFGF (A), bFGF + TM-311 (B), bFGF + HKa (C), and bFGF + HKa + TM-311 (D) are depicted. HKa inhibited bFGF-induced angiogenesis by ≈85%, and these effects were inhibited completely by mAb TM-311.
Discussion
These results demonstrate that HKa binds with high affinity to tropomyosin through interactions involving HKa D5, and that inhibition of this interaction blocks the induction of endothelial apoptosis and inhibition of angiogenesis by HKa. These observations demonstrate that the effects of HKa on proliferating endothelial cells require direct binding to tropomyosin.
In addition to identifying a previous uncharacterized endothelial cell-binding site for HKa, our studies provide insight into the biology of endothelial cells during angiogenesis by demonstrating that tropomyosin, a cytoskeletal protein, is preferentially exposed on the surface of proliferating cells. Exposure of tropomyosin on the endothelial surface was documented by direct visualization with confocal microscopy and by demonstrating that tropomyosin was accessible to biotinylation by N-hydroxysuccinimide (sulfo)-LC-biotin. Prior studies that have examined the binding of HK or HKa to endothelial cells have used confluent, static endothelial monolayers; however, our findings demonstrate that under these conditions tropomyosin is minimally exposed on the cell surface, and hence other binding proteins play a more prominent role in the binding of single- (12, 15, 29–32) or two-chain (13) HKa.
These findings are consistent with studies demonstrating that the endothelial cell cytoskeleton undergoes dramatic structural rearrangement during the transition between a quiescent and proliferative state (33–35). However, other than one report in which actin was demonstrated on the surface of cultured endothelial cells (36), there has been little evidence for exposure of cytoskeletal components on the endothelial surface. Hence, our results challenge the paradigm that cytoskeletal proteins exist solely within the confines of the endothelial plasma membrane under all conditions. Moreover, the observation that mAb TM-311 inhibits the antiangiogenic activity of HKa in the CAM assay suggests that tropomyosin is available also on the surface of angiogenic endothelial cells in vivo.
Vertebrate cells express a number of tropomyosin isoforms in a cell-specific manner. The expression of tropomyosin in nonmuscle cells has been studied most intensively in human fibroblasts, which express at least eight isoforms (27). Tropomyosin binds and stabilizes actin filaments, protecting them from severing or depolymerizing by factors such as gelsolin or actin depolymerizing factor (27, 37). Several groups have demonstrated a role for alterations in tropomyosin isoform expression in promoting cellular transformation (27, 38, 39), suggesting that tropomyosin may influence the state of cytoskeletal organization. We hypothesize that alteration in the expression and cellular localization of tropomyosin may contribute to the transition of endothelial cells from a quiescent to an angiogenic phenotype.
The identification of tropomyosin as an endothelial-binding site required for the antiangiogenic activity of HKa raises several questions concerning the mechanism(s) by which HKa induces endothelial apoptosis. Although HK and HKa express antiadhesive properties (40, 41), the ability of HKa to induce apoptosis of proliferating endothelial cells at concentrations below those generally used in defining its antiadhesive activity argues that additional mechanisms for its antiangiogenic activity exist (11). The studies reported here suggest that HKa may affect the endothelial cell cytoskeleton directly, perhaps causing secondary alterations in downstream signaling pathways dependent on reciprocal interactions between the cytoskeleton and adhesion receptors. Indeed, biomechanical influences mediated through the cytoskeleton play a critical role in processes such as cell-cycle entry, cellular growth, and apoptosis (33–35). Although both TM-311 and HK also bind to tropomyosin, neither induces endothelial apoptosis, suggesting that HKa binds to TM in a unique manner and induces ligand specific responses.
In a recent report, MacDonald et al. demonstrated crossreactivity between an antibody reactive with an endostatin-binding cyclic peptide and hTM3 and presented evidence for involvement of hTM3 in the antiangiogenic effects of endostatin in vivo (23). Both this antibody and mAb TM-311 recognized a ≈38-kDa endothelial cell protein, suggesting that TM-311, the tropomyosin isoform specificity of which has not been characterized, also may recognize hTM3. Because TM-311 blocked HKa-induced endothelial cell apoptosis, hTM3 also may mediate the antiangiogenic activity of HKa. However, in our studies TM-311 recognized endothelial proteins of ≈39 and ≈36 kDa (Fig. 4), and therefore the specific role of hTM3 in mediating the activity of HKa requires additional study. If HKa binds hTM3, then the nature of this interaction may differ from that of endostatin, because the affinity of HKa for tropomyosin (Kd ≈ 1.6 nM) is substantially higher than that of endostatin (Kd ≈ 100 μM; ref. 23). Moreover, although endostatin may require internalization before interacting with hTM3 (23), HKa is not internalized by endothelial cells (42), and our binding studies are consistent with direct binding of HKa to cell surface tropomyosin. Nevertheless, the observation that at least two antiangiogenic polypeptides bind to endothelial cells through interactions with tropomyosin suggests a potentially broader role for tropomyosin and perhaps other cytoskeletal proteins in mediating activity of naturally occurring angiogenesis inhibitors.
In summary, we have demonstrated that an interaction between HKa and endothelial tropomyosin underlies the antiangiogenic activity of HKa. Further efforts to characterize the biology of endothelial tropomyosin as well as the binding interaction between tropomyosin and HKa may yield insight not only into the regulation of angiogenesis but also the development of novel agents targeted toward arrest of pathologic angiogenesis.
Acknowledgments
This work was supported by National Cancer Institute Grant RO1 CA83134 and Department of the Army Grant DAMD17-00-1-0078.
Abbreviations
bFGF, basic fibroblast growth factor
HKa, two-chain (cleaved) high molecular weight kininogen
HK, single-chain high molecular weight kininogen
HKa D5, HKa domain 5
CAM, chick chorioallantoic membrane
hTM, human tropomyosin
This paper was submitted directly (Track II) to the PNAS office.
Kumar, G. A., McCrae, K. R. & Pang, Y. P. (2001) Abstr. Am. Chem. Soc. 222, 134 (abstr.).
References
- 1.Folkman J. (1995) Nat. Med. 1, 27-31. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. (1985) Adv. Cancer Res. 43, 175-203. [DOI] [PubMed] [Google Scholar]
- 3.Shweiki D., Itin, A., Soffer, D. & Keshet, E. (1992) Nature (London) 359, 843-845. [DOI] [PubMed] [Google Scholar]
- 4.Thomas K. A. (1996) J. Biol. Chem. 271, 603-606. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y. & Becker, D. (1997) Nat. Med. 3, 887-893. [DOI] [PubMed] [Google Scholar]
- 6.Sage E. H. (1997) Trends Cell Biol. 7, 182-186. [DOI] [PubMed] [Google Scholar]
- 7.Browder T., Folkman, J. & Pirie-Shepard, S. (2000) J. Biol. Chem. 275, 1521-1524. [DOI] [PubMed] [Google Scholar]
- 8.Holmgren L., O'Reilly, M. S. & Folkman, J. (1995) Nat. Med. 1, 149-153. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D. & Folkman, J. (1996) Cell 86, 353-364. [DOI] [PubMed] [Google Scholar]
- 10.Dameron K. M., Volpert, O. V., Tainsky, M. A. & Bouck, N. (1994) Science 265, 1582-1584. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J.-C., Claffey, K., Sakthivel, R., Darzynkiewicz, Z., Shaw, D. E., Leal, J., Wang, Y.-C., Lu, F. M. & McCrae, K. R. (2000) FASEB J. 14, 2589-2600. [DOI] [PubMed] [Google Scholar]
- 12.Hasan A. A. K., Cines, D. B., Herwald, H., Schmaier, A. H. & Muller-Esterl, W. (1995) J. Biol. Chem. 270, 19256-19261. [DOI] [PubMed] [Google Scholar]
- 13.Colman R. W., Pixley, R. A., Najamunnisa, S., Yan, W., Wang, J., Mazar, A. & McCrae, K. R. (1997) J. Clin. Invest. 100, 1481-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph K., Ghebrehiwet, B., Peerschke, E. I. B., Reid, K. B. M. & Kaplan, A. P. (1996) Proc. Natl. Acad. Sci. USA 93, 8552-8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herwald H., Dedio, J., Kellner, R., Loos, M. & Muller-Esterl, W. (1996) J. Biol. Chem. 271, 13040-13047. [DOI] [PubMed] [Google Scholar]
- 16.Hasan A. A. K., Zisman, T. & Schmaier, A. H. (1998) Proc. Natl. Acad. Sci. USA 95, 3615-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma K., Simantov, R., Zhang, J.-C., Silverstein, R., Hajjar, K. A. & McCrae, K. R. (2000) J. Biol. Chem. 275, 15541-15548. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U. K. (1970) Nature (London) 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 19.van Iwaarden F., deGroot, P. G. & Bouma, B. N. (1988) J. Biol. Chem. 263, 4698-4703. [PubMed] [Google Scholar]
- 20.Nguyen M., Shing, Y. & Folkman, J. (1993) Microvasc. Res. 47, 31-40. [DOI] [PubMed] [Google Scholar]
- 21.O'Reilly M. S., Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane, W. S., Flynn, E., Birkhead, J. R., Olsen, B. R. & Folkman, J. (1997) Cell 88, 277-285. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y.-H., Javaherian, K., Lo, K.-M., Chopra, R., Boehm, T., Lanciotti, J., Harris, B. A., Li, Y., Shapiro, R., Hohenester, E., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 10443-10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald N. J., Shivers, W. Y., Narum, D. L., Plum, S. M., Wingard, J. N., Fuhrmann, S. R., Liang, H., Holland-Linn, J., Tom Chen, D. H. & Sim, B. K. L. (2001) J. Biol. Chem. 276, 25190-25196. [DOI] [PubMed] [Google Scholar]
- 24.Yue T. L., Wang, X., Louden, C. S., Gupta, S., Pillarisetti, K., Gu, G. L., Hart, T. K., Lysko, P. G. & Feuerstein, G. Z. (1997) Mol. Pharmacol. 51, 951-962. [DOI] [PubMed] [Google Scholar]
- 25.Kesari K. V., Yoshizaki, N., Geng, X., Lin, J. J. C. & Das, K. M. (1999) Clin. Exp. Immunol. 118, 219-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lees-Miller J. P. & Helfman, D. M. (1991) BioEssays 13, 429-437. [DOI] [PubMed] [Google Scholar]
- 27.Lin J. J. C., Warren, K. S., Hamboldt, D. D. & Lin, J. L. C. (1997) Int. Rev. Cytol. 170, 1-38. [DOI] [PubMed] [Google Scholar]
- 28.Colman R. W., Jameson, B. A., Lin, Y., Johnson, D. & Mousa, S. A. (2000) Blood 95, 543-550. [PubMed] [Google Scholar]
- 29.Hasan A. A. K., Cines, D. B., Zhang, J. & Schmaier, A. H. (1994) J. Biol. Chem. 269, 31822-31830. [PubMed] [Google Scholar]
- 30.Herwald H., Hasan, A. A. K., Godovac-Zimmerman, J., Schmaier, A. H. & Muller-Esterl, W. (1995) J. Biol. Chem. 270, 14634-14642. [PubMed] [Google Scholar]
- 31.Dedio J. & Muller-Esterl, W. (1996) FEBS Lett. 399, 255-258. [DOI] [PubMed] [Google Scholar]
- 32.Dedio J., Jahnen-Dechent, W., Bachmann, M. & Muller-Esterl, W. (1998) J. Immunol. 160, 3534-3542. [PubMed] [Google Scholar]
- 33.Ingber D. E. (1997) Annu. Rev. Physiol. 59, 575-599. [DOI] [PubMed] [Google Scholar]
- 34.Ingber D. E., Prusty, D., Sun, Z., Betensky, H. & Wang, N. (1995) J. Biomech. 28, 1471-1484. [DOI] [PubMed] [Google Scholar]
- 35.Huang S. & Ingber, D. E. (2000) Exp. Cell Res. 261, 91-103. [DOI] [PubMed] [Google Scholar]
- 36.Dudani A. K. & Ganz, P. R. (1996) Br. J. Haematol. 95, 168-178. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa R., Yamashiro, S. & Matsumara, F. (1989) J. Biol. Chem. 264, 7490-7497. [PubMed] [Google Scholar]
- 38.Takenaga K., Nakamura, Y. & Sakiyama, S. (1988) Mol. Cell. Biol. 8, 3934-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pradad G. L., Fuldner, R. A. & Cooper, H. L. (1993) Proc. Natl. Acad. Sci. USA 90, 7039-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colman R. W. & Schmaier, A. H. (1997) Blood 90, 3819-3843. [PubMed] [Google Scholar]
- 41.Chavakis T., Kanse, S. M., Lupu, F., Hammes, H. P., Müller-Esterl, W., Pixley, R. A., Colman, R. W. & Preissner, K. T. (2000) Blood 96, 514-522. [PubMed] [Google Scholar]
- 42.Hasan A. A. K., Cines, D. B., Ngaiza, J. R., Jaffe, E. A. & Schmaier, A. H. (1995) Blood 85, 3134-3143. [PubMed] [Google Scholar]