Abstract
Telomerase reverse transcriptase (TRT) is a tumor-associated antigen expressed in the vast majority of human tumors and is presently one of the most promising target candidates for a therapeutic cancer vaccine. TRT is also expressed at low level in selected tissues and should be considered a self antigen. In the present study we sought to develop cytotoxic T lymphocytes (CTL) responses directed against human (h)TRT peptides with low relative affinity for which the available repertoire is to be preferentially spared from tolerance. This was accomplished by using analogue peptides of hTRT whose relative affinity for the MHC was increased by a targeted (→Tyr) substitution in position one. By immunizing HLA A2.1 transgenic mice with these analogue peptides, we identified one such low relative affinity peptide (p572) that is endogenously processed and presented by HLA A2.1 in tumor cells, and is recognized by specific CTL. We used the highly immunogenic analogue peptide to successfully induce TRT-specific CTL in cancer patients and normal donors. CTL against p572-lysed human and mouse tumor cells but not activated autologous B cells. This peptide represents, therefore, an important candidate component of a cancer vaccine based on a TRT substrate and validates the strategy of targeting peptides with low affinity for the MHC for cancer immunotherapy.
Therapeutic vaccines against cancer aim mainly at inducing cytotoxic T lymphocytes (CTL) capable of recognizing and eliminating tumor cells. Proteins from tumor cells whose peptides are specifically recognized by CTL are referred to as tumor-associated antigens (TAA). During the past decade, considerable effort has been made to identify TAA, but most TAA identified to date are proteins uniquely expressed in a given type of tumor. Although this restricts potentially applicable immunotherapies to a limited set of malignancies (1–3), great progress could be made if one could target an antigen present in all types of tumor.
Telomerase is a ribonucleoprotein that mediates RNA-dependent synthesis of telomeric DNA maintaining telomere length and chromosomal stability (4, 5). Telomerase activation is sufficient for immortalization, a key event in the process of malignant transformation (6). Over 85% of all types of human tumors express high telomerase activity (7, 8). In contrast, normal tissues display no or little telomerase activity (7, 8). We and others have recently demonstrated that human telomerase reverse transcriptase (hTRT) represents a source of MHC class I peptides recognized by CD8+ T cells on tumor cells (9, 10), and showed that CTL generated from cancer patients or healthy individuals by in vitro immunization could kill tumor cells of different histological origin (9–11). Experiments in mice showed independently that vaccination with dendritic cells transfected with murine TRT mRNA induces tumor protective CTL responses (12).
Because most TAA are self antigens, tolerance that normally protects the individual from the development of autoimmunity stands as a major potential obstacle in the development of T cell responses capable of eradicating tumors in vivo (13, 14). TRT is no exception because it is expressed early in ontogeny and at low level in selected normal tissues with high replicative activity (15, 16). As shown in the mouse (17, 18), one may expect deletion of CTL precursors that recognize the MHC/TRT peptide complexes with high affinity. Thus, the affinity of a given peptide for the MHC molecule appears to be critical for how central tolerance shapes the available T cell repertoire (19, 20). These studies demonstrated that peptides with low affinity for the MHC molecule, which form unstable complexes, allow specific T cells to escape tolerance.
Here, we report on the identification of a 9-mer peptide (572RLFFYRKSV580) from the hTRT sequence with low affinity for the HLA A2.1 molecule that is efficiently processed and presented in both human and mouse tumor cells. We found that the immunogenicity of peptide p572 could be greatly enhanced by a single amino acid (Arg → Tyr) substitution in position one which augmented its relative affinity for the HLA A2.1 molecule. We used the analogue pY572 peptide, and succeeded in generating wild-type p572-specific CTL both in normal individuals and cancer patients.
Materials and Methods
Mice.
HHD transgenic mice, which express a chimeric HLA A2.1/H2-Db MHC class I molecule, are on a C57BL/6 background and have been described (21). Mice were bred and maintained under specific pathogen-free conditions in the vivarium of the Univ. of California at San Diego or the Institut Pasteur (Paris). All experimental procedures were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Blood Samples.
Prostate cancer patients were recruited through the Division of Urology (Univ. of California at San Diego). Blood from these patients was obtained by venipuncture. HLA-A2+ individuals were selected by flow cytometry screening using the anti-HLA A2 monoclonal antibody BB7.2. Buffy coats from HLA A2+ normal donors were purchased from the San Diego Blood Bank or the Etablissment Francais du Sang (Paris). Experiments were performed in accordance with an approved Institutional Review Board protocol.
Cell Lines.
The human HeLa, T2, U266, and HSS cell lines were purchased from the American Type Culture Collection. The murine EL4–HHD, RMAS–HHD transfectants, and their parental cell lines have been described (21). The HeLa–HHD transfectants were generated by transfection with a plasmid encoding the HHD chimeric class I molecule as described (21).
Peptides.
The synthetic peptides described in Table 1 were purchased from the Biopolymer Synthesis Center (CalTech, Pasadena, CA) or Multiple Peptide Systems (San Diego). The synthetic peptides 128TPPAYRPPNAPIL140 of the hepatitis B virus core antigen, 58GILGFVFTL66 of the matrix antigen of influenza virus, and 476ILKEPVHGV484 of HIV type 1 reverse transcriptase, were purchased from Neosystem (Strasburg, France).
Table 1.
Immunogenicity of selected hTRT HLA A2.1-binding peptides
| Peptide | Sequence | Relative avidity | Immunogenicity |
|---|---|---|---|
| p152 | LLARCALFV | 15 | |
| pY152 | YLARCALFV | 5 | 50, 36, 14, 0, 88, 16, 36, 83 |
| p407 | VLLKTHCLP | 10 | |
| pY407 | YLLKTHCLP | 10 | 0, 0, 0, 0 |
| p555 | ELLRSFFYV | 35 | |
| pY555 | YLLRSFFYV | 3 | 72, 46, 12, 0, 21, 18, 27, 97 |
| p572 | RLFFYRKSV | 30 | |
| pY572 | YLFFYRKSV | 1.9 | 45, 24, 9, 44, 92, 21, 73 |
| p675 | LLGASVLGL | >30 | |
| pY675 | YLGASVLGL | 2 | 17, 12, 0, 0 |
| p724 | RLYEVIASI | >30 | |
| pY724 | YLYEVIASI | 3.5 | 6, 0, 0, 0 |
| p1072 | WLCHQAFLL | >30 | |
| pY1072 | YLCHQAFLL | 4 | 17, 0, 0, 0 |
| p540 | ILAKFLHWL | 2.9 | |
| p865 | RLVDDFLLV | 2.5 |
The denomination of peptides denotes the position of the first residue in the predicted amino acid sequence of hTRT (8).
The relative avidity is measured in arbitrary units as described in Materials and Methods.
Data represent the percent (%) specific lysis by primary CTL cultures generated from individual HHD mice immunized with the indicated analogue peptide. Values refer to % lysis of RMAS-HHD cells pulsed with the counterpart wild-type peptide (10−6 M) minus the % lysis of RMAS-HHD cells pulsed with the HLA A2-binding 58GILGFVFTL66 peptide of Influenza virus matrix antigen used as control at an E/T ratio of 50:1. Nonspecific lysis on target cells pulsed with the irrelevant peptide was between 0 and 13%.
p540 and p865 representing previously described high-affinity hTRT peptide (9) are shown by comparison.
HLA A2.1 Binding Assay.
The relative avidity of hTRT peptides for HLA A2.1 was measured by using a MHC stabilization assay on T2 cells in comparison with a reference peptide as described (22). Results are expressed as values of relative avidity, that is the ratio of the concentration of test peptide necessary to reach 20% of the maximal binding by the reference peptide over that of the reference peptide, thus the lower the value the stronger the binding.
Generation of Murine and Human Effector CTL.
Murine peptide-specific effector CTL were generated by in vitro stimulation of spleen cells from HHD transgenic mice injected s.c. at the base of the tail with hTRT peptides along with the I-Ab MHC class II helper peptide 128–140 of the hepatitis B virus core protein in incomplete Freund's adjuvant as described (9). Long-term CTL lines were maintained in culture by weekly restimulation with irradiated, peptide-pulsed syngeneic spleen cells in RPMI medium 1640 containing 10% FBS, 2 mM glutamine, 5 × 10−5 M 2-mercaptoethanol, 50 μg/ml streptomycin, and 50 μg/ml penicillin (complete medium), and supplemented with 40 international units/ml of recombinant human IL-2 or 10% supernatant from Con A-stimulated rat spleen cells. Human CTL were generated from peripheral blood mononuclear cells (PBMC) stimulated in vitro with autologous, irradiated, peptide-pulsed adherent cells in the presence of IL-7 and IL-2 as described (9). Effector CTL were assessed in a standard 51Cr-release assay 4–6 days after restimulation (9).
Tetramer Staining.
HLA A2.1 tetramers containing hTRT p572, hTRT p865, or the analogue peptide pY572 were obtained from the National Institute of Allergy and the Infectious Diseases Tetramer Facility and the National Institutes of Health AIDS Research and Reference Reagent Program. Briefly, 4 or 5 days after stimulation in culture, cells (0.5 × 106) were incubated with phycoerythrin-conjugated A2.1/peptide tetramers (20 μg/ml) and monoclonal antibody 53–6.7 (2 μg/ml) against murine CD8α conjugated with fluorescein isothiocyanate (BD PharMingen, San Diego) in Hank's balanced saline solution containing 0.1% BSA and 0.05% sodium azide for 30 min at 4°C. Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). Twenty thousand events were collected and analyzed by using the cellquest software (Becton Dickinson).
Results
Immunogenicity of Analogue hTRT Peptides with Low Relative Affinity for HLA A2.1.
Searching for hTRT peptides binding to HLA A2.1 with low affinity we selected seven 9-mers containing a predicted binding motif (23). Peptides with a putative low binding affinity were identified by using the software of the Bioinformatics and Molecular Analysis Section (National Institutes of Health, Washington, DC) available at http://bimas.dcrt.nih.gov/molbio/hla_bind/index.html. The relative binding affinities of the selected peptides were evaluated in comparison with a canonical high-affinity peptide of HIV type 1 reverse transcriptase (Table 1). The relative affinity of these peptides for the HLA A2.1 molecule was between 3- and >10-fold lower than that of the two hTRT peptides, p540 and p865, previously reported as high relative affinity MHC class I binders (Table 1).
The immunogenicity of peptides recognized by CD8+ T cells correlates with their affinity for the MHC class I molecule (24). Consequently, low relative affinity peptides were expected to have reduced immunogenicity, hence limiting our ability to study them. To circumvent this problem, we synthesized for each of the seven hTRT peptides 9-mer analogues in which the first residue was substituted with a Tyr to increase their binding affinity for HLA A2.1. Analogue peptides of naturally occurring, HLA A2.1-binding peptides, with Tyr substitutions in position one have been shown to display a higher relative affinity for the MHC than their wild-type counterparts (23, 25). Six of the seven analogue peptides bound to HLA A2.1 with higher relative affinity (>3.5 folds) than their wild-type counterpart. In these instances, the relative affinity was comparable to that reported for the high-affinity peptides p540 and p865 (Table 1). In vivo immunogenicity was assessed in HHD transgenic mice (21). These mice are H-2 Db−/−, β2microglobulin−/− and express a chimeric MHC class I molecule with the α1 and α2 domains of HLA A2.1 and the α3 domain of H-2 Db, to preserve the interaction with murine CD8, and covalently linked with the human β2 microglobulin light chain. Mice were immunized with a mixture of analogue peptide together with a T helper peptide (22). After in vitro restimulation, the ensuing CTL response was screened against target cells pulsed with the wild-type peptide counterpart. The results showed that not all peptides were equally immunogenic (Table 1). All of the mice immunized with the pY572 peptide generated CTL able to lyse specifically peptide pulsed target cells. All but one mouse immunized with pY152 and pY555 were also able to mount specific CTL responses. The other peptides yielded variable CTL responses. Only 50% of the mice immunized with pY675, and 25% immunized with pY724 and pY1072, mounted a specific CTL response. No specific response was generated in mice immunized with pY407. Thus, three of seven analogue peptides generated consistent CTL responses against their wild-type counterpart.
hTRT p572 Is Endogenously Processed and Presented in Tumor Cells.
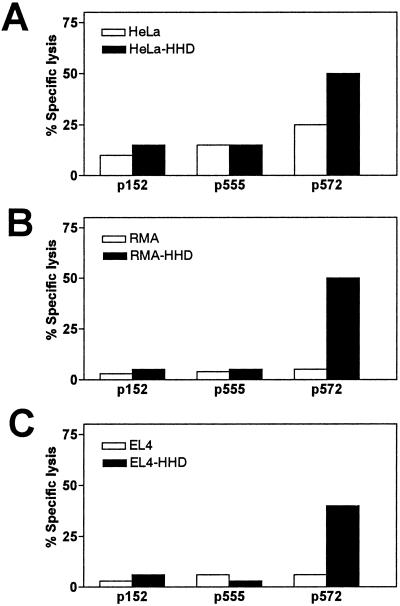
The second step of the screening process consisted in assessing presentation of endogenously synthesized hTRT peptides in the context of the HLA A2.1 molecule. To this end, effector CTL specific for peptide p572, p152, and p555 were tested for their ability to lyse telomerase-positive human tumor cells (26). HeLa cells transfected with the chimeric HHD gene were lysed specifically by p572-specific CTL as compared with the HLA A2.1− parental HeLa cells (Fig. 1A). On the other hand, CTL specific for p152 or p555 failed to lyse the HeLa–HHD transfectants above the level of parental HLA A2.1− HeLa cells (Fig. 1A). These results suggest that p572 is a natural HLA A2.1 ligand endogenously processed and presented on human cancer cells.
Fig 1.
Low-affinity peptide p572 is processed from endogenous TRT and presented by HLA A.2.1 in human and murine tumor cells. Effector CTL obtained from HHD mice (described in Table 1) able to recognize wild-type TRT peptide pulsed target cells, (p155, p555, or p572), were assayed for lytic activity against the following TRT+ tumor cell lines: human HeLa parental cells and HeLa–HHD transfectants (A); murine RMA parental cells and the RMA-HHD transfectants (B); and murine EL4 and the EL4–HHD transfectants (C). Experiments were performed at an E/T ratio of 70:1. Data corresponds to one representative experiment out of three experiments performed by using different CTL cultures with similar results.
Comparison of the human and mouse TRT amino acid sequences revealed that p572 is conserved between the two species. Therefore, we decided to see whether the p572 epitope is processed from mouse TRT and analyzed the lytic activity of the p572-specific CTL against two telomerase-positive murine lymphoma cells (EL4 and RMA) (12) transfected with HHD. As shown, EL4–HHD and RMA-HHD were specifically lysed by p572-specific CTL as compared with the parental EL4 and RMA cells (Fig. 1 B and C), indicating that the HHD chimeric class I molecule presents the p572 epitope borne out of the endogenous processing of murine TRT.
Analysis of the p572-Specific Repertoire Crossreactive with pY572.
The use of analogue peptides to generate heightened CTL responses against naturally occurring peptides hinges on the existence of crossreactivity between the CD8+ T cell precursors against the wild type and the analogue structures. Although our experiments suggest this to be the case between p572- and pY572-reactive CTL (Table 1 and Fig. 1), we sought full characterization and formal proof. We reasoned that a logical approach to explore this issue would be to use as cellular probes polyclonal CTL generated by immunizing HHD mice with the wild-type p572 peptide as these cells would best represent the repertoire able to recognize the wild-type p572 structure in the context of the MHC class I molecule.
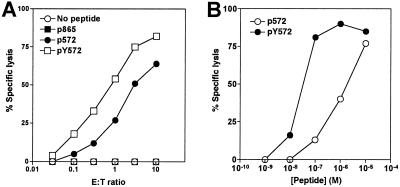
First, we compared the antigenicity of the two p572 and pY572 peptides. To this end, a polyclonal CTL line reactive with p572 and generated from spleen cells of HHD mice immunized with p572 was tested in a cytotoxicity assay using RMAS-HHD transfectants pulsed with different peptides as targets (Fig. 2A). These CTL were able to lyse targets pulsed with both p572 and pY572 peptides, but did not recognize targets pulsed with a different HLA A2.1 peptide, hTRT p865 (Fig. 2A). Remarkably, lysis of targets pulsed with the analogue pY572 peptide was much greater than with the wild-type peptide as the same level of lysis of p572 targets required more than 3-fold excess CTL (Fig. 2A). Additionally, a peptide dose–response study was performed. Briefly, p572-specific CTL were used to lyse the HLA A2.1+/TAP− T2 cells (TAP, transfer associated with antigen processing) pulsed with varying amounts of either peptide as targets (Fig. 2B). We found that 50% lysis required more than a 10-fold higher amount of p572 than pY572. These results indicate that the high relative affinity analogue peptide pY572 possesses greater antigenicity than the corresponding wild-type peptide.
Fig 2.
Antigenicity of low-affinity peptide p572 and high-affinity peptide pY572 peptides. Effector CTL from HHD transgenic mice primed with p572 peptide and restimulated in vitro with p572 for 8 weeks were assayed for lytic activity against RMAS-HHD or RMAS-HHD cells pulsed with 10−6 M peptide at the indicated E/T ratio (A); or T2 cells pulsed with increasing concentrations of p572 or pY572 peptides at an E/T ratio of 30:1. Lysis of nonpulsed T2 cells at this E/T ratio was ≤ 2% (B). Data correspond to one representative experiment out of three independent experiments with similar result.
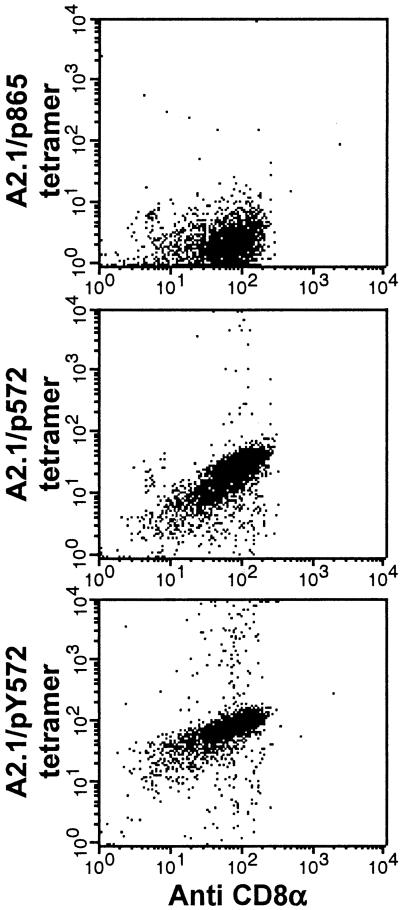
Next, we investigated the extent to which the T cell repertoire, specific for the wild-type peptide, is crossreactive with the analogue peptide. To this end, we sought to determine the proportion of p572-specific CD8+ T cells present in the polyclonal CTL population able to bind both HLA A2.1/p572 hTRT and A2.1/pY572 hTRT tetramers. We found that virtually all of the p572-specific CD8+ T cells bound both the wild type and the analogue peptide containing tetramers, but not a tetramer containing the hTRT p865 peptide used as a control (Fig. 3). This finding suggests that within the limits of the analysis in HHD mice and after expansion in vitro, the available repertoire for TRT p572 is crossreactive with the analogue peptide pY572.
Fig 3.
Tetramer binding of p572 peptide-specific CTL. Effector CTL described in Fig. 2 were stained with phycoerytherin-conjugated A2.1/p572, A2.1/pY572, or A2.1/p865 tetramers and fluorescein isothiocyanate-conjugated anti-CD8 monoclonal antibody. Data correspond to one representative experiment out of three independent experiments with similar result.
Interestingly, the binding intensity of the A2.1/p572 hTRT tetramer (mean fluorescence intensity = 46) was 5-fold lower than that of the A2.1/pY572 hTRT tetramer (mean fluorescence intensity = 251) (Fig. 3). The intensity of binding of MHC class I/peptide tetramers to CD8+ T cells measured by fluorescence activated cell sorting has been shown to correlate with the affinity of interaction between the TCR and the MHC Class I/peptide complex (18, 27, 28). Therefore, this result together with the peptide titration experiments (Fig. 2B) may suggest that the relative affinity of the p572-specific TCR for the HLA A2.1/pY572 complex is higher than that for the HLA A2.1/p572 complex. An alternative possibility would be that the differences observed in binding intensity could be caused by a different degree of peptide occupancy of the HLA A2.1 molecules in the tetamers and on target cells, reflecting different intrinsic relative affinities for HLA A2.1 by the two peptides.
CTL Responses Against hTRT p572 in Cancer Patients and in Normal Individuals.
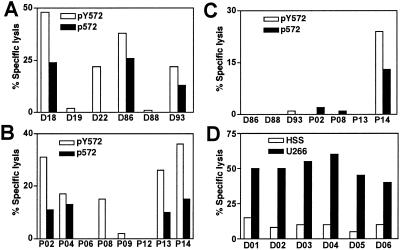
We tested for the presence of p572-specific CD8+ T cells in the human repertoire, and their possible expansion and differentiation into CTL on antigen stimulation. To this end, we used PBMC from HLA A2+ donors in an in vitro immunization protocol. A total of six normal donors and eight prostate cancer patients were analyzed. In vitro immunization was carried out with both the analogue pY572 peptide and the wild-type p572 as immunogens. After 4 rounds of in vitro restimulation, cultures were tested for their lytic activity against T2 target cells pulsed with either the analogue pY572 peptide or the wild-type p572 peptide. Immunization with the analogue pY572 peptide yielded CTL in four of six normal donors and five of eight prostate cancer patients (Fig. 4 A and B). Importantly, the majority of the CTL lines also lysed target cells pulsed with the wild-type p572 peptide, albeit to a lesser extent. Only one of the responder normal donors CTL and one of the responder prostate cancer patients CTL failed to recognize the wild-type p572 peptide. In contrast, immunization with the wild-type p572 peptide generated a specific CTL response in one of seven individuals only (Fig. 4C).
Fig 4.
Generation of p572-specific CTL from normal donors and prostate cancer patients. PBMC from HLA A2+ individuals were stimulated with peptide-pulsed autologous antigen presenting cells. After four rounds of weekly stimulation, effector cells were assayed for lytic activity against T2 cells or T2 cells pulsed with the indicated peptide at an E/T ratio of 50:1 or 100:1. Data represent the specific lysis on peptide pulsed targets minus that of nonpulsed cells. Background lysis on nonpulsed T2 cells was lower than 8% in all cases except for D93, in which it was 20%. (A) Analysis of CTL obtained from six different normal donors by in vitro stimulation with the analogue pY572 peptide. (B) Analysis of CTL obtained from eight different prostate cancer patients by in vitro stimulation with pY572 peptide. (C) Analysis of CTL obtained from three normal donors and four prostate cancer patients by in vitro stimulation with the wild-type peptide p572. (D) CTL obtained from six normal donors after stimulation with the analogue pY572 peptide and three subsequent rounds of weekly restimulation with the wild-type p572 peptide were assayed against HLA A2+ hTRT+ U266 cells and HLA A2− hTRT+ HSS cells.
Finally, we tested the ability of p572-specific human CTL to lyse human tumor cells. CTL lines were generated from the PBMC of six normal donors by a first round of in vitro immunization with the analogue pY572 peptide followed by 3 rounds of restimulation with the wild-type p572 peptide, a protocol adopted to maximize reactivity against the wild-type peptide. The six peptide-specific CTL were used as effectors against the myeloma cell line U266 (hTRT+/HLA A2+) and HSS (hTERT+/HLA A2−) used as a control. All six CTL lines lysed U266 cells above the levels of lysis of HSS cells (Fig. 4D), implying specificity of killing.
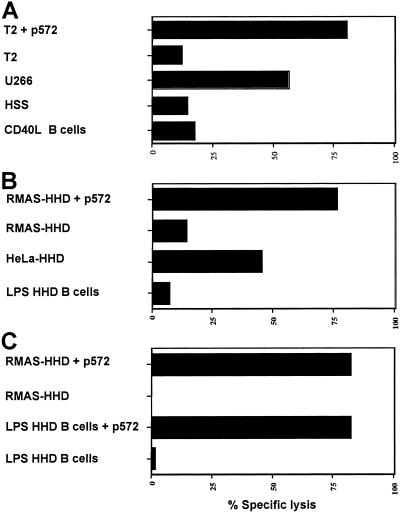
CTL Recognizing hTRT p572 Do Not Lyse Activated B Lymphocytes.
It has been reported that telomerase is expressed in B lymphocytes undergoing activation and cell replication, but not in naïve and memory B cells (29). To address the legitimate concern that CTL recognizing p572 could lyse nontransformed activated B lymphocytes, 51Cr-release experiments were performed by using activated B lymphocytes as targets. To this end, autologous B lymphocytes from a normal donor were isolated and activated through CD40 engagement. Human p572-specific CTL did not lyse activated autologous B lymphocytes (Fig. 5A). The possibility of untoward effects on B lymphocytes was further tested by using CD19+ spleen lymphocytes from HHD mice after activation with lipopolysaccharide. Cell blasts were then used as targets in cytotoxic assays using HHD CTL lines raised against the wild-type p572 peptide or the analogue pY572 peptide. In both instances no lysis of HHD B cell targets occurred even at a 90:1 effector/target (E/T) ratio (Fig. 5 B and C). However, activated B lymphocytes were susceptible to lysis after pulsing with peptide (Fig. 5C). Of note, the CTL lysed HeLa–HHD transfectant tumor cells through the endogenously synthesized and processed hTRT (Fig. 5B). Collectively, this finding indicates that actively replicating B lymphocytes are likely not target of anti-p572 CTL.
Fig 5.
p572-specific CTL do not lyse activated B lymphocytes. (A) Effector CTL from one of the donors described in Fig. 4D were assayed against autologous B lymphocytes activated for 48 h with trimeric CD40 L (40 μg/ml) (52) and the cell lines indicated, either pulsed or nonpulsed with the p572 peptide. A CTL line originated from pY572-primed HHD mice and maintained by weekly restimulation with p572 (B) or the CTL described in Fig. 2 raised from p572-primed HHD mice (C) were assayed for lytic activity against spleen B lymphocytes from HHD mice incubated with lipopolysaccharide (20 μg/ml) for 48 h and the cell lines indicated, either pulsed or nonpulsed with the p572 peptide. Data represent the percent specific lysis at an E/T ratio of 90:1.
Discussion
Knowledge on peptide binding motifs for particular MHC class I alleles has been successfully used to identify high relative affinity immunogenic peptides for a variety of tumor antigens, including the MAGE-3 melanoma antigen (30), human papilloma virus (31), p53 (32), HER-2/neu (33), MUC-1 (34), and more recently hTRT (9, 10). In the present study, we turned our attention to hTRT peptides with low relative affinity reasoning that, in case antigenicity and immunogenicity is proven, these peptides may offer advantages over the high-affinity peptides. Because the effect of self-tolerance on the specific T cell repertoire depends on the amount of antigen and on the affinity of a particular peptide for the MHC (19, 35, 36), it is reasonable to expect that the T cells specific for peptides with low relative affinity for the MHC would be less subject to self tolerance than T cells specific for higher affinity peptides (19, 20). If this assumption is correct, the T cell repertoire specific for low-affinity hTRT peptides could be preferentially preserved and available for tumor rejection responses. Our results indicate that CTL precursors specific for p572, a peptide with low relative affinity for the MHC, exist both in humans and HHD transgenic mice. Notably, because p572 is a true self antigen, the fact that CTL responses could be generated in vitro and in vivo argues that central tolerance had little adverse effect on CD8+ T cells recognizing this epitope.
A second advantage offered by an hTRT peptide with low affinity for the MHC is a diminished potential risk of autoimmune side effects, e.g., on mature hemopoietic cells undergoing replication as these express telomerase (37). Whereas telomerase expression in actively replicating cells can predictably generate p572 peptides from the endogenously synthesized TRT, these peptides may have a short life because of their intrinsic low affinity for the MHC. This, combined with the fact that telomerase activity in actively replicating hemopoietic cells is derepressed for a short period (29), argues that in vivo immunization with pY572 will probably not be followed by autoimmune sequelae. In line with this conclusion are the data presented here that activated B lymphocytes are not lysed by murine or human CTL against p572 whether these had been induced by immunization with p572 or pY572. The reason why activated B lymphocytes are not lysed by CTL against p572 can only be speculated. A simple explanation could be that in activated B lymphocytes the induction of telomerase activity may not depended on a net hTRT protein increase as recently demonstrated for activated CD4+ T lymphocytes (38).
We have shown that the antigenicity and immunogenicity of the p572 peptide was clearly heightened by the Arg → Tyr substitution in position one. The antigenicity and immunogenicity of MHC class I-restricted peptides can be positively affected by targeted amino acid substitutions at canonical positions known to be implicated in MHC binding (39) or TCR contact (40). Position one is not the most critical for peptide binding to the MHC molecule nor for TCR contact with the MHC/peptide complex, even though it was shown to be involved in binding to the HLA A2.1 molecule and in at least one instance in contact with the CDR1α loop of the TCR (41–45). Our results show that the Arg → Tyr substitution in position one increases the affinity of interaction for the HLA A2.1 molecule approximately 15-fold and that the analogue pY572 peptide displays, as a result, higher antigenicity and immunogenicity. This finding is in agreement with previous studies showing that targeted modifications of residues implicated in primary or secondary interactions with the MHC molecule augment considerably the affinity for the MHC molecule and the overall antigenicity and immunogenicity of the MHC/peptide complex (46–48). These reports also suggested that the increased MHC binding by analogue peptides stabilizes the TCR-MHC/peptide complex interaction. On the other hand, it has been shown that targeted modifications of TCR contact residues increase antigenicity and immunogenicity without increasing MHC binding (40). In particular, analogues of p53 natural epitopes generated by substitutions in position 7 were able to mobilize otherwise nonresponsive CTL both in mouse and humans, possibly by enhancing TCR contact (49, 50). In light of the above, we cannot exclude that the enhanced immunogenicity of the analogue peptide pY572 is the result not only of a more stable MHC/peptide complex but also of a secondary increase in TCR contact by the MHC/peptide complex.
The use of analogue peptides with selected residue modifications relies on the assumption that the CD8+ T cell repertoires specific for the two peptides are broadly overlapping. We used tetramers containing either the wild-type p572 peptide or the analogue peptide pY572 to show that in HHD transgenic mice virtually all of the CD8+ T cells that recognize the p572 peptide are crossreactive with the analogue peptide. Thus, within the limitations of the present observation, it appears that the receptors on T cells recognizing the wild-type p572 peptide also see the pY572 peptide. This is at variance with a previous report (51) showing that 20% of MART-1-specific CTL clones from in vitro expanded human CTL did not recognize a highly antigenic analogue decapeptide with an empirically determined Glu → Ala substitution in position one. The difference between the two studies may be related to the different nature of the peptides and/or the amino acid substitutions in question. Alternatively, it may be due to the different size of the CD8+ T cell repertoire in humans and HLA A2.1 transgenic mice.
In conclusion, we have identified and characterized the first low-affinity HLA A2.1 restricted epitope (p572) of hTRT, a ribonucleoprotein expressed in the vast majority of human tumors. Based on the demonstration presented here, we predict that the specific CD8+ T cell repertoire against this epitope may be more intact than that against high-affinity hTRT peptides and that CTL precursors for p572 can be expanded in cancer patients by using the analogue peptide. Thus, the high relative affinity analogue peptide may more efficiently mobilize and activate CD8+ T cells crossreacting with the wild-type peptide to incite an antitumor response. These results extend the number of hTRT epitopes that can potentially be targeted by CTL with implications for the design of an hTRT-based vaccine. A vaccine comprising p572 together with the high-affinity epitopes previously identified (9) may have greater chances of success than vaccines comprising high-affinity epitopes only as self tolerance is expected to exert its toll on the T cell repertoire differently depending on the affinity for the MHC of hTRT peptides. Furthermore, this study suggests that the strategy used here to directly target low-affinity peptides can be applied to the study of other tumor antigens and self antigens.
Acknowledgments
The work was supported by National Institutes of Health Grants RO1 CA 84062 (to M.Z.) and MO1 RROO827 (to the General Clinical Research Center of the University of California at San Diego).
Abbreviations
CTL, cytotoxic T lymphocytes
E/T, effector/target
hTRT, human telomerase reverse transcriptase
PBMC, peripheral blood mononuclear cells
TAA, tumor-associated antigens
TCR, T cell receptor
References
- 1.Van Pel A., van der Bruggen, P., Coulie, P. G., Brichard, V. G., Lethe, B., van den Eynde, B., Uyttenhove, C., Renauld, J. C. & Boon, T. (1995) Immunol. Rev. 145, 229-250. [DOI] [PubMed] [Google Scholar]
- 2.Van den Eynde B. J. & van der Bruggen, P. (1997) Curr. Opin. Immunol. 9, 684-693. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S. A. (1997) Immunol. Today 18, 175-182. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn E. H. (1992) Annu. Rev. Biochem. 61, 113-129. [DOI] [PubMed] [Google Scholar]
- 5.Counter C. M., Avilion, A. A., LeFeuvre, C. E., Stewart, N. G., Greider, C. W., Harley, C. B. & Bacchetti, S. (1992) EMBO J. 11, 1921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerson M., Counter, C. M., Eaton, E. N., Ellisen, L. W., Steiner, P., Caddle, S. D., Ziaugra, L., Beijersbergen, R. L., Davidoff, M. J., Liu, Q., et al. (1997) Cell 90, 785-795. [DOI] [PubMed] [Google Scholar]
- 7.Kim N. W. (1997) Eur. J. Cancer 33, 781-786. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T. M., Morin, G. B., Chapman, K. B., Weinrich, S. L., Andrews, W. H., Lingner, J., Harley, C. B. & Cech, T. R. (1997) Science 277, 955-959. [DOI] [PubMed] [Google Scholar]
- 9.Minev B., Hipp, J., Firat, H., Schmidt, J. D., Langlade-Demoyen, P. & Zanetti, M. (2000) Proc. Natl. Acad. Sci. USA 97, 4796-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonderheide R. H., Hahn, W. C., Schultze, J. L. & Nadler, L. M. (1999) Immunity 10, 673-679. [DOI] [PubMed] [Google Scholar]
- 11.Vonderheide R. H., Schultze, J. L., Anderson, K. S., Maecker, B., Butler, M. O., Xia, Z., Kuroda, M. J., von Bergwelt-Baildon, M. S., Bedor, M. M., Hoar, K. M., et al. (2001) Cancer Res. 61, 8366-8370. [PubMed] [Google Scholar]
- 12.Nair S. K., Heiser, A., Boczkowski, D., Majumdar, A., Naoe, M., Lebkowski, J. S., Vieweg, J. & Gilboa, E. (2000) Nat. Med. 6, 1011-1017. [DOI] [PubMed] [Google Scholar]
- 13.Nanda N. K. & Sercarz, E. E. (1995) Cell 82, 13-17. [DOI] [PubMed] [Google Scholar]
- 14.Houghton A. N. (1994) J. Exp. Med. 180, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim N. W., Piatyszek, M. A., Prowse, K. R., Harley, C. B., West, M. D., Ho, P. L., Coviello, G. M., Wright, W. E., Weinrich, S. L. & Shay, J. W. (1994) Science 266, 2011-2015. [DOI] [PubMed] [Google Scholar]
- 16.Broccoli D., Young, J. W. & de Lange, T. (1995) Proc. Natl. Acad. Sci. USA 92, 9082-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theobald M., Biggs, J., Hernandez, J., Lustgarten, J., Labadie, C. & Sherman, L. A. (1997) J. Exp. Med. 185, 833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez J., Lee, P. P., Davis, M. M. & Sherman, L. A. (2000) J. Immunol. 164, 596-602. [DOI] [PubMed] [Google Scholar]
- 19.Liu G. Y., Fairchild, P. J., Smith, R. M., Prowle, J. R., Kioussis, D. & Wraith, D. C. (1995) Immunity 3, 407-415. [DOI] [PubMed] [Google Scholar]
- 20.Harrington C. J., Paez, A., Hunkapiller, T., Mannikko, V., Brabb, T., Ahearn, M., Beeson, C. & Goverman, J. (1998) Immunity 8, 571-580. [DOI] [PubMed] [Google Scholar]
- 21.Pascolo S., Bervas, N., Ure, J. M., Smith, A. G., Lemonnier, F. A. & Perarnau, B. (1997) J. Exp. Med. 185, 2043-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firat H., Garcia-Pons, F., Tourdot, S., Pascolo, S., Scardino, A., Garcia, Z., Michel, M.-L., Jack, R., Jung, G., Kostmatopoulos, K., et al. (1999) Eur. J. Immunol. 29, 3112-3121. [DOI] [PubMed] [Google Scholar]
- 23.Ruppert J., Sidney, J., Celis, E., Kubo, R. T., Grey, H. M. & Sette, A. (1993) Cell 74, 929-937. [DOI] [PubMed] [Google Scholar]
- 24.Sette A., Vitiello, A., Reherman, B., Fowler, P., Nayersina, R., Kast, W. M., Melief, C. J., Oseroff, C., Yuan, L., Ruppert, J., et al. (1994) J. Immunol. 153, 5586-5592. [PubMed] [Google Scholar]
- 25.Tourdot S., Scardino, A., Saloustrou, E., Gross, D. A., Pascolo, S., Cordopatis, P., Lemonnier, F. A. & Kosmatopoulos, K. (2000) Eur. J. Immunol. 30, 3411-3421. [DOI] [PubMed] [Google Scholar]
- 26.Morin G. B. (1989) Cell 59, 521-529. [DOI] [PubMed] [Google Scholar]
- 27.Busch D. H. & Pamer, E. G. (1999) J. Exp. Med. 189, 701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee C., Savage, P. A., Lee, P. P., Davis, M. M. & Greenberg, P. D. (1999) J. Immunol. 162, 2227-2234. [PubMed] [Google Scholar]
- 29.Weng N. P., Granger, L. & Hodes, R. J. (1997) Proc. Natl. Acad. Sci. USA 94, 10827-10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celis E., Tsai, V., Crimi, C., DeMars, R., Wentworth, P. A., Chesnut, R. W., Grey, H. M., Sette, A. & Serra, H. M. (1994) Proc. Natl. Acad. Sci. USA 91, 2105-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kast W. M., Brandt, R. M., Sidney, J., Drijfhout, J. W., Kubo, R. T., Grey, H. M., Melief, C. J. & Sette, A. (1994) J. Immunol. 152, 3904-3912. [PubMed] [Google Scholar]
- 32.Theobald M., Biggs, J., Dittmer, D., Levine, A. J. & Sherman, L. A. (1995) Proc. Natl. Acad. Sci. USA 92, 11993-11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisk B., Blevins, T. L., Wharton, J. T. & Ioannides, C. G. (1995) J. Exp. Med. 181, 2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brossart P., Heinrich, K. S., Stuhler, G., Behnke, L., Reichardt, V. L., Stevanovic, S., Muhm, A., Rammensee, H. G., Kanz, L. & Brugger, W. (1999) Blood 93, 4309-4317. [PubMed] [Google Scholar]
- 35.Oehen S. U., Ohashi, P. S., Burki, K., Hengartner, H., Zinkernagel, R. M. & Aichele, P. (1994) Cell Immunol. 158, 342-352. [DOI] [PubMed] [Google Scholar]
- 36.Kurts C., Sutherland, R. M., Davey, G., Li, M., Lew, A. M., Blanas, E., Carbone, F. R., Miller, J. F. & Heath, W. R. (1999) Proc. Natl. Acad. Sci. USA 96, 12703-12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiyama E., Yokoyama, T., Tatsumoto, N., Hiyama, K., Imamura, Y., Murakami, Y., Kodama, T., Piatyszek, M. A., Shay, J. W. & Matsuura, Y. (1995) Cancer Res. 55, 3258-3262. [PubMed] [Google Scholar]
- 38.Liu K., Hodes, R. J. & Weng, N. (2001) J. Immunol. 166, 4826-4830. [DOI] [PubMed] [Google Scholar]
- 39.Krausa P., Brywka, M., III, Savage, D., Hui, K. M., Bunce, M., Ngai, J. L., Teo, D. L., Ong, Y. W., Barouch, D., Allsop, C. E., et al. (1995) Tissue Antigens 45, 223-231. [DOI] [PubMed] [Google Scholar]
- 40.Slansky J. E., Rattis, F. M., Boyd, L. F., Fahmy, T., Jaffee, E. M., Schneck, J. P., Margulies, D. H. & Pardoll, D. M. (2000) Immunity 13, 529-538. [DOI] [PubMed] [Google Scholar]
- 41.Madden D. R., Garboczi, D. N. & Wiley, D. C. (1993) Cell 75, 693-708. [DOI] [PubMed] [Google Scholar]
- 42.Madden D. R. (1995) Annu. Rev. Immunol. 13, 587-622. [DOI] [PubMed] [Google Scholar]
- 43.Garboczi D. N., Ghosh, P., Utz, U., Fan, Q. R., Biddison, W. E. & Wiley, D. C. (1996) Nature (London) 384, 134-141. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y. H., Smith, K. J., Garboczi, D. N., Utz, U., Biddison, W. E. & Wiley, D. C. (1998) Immunity 8, 403-411. [DOI] [PubMed] [Google Scholar]
- 45.Hausmann S., Biddison, W. E., Smith, K. J., Ding, Y. H., Garboczi, D. N., Utz, U., Wiley, D. C. & Wucherpfennig, K. W. (1999) J. Immunol. 162, 5389-5397. [PubMed] [Google Scholar]
- 46.Valmori D., Fonteneau, J. F., Valitutti, S., Gervois, N., Dunbar, R., Lienard, D., Rimoldi, D., Cerundolo, V., Jotereau, F., Cerottini, J. C., et al. (1999) Int. Immunol. 11, 1971-1980. [DOI] [PubMed] [Google Scholar]
- 47.Valmori D., Fonteneau, J. F., Lizana, C. M., Gervois, N., Lienard, D., Rimoldi, D., Jongeneel, V., Jotereau, F., Cerottini, J. C. & Romero, P. (1998) J. Immunol. 160, 1750-1758. [PubMed] [Google Scholar]
- 48.Overwijk W. W., Tsung, A., Irvine, K. R., Parkhurst, M. R., Goletz, T. J., Tsung, K., Carroll, M. W., Liu, C., Moss, B., Rosenberg, S. A. & Restifo, N. P. (1998) J. Exp. Med. 188, 277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann T. K., Loftus, D. J., Nakano, K., Maeurer, M. J., Chikamatsu, K., Appella, E., Whiteside, T. L. & DeLeo, A. B. (2002) J. Immunol. 168, 1338-1347. [DOI] [PubMed] [Google Scholar]
- 50.Tangri S., Ishioka, G. Y., Huang, X., Sidney, J., Southwood, S., Fikes, J. & Sette, A. (2001) J. Exp. Med. 194, 833-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valmori D., Gervois, N., Rimoldi, D., Fonteneau, J. F., Bonelo, A., Lienard, D., Rivoltini, L., Jotereau, F., Cerottini, J. C. & Romero, P. (1998) J. Immunol. 161, 6956-6962. [PubMed] [Google Scholar]
- 52.Yotnda P., Garcia, F., Peuchmaur, M., Grandchamp, B., Duval, M., Lemonnier, F., Vilmer, E. & Langlade-Demoyen, P. (1998) J. Clin. Invest. 102, 455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]