Abstract
The mechanism by which tumor necrosis factor-α (TNF) differentially modulates type I diabetes mellitus in the nonobese diabetic (NOD) mouse is not well understood. CD4+CD25+ T cells have been implicated as mediators of self-tolerance. We show (i) NOD mice have a relative deficiency of CD4+CD25+ T cells in thymus and spleen; (ii) administration of TNF or anti-TNF to NOD mice can modulate levels of this population consistent with their observed differential age-dependent effects on diabetes in the NOD mouse; (iii) CD4+CD25+ T cells from NOD mice treated neonatally with TNF show compromised effector function in a transfer system, whereas those treated neonatally with anti-TNF show no alteration in ability to prevent diabetes; and (iv) repeated injection of CD4+CD25+ T cells into neonatal NOD mice delays diabetes onset for as long as supplementation occurred. These data suggest that alterations in the number and function of CD4+CD25+ T cells may be one mechanism by which TNF and anti-TNF modulate type I diabetes mellitus in NOD mice.
There is mounting evidence that CD4+CD25+ T cells represent an important mechanism for the maintenance of self-tolerance (1, 2). CD4+CD25+ T cells prevent experimentally induced organ-specific autoimmune disease (3). Removal of these cells from mice that normally do not develop autoimmune disease can result in autoimmunity (4, 5). These cells need cell-to-cell contact to exert their suppressive effect. Whether or not a soluble factor is involved seems to depend on the experimental system used (6, 7). These cells constitutively express CTLA-4 (3, 6, 7) and the glucocorticoid-induced tumor necrosis factor receptor (GITR; refs. 8 and 9). CTLA-4 can negatively regulate T cell activation (10) either by release of transforming growth factor-β by a cell expressing CTLA-4 or by abrogating activation signals through CD28. Stimulation or blocking of GITR on CD4+CD25+ T cells can also modify their suppressive ability (8).
Tumor necrosis factor-α (TNF) has been shown to have a role in many inflammatory diseases and is a major mediator of inflammation. The effects of TNF in disease, however, are often contradictory and puzzling (11–13). Our laboratory and others have shown that elevation of TNF levels during the neonatal period in nonobese diabetic (NOD) mice intensifies the diabetic process (11, 12). Injection (i.p.) of TNF into newborn NOD mice every other day for 3 weeks led to an acceleration of diabetes onset and an increased incidence of disease (11). Injection of neutralizing anti-TNF into newborn NOD mice resulted in complete prevention of disease. Interestingly, if TNF treatment was started in the adult NOD mouse, disease onset was delayed, and the incidence of diabetes was decreased or abrogated (14–16). Anti-TNF administered during the young adult period did not yield complete protection from diabetes (11) and may even accelerate the diabetic process (17, 18). Similar findings regarding the effect of TNF at different maturation states of the immune system have been reported (19). A defined mechanism explaining TNF and anti-TNF's temporally differential effect on type I diabetes mellitus (T1DM) in NOD mice has not yet been described.
The NOD mouse is a spontaneous animal model of T1DM. To date, most studies on CD4+CD25+ T cells were performed with in vitro models or induced animal models of autoimmunity. In this study, we examined the possibility that neonatal or young adult i.p. injection of TNF and anti-TNF could modulate the CD4+CD25+ T regulatory population and, by this effect, influence the onset of T1DM in the NOD mouse.
Materials and Methods
Mice.
NOD.Lt (NOD) and B6.NOD-H2g7 (B6.NOD) mice were obtained from The Jackson Laboratory and bred in the Stanford University Animal Facility under barrier isolation conditions. Diabetes incidence in the colony is currently 80% in females by 22 weeks. NOR, C57BL/6, NZB, NZW, BALB/c, and DBA2 mice were bred in the same manner. All animals used were female and nondiabetic, unless specifically noted. Mice were injected neonatally with TNF or anti-TNF i.p. as described (11). Dosages are given in the legend to Fig. 3. There was no significant difference in weight gain between control and treated animals during or after the therapeutic period.
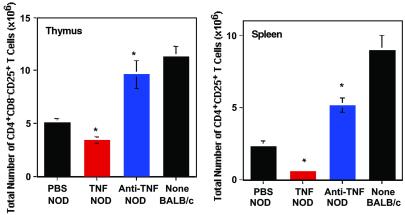
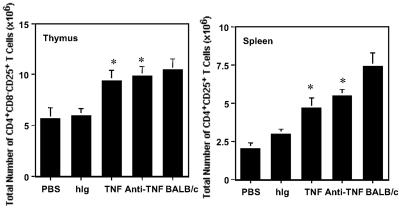
Fig 3.
Administration of TNF neonatally to NOD mice significantly decreases the CD4+CD25+ T regulatory cells in the thymus (Left) and spleen (Right), whereas neonatal administration of anti-TNF increases the CD4+CD25+ T regulatory population relative to control NOD mice. Effect of 1 μg of TNF (red) injected i.p. on alternate days for 3 weeks starting 1 day after birth on the number of CD4+CD25+ T regulatory cells in the thymus and spleen. Effect of 20 μg/g body weight of anti-TNF (blue; maximum dose of 100 μg) injected i.p. on alternate days for 3 weeks starting 1 day after birth on the number of CD4+CD25+ T regulatory cells in the thymus and spleen. The data are representative of the mean ± SEM of six mice from at least two separate experiments. Significant differences (P < 0.05) between PBS (and hIg) controls vs. treated mice are noted with an asterisk. Hamster Ig-treated mice were not significantly different from PBS-treated mice (data not shown). The mice are all female and 3–5 weeks of age.
Assessment of Diabetes.
Mice were monitored for glycosuria by using Chemstrip (Roche Diagnostics). Mice were considered diabetic when they had two consecutive positive readings. The onset of diabetes was dated from the first of the sequential measurements. Diabetes incidence is expressed as a percentage (mice with diabetes divided by total mice treated in the group).
Antibodies and Reagents.
Biotinylated anti-CD25 (clone 7D4), FITC-conjugated anti-CD25, phycoerythrin-conjugated anti-CTLA-4 (clone 4F10), and FITC-streptavidin and cychrome-conjugated anti-CD4 were purchased from PharMingen. Tricolor-conjugated anti-CD8 and FITC-conjugated anti-CD4 were purchased from Caltag (South San Francisco). TNF was purchased from R. Contreras (University of Gent, Belgium). Anti-TNF (a hamster IgG monoclonal antibody) was a generous gift from R. Schreiber (Washington University, St. Louis; ref. 20). Hamster IgG (hIg; Pierce) is an Ig control for the anti-TNF.
Flow Cytometric Analyses.
Single cell suspensions from the thymus, spleen, pancreatic lymph node, and inguinal lymph node were obtained by pressing the tissue between glass slides and forcing the resultant suspension through nylon mesh. Cell number was determined by using a hemocytometer and trypan blue exclusion to determine cell viability (>95% viable). For measurement of CD4+CD25+ T cells, 106 cells were incubated with biotinylated anti-CD25 followed by FITC-anti-CD4 and streptavidin–phycoerythrin. Fc block (PharMingen) was used to prevent nonspecific binding. Cells were analyzed with a FACScan flow cytometer using CELLQUEST (version 3.2; Becton Dickinson).
Purification of CD4+CD25+ T Cells and T Cell Transfer.
Lymph nodes (inguinal, popliteal, cervical, submandibular, axillary, and brachial) and spleen were harvested from 4-week-old female NOD mice that had been treated on alternate days from birth for 3 weeks with TNF, anti-TNF, or hIg. Single cell suspensions were prepared, the red blood cells were disrupted with ACK lysis buffer, and CD4+CD25+ T cells were purified as described (21). Purity ranged from 86 to 92%. Purified populations in PBS were injected into the tail vein of NOD.scid mice at 4–8 weeks of age. Spleens from recently diabetic NOD mice were harvested, and 10,200,000 female diabetic spleen cells were injected into NOD.scid mice to induce diabetes. A total of 10,200,000 cells, in a volume of 150 μl, was injected into all mice receiving cells. Experimental groups of NOD.scid mice received 107 diabetic spleen cells plus 200,000 CD4+CD25+ T cells (positive fraction) or 200,000 CD4+CD25− T cells (negative fraction) from NOD mice treated neonatally with TNF, anti-TNF, or hIg. An additional group of NOD.scid mice received 150 μl of PBS (carrier control).
Injection of CD4+CD25+ T Cells into Neonatal NOD Mice.
CD4+CD25+ or CD4+CD25− cells (200,000, purified as above) or PBS (carrier control) in a volume of 50 μl were injected i.p. into newborn NOD mice during weeks 1, 2, and 3. After week 3, all injections were i.v.
Statistics.
Significant differences between groups at a given time were assessed using a Mann–Whitney or Student's t test. Differences in the percentage of mice becoming diabetic at a specific time were compared using Fisher's exact test. All statistical tests were performed using SIGMASTAT (Jandel, San Rafael, CA). A criterion of P < 0.05 was accepted as significant in all statistical tests.
Results
NOD Mice Have a Relative Deficiency of CD4+CD25+ T Regulatory Cells.
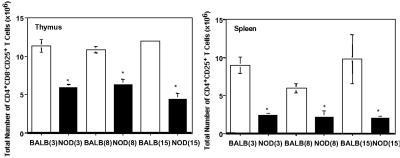
To determine whether a deficiency of CD4+CD25+ T regulatory cells could play a role in autoimmunity in the NOD mouse, the number of CD4+CD25+ T cells was determined in the primary (thymus) and peripheral lymphoid (spleen, pancreatic lymph node, inguinal lymph node) organs in NOD and BALB/c mice at 3, 8, and 15 weeks (Fig. 1 and data not shown). The main difference between the two mouse strains was noted in the thymus and spleen. A statistically nonsignificant trend toward decreased numbers of CD4+CD25+ T cells in the pancreatic lymph node of 8- and 15-week-old NOD mice was noted when compared with BALB/c mice. No differences were observed in the inguinal lymph node.
Fig 1.
NOD mice have a decreased number of CD4+CD25+ T regulatory cells in spleen and thymus when compared directly to BALB/c mice. Each data point represents the mean of six mice per time point from at least two separate experiments. Filled bars, mean ± SEM of the number of CD4+CD25+ T cells in NOD mice. Empty bars, mean ± SEM of the number of CD4+CD25+ T cells from BALB/c mice. Significant differences in comparing NOD and BALB/c at 3, 8, and 15 weeks are noted with an asterisk. The age of the mouse is noted in parentheses.
When comparing NOD and BALB/c mice, significant differences were noted in thymic and splenic weights in the absence of a significant difference in body weight (Table 1). The diminished size of the NOD mouse thymus has been alluded to in the literature (22) but has not been fully characterized. NOD mice, when compared with BALB/c mice at 3, 8, and 15 weeks, had a significantly smaller thymus only at an early age (3 weeks; P = 0.0086), and a significantly diminished spleen size at 3 and 8 weeks (3 weeks, P = 0.0003; 8 weeks, P = 0.015; and 15 weeks, P = 0.056).
Table 1.
NOD.Lt mice have a significant difference in the size of their thymus and spleen when compared with BALB/c mice
| Thymus mean weight in mg ± SEM (n = 6) | Spleen mean weight in mg ± SEM (n = 6) | |||||
|---|---|---|---|---|---|---|
| Mice | 3 weeks | 8 weeks | 15 weeks | 3 weeks | 8 weeks | 15 weeks |
| BALB/c | 121 ± 3 | 79 ± 12 | 55 ± 9 | 135 ± 5 | 123 ± 10 | 200 ± 62 |
| NOD.Lt | 74 ± 3 | 80 ± 7 | 57 ± 4 | 77 ± 7 | 79 ± 9 | 104 ± 7 |
, P < 0.05.
As a result of these findings, we sought to account for the observed weight difference by determining the total number of CD4+CD25+ T regulatory cells in each organ examined. Total CD4+CD25+ T cell number was determined by multiplying the percent CD4+CD25+ T cells by total organ cell number. The derived data show highly significant differences in the spleen as well as in the thymus (Fig. 1). These data suggest that the relative deficiency of regulatory CD4+CD25+ T cells in NOD mice could result in an inability to maintain peripheral tolerance.
CD4+CD25+ T Regulatory Cells Are Decreased in the Thymus of Additional Strains of Autoimmune Prone Mice.
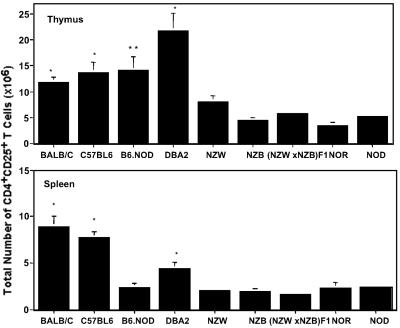
To determine whether a relative deficiency of CD4+CD25+ T regulatory cells is specific to NOD mice, we determined the number of CD4+CD25+ T cells in several additional strains (Fig. 2) of mice. These additional strains may be thought of as representative of a “gradient” of autoimmune disease susceptibility. In addition to NOR mice (a NOD congenic strain that develops insulitis but not diabetes), we examined NZB (autoimmune hemolytic anemia), NZW (normal when young, nephritis when old), and (NZB×NZW)F1 mice (a spontaneous model for systemic lupus erythematosus). The NZB mouse also shares a promoter defect in the FcγIlb gene with the NOD mouse that is associated with reduced expression and function of the Fc receptor FcγII (23) as well as structural abnormalities of thymic architecture (22). DBA2, C57BL/6, and B6.NOD mice were used as additional normal controls. The B6.NOD is a NOD congenic on the B6 background that carries IAg7. It develops an occasional peri-islet infiltrate and does not get diabetes.
Fig 2.
Comparison of the CD4+CD25+ T regulatory population in the thymus and spleen of autoimmune prone and additional control mice. The relative number of CD4+CD25+ T regulatory cells for the designated mouse strain (see x axis label is displayed. The data are representative of the mean ± SEM from six mice and at least two separate experiments. All mice are female and 3–4 weeks of age. *, a statistically significant difference (P < 0.05) when compared with NZW, NZB, (NZBxNZW)F1, NOR, and NOD mice. **, a statistically significant difference (P < 0.05) when compared with NZB, (NZB×NZW)F1, NOR, and NOD mice.
BALB/c, C57BL6, and DBA2 mice all have significantly higher numbers of regulatory T cells in the thymus when compared with NZW, NZB, (NZB×NZW)F1, NOR, and NOD mice (Fig. 2). B6.NOD also has significantly larger numbers of CD4+CD25+ T cells in the thymus compared with NZB, (NZB×NZW)F1, NOR, and NOD mice. However, there is no significant difference in thymic numbers of CD4+CD25+ T cells when comparing B6.NOD and NZW mice (P = 0.058).
The relationship between autoimmunity and numbers of CD4+CD25+ T cells in the spleen is less clear (Fig. 2). BALB/c, C57BL6, and DBA2 have significantly higher numbers of splenic CD4+CD25+ T cells when compared with autoimmune prone strains. However, B6.NOD mice had splenic numbers of CD4+CD25+ T cells similar to those of the autoimmune prone strains. This may reflect an effect of the replacement of the H2b haplotype with the H2g7 haplotype, and requires further study.
These data suggest that a relative deficiency of CD4+CD25+ T cells may be common to the thymus in several strains of mice susceptible to autoimmune disease [NZW, NZB, (NZB×NZW)F1, NOR, and NOD], relative to control mice (BALB/c, C57BL/6, and DBA2). Only a trend toward a decreased number of CD4+CD25+ T cells is observed in the spleens of autoimmune prone mice. The (NZB×NZW)F1 mice did not display a dramatic drop in regulatory cell number relative to their individual parental strains.
Neonatal Administration of TNF-α Decreases, Whereas Neonatal Anti-TNF-α Increases, the Number of CD4+CD25+ T Regulatory Cells in the Thymus and Spleen of NOD Mice.
Injection of neonatal NOD mice with TNF accelerates the onset and increases the incidence of diabetes, whereas neonatal injection of anti-TNF completely abrogates diabetes (11). To determine whether the numbers of CD4+CD25+ T regulatory cells could represent one mechanism by which TNF and anti-TNF modulate diabetes in NOD mice, we measured these cells in mice treated with either TNF or anti-TNF. In NOD mice, at 4 weeks, the CD4+CD25+ T cell population in TNF-treated mice (Fig. 3) was reduced by 76% in the spleen (P = 0.0019) and 33% in the thymus (P = 0.005) compared with NOD mice injected with PBS (carrier control). In contrast, NOD mice injected with anti-TNF (Fig. 3) demonstrated a significant increase in the number of CD4+CD25+ T regulatory cells in both the thymus (≈90%; P = 0.007) and spleen (≈123%; P = 0.0012). Administration of TNF or anti-TNF to neonatal mice did not result in a significant change in the size of the thymus or spleen of NOD mice (data not shown). These data implicate CD4+CD25+ T cells as one mechanism by which neonatal administration of TNF and anti-TNF regulates diabetes in the NOD mouse.
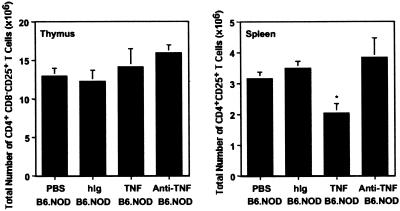
To determine whether TNF regulation of CD4+CD25+ T cell number is specific to the NOD mouse, we also injected neonatal B6.NOD mice with either TNF or anti-TNF (Fig. 4). Previous experiments in our laboratory showed that B6.NOD mice injected neonatally with TNF did not develop diabetes over a 50-week period, did not develop significant insulitis (only an occasional mouse with peri-islet infiltration at the vascular pole), and did not exhibit an enhanced response to islet cell antigen GAD65 (glutamic acid decarboxylase 65; data not shown). In Fig. 4, we show that B6.NOD mice injected neonatally with TNF had significantly decreased numbers of CD4+CD25+ T cells in the spleen (P = 0.0008) compared with B6.NOD mice treated with either the carrier control (PBS) or isotype control (hIg). There was no effect on the number of CD4+CD25+ T cells in the thymus of B6.NOD mice treated with TNF, or in the thymus or spleen of animals treated with anti-TNF (Fig. 4). These data suggest that TNF can regulate the number of CD4+CD25+ T cells in other mouse strains, but there are apparently compensatory mechanisms (i.e., maintenance of thymic numbers or output) that can overcome the potential loss of regulatory ability (i.e., decrease in CD4+CD25+ T cells in spleen).
Fig 4.
Administration of TNF to neonatal B6.NOD mice decreases the number of CD4+CD25+ T cells in the spleen. Anti-TNF has no effect on the number of regulatory T cells in the thymus or spleen. TNF or anti-TNF was administered to neonatal B6.NOD mice as described in Fig. 3. The experimental groups are described on the x axis of the figure. The data represent the mean ± SEM of six mice from at least two separate experiments. Statistically significant differences (P < 0.05) between PBS-treated and TNF-treated mice are noted with an asterisk. The mice are all female and 3–4 weeks of age.
We also examined splenic CTLA-4 expression on CD4+CD25+ T cells from NOD mice treated neonatally with TNF or anti-TNF. Although constitutive CTLA-4 expression was observed in these T cells, there was no change in mean fluorescence level of CTLA-4 expression when comparing CD4+CD25+ T cells from TNF, anti-TNF, hIg, or PBS control-treated mice (data not shown). It is possible that a change in CTLA-4 expression was not detected because of the rapid internalization of molecules expressed on the cell surface (10, 24).
Administration of Either TNF or Anti-TNF to Young Adult NOD Mice Increases the Number of CD4+CD25+ T Cells in the Thymus and Spleen.
TNF or anti-TNF administered to the young adult NOD mouse (i.e., 4–5 weeks of age; ref. 11) both result in a decreased incidence of diabetes relative to control NOD mice. To determine whether CD4+CD25+ T cells could represent a mechanism by which T1DM could be modulated by adult TNF or anti-TNF administration, we examined the levels of CD4+CD25+ T cells in 8-week-old NOD mice that were treated for 3 weeks, utilizing a previously established protocol (11) with either TNF or anti-TNF. In the thymus and spleen, the numbers of CD4+CD25+ T cells were increased in mice treated with either TNF or anti-TNF. The increase in thymic CD4+CD25+ T cell number is significantly greater than the number in NOD mice treated with either PBS or hIg (carrier or isotype control; Fig. 5). Neonatal TNF treatment seems to be associated with decreased effector function of CD4+CD25+ T cells (Fig. 6). It is possible that young adult NOD mice treated with TNF would also have CD4+CD25+ T cells with decreased effector function. As an earlier onset of T1DM is not observed in these animals, it is possible that a decrease in effector function is offset by the observed increase in the overall number of cells observed in the thymus (Fig. 5), thus highlighting either the key role of the thymus in producing these cells or the abilities of these cells to expand in the periphery. It is also possible that the CD4+CD25+ T cells become less sensitive to TNF, allowing cell survival, as the NOD mouse ages; more regulatory cells may also be exported from the thymus as more are produced. Nevertheless, these data reinforce the hypothesis that TNF or anti-TNF can modulate T1DM in the neonatal and young adult NOD mouse by modulation of the level of CD4+CD25+ T cells.
Fig 5.
Administration of TNF or anti-TNF to the young adult NOD mouse significantly increases the number of CD4+CD25+ T regulatory cells in the thymus and spleen. Effect of i.p. injection of either 1 μg of TNF or 100 μg of anti-TNF every other day for 3 weeks on the total number of CD4+CD25+ T cells in thymus and spleen. The data are representative of the mean ± SEM from two experiments, each data point consisting of six mice. An asterisk indicates that there is a statistically significant increase in numbers of CD4+CD25+ T cells when compared with hIg- or PBS-treated NOD.
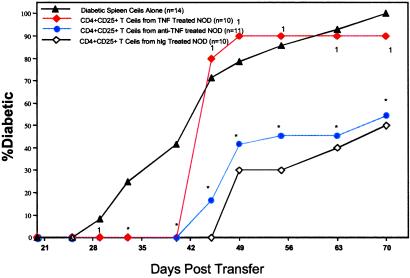
Fig 6.
CD4+CD25+ T regulatory cells from NOD mice treated with TNF can delay the onset of diabetes, but are not as effective in preventing its onset as regulatory cells from NOD mice treated with anti-TNF or hIg. ▴, the percentage of diabetes in NOD.scid mice that had been injected with 107 + 200,000 diabetic spleen cells alone. Red ♦, 107 diabetic spleen cells + 200,000 CD4+CD25+ T cells from NOD mice treated neonatally with TNF. Blue •, 200,000 CD4+CD25+ T regulatory cells from anti-TNF-treated mice + 107 diabetic spleen cells. ⋄, 200,000 CD4+CD25+ T cells from hIg-treated mice + 107 diabetic spleen cells. All data points represent n = 10, except diabetic spleen cells alone (▴), which represents n = 14, and anti-TNF (•), which represents n = 11. Data from NOD.scid mice receiving the CD4+CD25− fraction are not shown, as the progression of diabetes in the NOD.scid mouse was not significantly different from those mice receiving spleen cells alone. Asterisk denotes a significant difference (P < 0.05) from NOD.scid mice receiving diabetic spleen cells alone (▴). A 1 denotes no significant difference from NOD.scid mice receiving diabetic spleen cells alone (▴). The data are representative of two independent experiments.
CD4+CD25+ T Regulatory Cells from NOD Mice Injected Neonatally with TNF Do Not Effectively Prevent Diabetes Onset When Compared with Regulatory Cells from Anti-TNF-Treated Mice.
To determine whether the CD4+CD25+ T regulatory cells from NOD mice treated neonatally with TNF or anti-TNF were functional and able to suppress autoimmune disease, we elected to cotransfer equal numbers of CD4+CD25+ T regulatory cells (i.e., 200,000 CD4+CD25+ T cells from TNF, anti-TNF, or hIg-treated NOD mice) with 107 diabetic spleen cells from NOD female mice into NOD.scid female mice. Diabetes onset was compared with NOD.scid mice receiving 10,200,000 diabetic spleen cells alone. Data from NOD.scid mice receiving the negative fraction (CD4+CD25− cells) from TNF, anti-TNF, or hIg-treated NOD mice are not shown, as there was no difference in diabetes onset or incidence between these groups and NOD.scid receiving diabetic spleen cells alone.
CD4+CD25+ T cells from TNF, anti-TNF, and hIg-treated mice significantly delayed diabetes transfer (Fig. 6). The delay in diabetes onset in NOD.scid mice caused by 200,000 CD4+CD25+ T cells from anti-TNF or hIg-treated NOD mice was maintained through day 70 (P < 0.001). In contrast, in NOD.scid mice given CD4+CD25+ T cells from TNF-treated mice, a rapid onset of diabetes occurred between 40 and 45 days. After 45 days, there was no statistical difference in diabetes incidence between NOD.scid mice receiving diabetic spleen cells alone and NOD.scid mice receiving CD4+CD25+ T cells from TNF-treated NOD mice. By 91 days, all treatment groups had >90% incidence of T1DM. These results show that (i) CD4+CD25+ T cells from NOD mice can play a role in the regulation of T1DM, (ii) neonatal treatment of NOD mice with TNF results in compromised effector function of CD4+CD25+ T cells, and (iii) administration of anti-TNF to neonatal NOD mice does not seem to alter the capability of CD4+CD25+ T cells to suppress autoimmunity, relative to regulatory T cells from control NOD mice.
Injection of CD4+CD25+ T Cells Into NOD Mice During the Neonatal Period Delays Onset of T1DM.
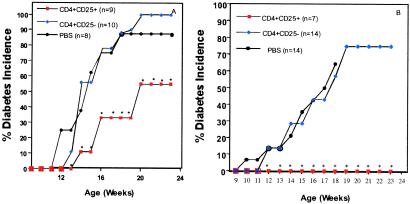
As neonatal injection of anti-TNF increased the number of CD4+CD25+ T cells and prevented diabetes, we decided to increase the numbers of these cells in untreated NOD mice. Neonatal NOD mice were given three i.p. injections (200,000 CD4+CD25+ or CD4+CD25− T cells once a week for 3 weeks). There was no difference in body weight between CD4+CD25+, CD4+CD25−, or PBS-injected NOD mice at weeks 3 and 10. No gross differences in body weight were observed during the experimental period until the mice became diabetic. Slightly increasing the number of CD4+CD25+ T cell population in NOD mice, by direct supplementation with a total of 600,000 regulatory T cells during the neonatal period, resulted in a significant delay in the onset of diabetes and a decreased incidence at 26 weeks (Fig. 7A). This is consistent with the effect of anti-TNF in increasing regulatory cell number.
Fig 7.
Injection (i.p.) of CD4+CD25+ T cells into neonatal NOD mice delays the onset of diabetes. (A) Percent diabetes in NOD mice injected intraperitoneally with 200,000 CD4+CD25+ or CD4+CD25− cells, once a week for 3 weeks or (B) NOD mice injected twice a week for 7 weeks and once a week for 9 weeks (weeks 1–3 were i.p. injections and weeks 4–16 were i.v. injections). •, the percentage of diabetes in NOD mice injected i.p. with PBS (carrier control). ▪, the percentage of diabetes in NOD mice injected i.p. with CD4+CD25+ T cells. ♦, the percentage of diabetes in NOD mice injected i.p. with CD4+CD25− T cells. The sample size is noted on the figure in parentheses.
We then speculated that if we were able to increase the number of CD4+CD25+ T cells in NOD mice to levels similar to those observed in nonautoimmune prone strains, we could have a more profound effect on diabetes incidence and onset. Little is known about the half-life of the injected CD4+CD25+ T cells, and it is unclear whether these cells expand in the periphery. To ensure the presence of an increased number of “active” regulatory T cells, we injected NOD mice with CD4+CD25+ T cells for a total of 18 weeks. Fig. 7B shows diabetes incidence in NOD mice that received two injections/week of 200,000 CD4+CD25+ or CD4+CD25− T cells for 7 weeks (weeks 1–3 were i.p. injections, and the remaining injections were i.v.), followed by 11 weeks of one injection/week of 200,000 CD4+CD25+ or CD4+CD25− T cells. Diabetes was again significantly delayed (at 12 weeks P < 0.003; Fig. 7B) by CD4+CD25+ T cell transfer. To ensure that we were increasing the numbers of CD4+CD25+ T cells in treated mice, six mice were killed from the group injected with supplemental CD4+CD25+ T cells, and four mice were killed from the PBS-treated group. The mice injected with CD4+CD25+ T cells showed a 113% increase in the number of regulatory T cells at week 18 in the spleen (vs. a 123% increase observed in the spleen with neonatal anti-TNF therapy; Fig. 3). There was no significant change in the number of regulatory cells in the thymus (data not shown). The data suggest that the transfers delivered adequate numbers of CD4+CD25+ T cells to the spleens of the NOD mice. However, complete prevention of diabetes (as seen with neonatal anti-TNF treatment of the NOD mouse) was observed only if there was repeated injection of CD4+CD25+ T cells, suggesting that a “fresh source” of functional regulatory T cells is necessary for the prevention of diabetes, and that additional mechanisms may play a role in the prevention of insulitis (see below). These data implicate the thymus as an important source of these cells for the periphery.
We examined the pancreas of NOD mice treated with CD4+CD25+ T cells. One of six mice showed no lymphocytic infiltrate. The remaining five mice showed an average of 62% of the examined islets (range: 20–100%) with intra-islet infiltration. These data are consistent with the CD4+CD25+ T cells not preventing lymphocytic infiltration of the pancreas but apparently suppressing the activity of potentially pathogenic cells.
Discussion
Patients with T1DM have been shown to have decreased levels of CD4+CD25+ T cells, when examining resting peripheral blood mononuclear cells, compared with normal controls and patients with T2DM (25). NOD mice, a spontaneous model of T1DM, also have an apparent deficiency in the number of CD4+CD25+ T cells, seen primarily in the thymus and spleen (Figs. 1 and 2). This deficiency of CD4+CD25+ regulatory T cells (especially in the thymus) seems to be a property of other strains of mice susceptible to autoimmune disease (Fig. 2). Deficiency of these cells is associated with an inability to maintain peripheral tolerance to self-antigen, as boosting the numbers of these cells in NOD mice by either neonatal anti-TNF injections or by direct supplementation of CD4+CD25+ T cells (Figs. 3 and 7) suppresses the disease process. Because neonatal anti-TNF injection results in an increase of peripheral CD4+CD25+ T cells, this suggests that endogenous TNF levels in the thymus (26) may play an important part in regulating the numbers of these T cells exiting the thymus and reaching the periphery. NOD mice [and (NZB×NZW)F1 mice] have several shared thymic structural abnormalities (22, 27) as well as decreased levels of CD4+CD25+ T cells (Fig. 2). The level of TNF expression (i.e., in the thymus, either absolute or relative to competing cytokines such as IL-10 and transforming growth factor-β) may be important in intrathymic T cell selection and in production and release of CD4+CD25+ T cells. These levels are currently unknown and difficult to measure (26), but they are likely to be central to our understanding of these processes.
TNF is a cytokine with pleiotropic effects, and its action on other cell types (i.e., dendritic cells, macrophages) involved in the autoimmune disease process cannot be ruled out. Here, we show that a purified population of CD4+CD25+ T cells isolated from NOD mice treated neonatally with TNF and injected into NOD.scid have a compromised effector function. Alteration of the function of these regulatory cells was not observed in CD4+CD25+ T cells from anti-TNF-treated mice. In addition, administration of anti-TNF to neonatal NOD mice dramatically increased the number of CD4+CD25+ T cells (Fig. 3). Because increasing the number of CD4+CD25+ T cells in NOD mice, which are deficient in these cells (Fig. 7), leads to a significant delay in the onset of T1DM, this result is consistent with the observed effect of neonatal anti-TNF on diabetes progression. Taken together, these data implicate TNF in the regulation of the numbers and function of CD4+CD25+ T cells.
Recent evidence has shown that GITR is constitutively expressed on CD4+CD25+ T cells (8, 9), that stimulation of GITR can abrogate CD4+CD25+ T cell-mediated suppression, that TRANCE-RANK signals can regulate the levels of CD4+CD25+ T cells in the pancreatic lymph node (28), and that TNF can regulate levels of RANK-L and RANK (29). Interestingly, GITR, TRANCE-RANK, and TNF can all activate NF-κB. It is not yet clear how these differing signals could differentially regulate NF-κB, potentially affecting the survival and function of CD4+CD25+ T cells.
A statistically nonsignificant trend toward decreased numbers of CD4+CD25+ T cells in the pancreatic lymph node was noted in nondiabetic NOD mice when compared with BALB/c mice at 8 and 15 weeks. No differences were noted in the inguinal lymph node. Because the CD4+CD25+ T cell population includes both regulatory T cells and recently activated and memory T cells, it is difficult with currently available reagents and methodology to differentiate between these subpopulations.
Repeated injections of CD4+CD25+ T cells were able to provide sustained protection from the onset of T1DM, and the time to onset of diabetes following the last injection of CD4+CD25+ T cells was consistently 8–9 weeks, suggesting a limited lifespan of these cells. This aspect needs further study to determine the fate of injected CD4+CD25+ T cells, their localization in the periphery, and their half-life.
In summary, these data show, in vivo, that the number and function of CD4+CD25+ regulatory T cells is influenced by the inflammatory cytokine milieu, and that direct or indirect effects on regulatory T cells may be a principal mechanism by which TNF regulates autoimmunity in the neonatal NOD mouse. If suitable means are found, modulation of the CD4+CD25+ T cell level may be a viable therapeutic option to modulate tolerance in autoimmune disease states, and the level itself may be a valuable marker to measure the ability of an individual to maintain the tolerant state.
Acknowledgments
We thank Lucino Hidalgo and Mary Vadeboncoeur for assistance with the experimental animals. This work was supported by the Juvenile Diabetes Foundation, the National Institutes of Health Grant DK51705, National Research Service Award Grant F32 DE05713-03 (to A.J.W.), and the Lee HySan Foundation (to H.H.).
Abbreviations
NOD, nonobese diabetic mouse
GITR, glucocorticoid-induced TNF receptor
TNF, tumor necrosis factor-α
T1DM, type I diabetes mellitus
References
- 1.Sakaguchi S. (2000) Cell 101, 455-458. [DOI] [PubMed] [Google Scholar]
- 2.Shevach E. (2001) J. Exp. Med. 193, F41-F45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon B., Lenschow, D. J., Rhee, L., Ashourian, N., Singh, B., Sharpe, A. & Bluestone, J. A. (2000) Immunity 12, 431-440. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 60, 1151-1164. [PubMed] [Google Scholar]
- 5.Suri-Payer E., Amar, A. Z., Thornton, A. M. & Shevach, E. (1998) J. Immunol. 60, 1212-1218. [PubMed] [Google Scholar]
- 6.Takahashi T., Tomoyuki, T., Yamazaki, S., Uede, T., Shimizu, J., Sakaguchi, N., Mak, T. W. & Sakaguchi, S. (2000) J. Exp. Med. 192, 303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read S., Malmström, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135-142. [DOI] [PubMed] [Google Scholar]
- 9.McHugh R. S., Whitters, M. J., Piccirillo, C. A., Young, D. A., Shevach, E. M., Collins, M. & Byrne, M. C. (2002) Immunity 16, 311-323. [DOI] [PubMed] [Google Scholar]
- 10.Walunas T. L., Lenschow, D. L., Bakker, C. Y., Linsley, P. S., Freeman, G. J., Green, J. M., Thompson, C. B. & Bluestone, J. A. (1994) Immunity 5, 405-413. [DOI] [PubMed] [Google Scholar]
- 11.Yang X. D., Tisch, R., Singer, S. M., Cao, Z. A., Liblau, R. S., Schreiber, R. D. & McDevitt, H. O. (1994) J. Exp. Med. 180, 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green E. A. & Flavell, R. A. (1999) Immunol. Rev. 169, 11-22. [DOI] [PubMed] [Google Scholar]
- 13.Kassiotis G. & Kollias, G. (2001) J. Clin. Invest. 107, 1507-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob C. O., Aiso, S., Michie, S. A., McDevitt, H. O. & Acha-Orbea, H. (1990) Proc. Natl. Acad. Sci. USA 87, 968-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewal I. S., Grewal, K. D., Wong, F. S., Picarella, D. E., Janeway, C. A., Jr. & Flavell, R. A. (1996) J. Exp. Med. 184, 1963-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christen U., Wolfe, T., Mohrle, U., Hughes, A. C., Rodrigo, E., Green, E. A., Flavell, R. A. & von Herrath, M. G. (2001) J. Immunol. 166, 7023-7032. [DOI] [PubMed] [Google Scholar]
- 17.Cope A. P., Liblau, R. S., Yang, X. D., Congia, M., Laudanna, L., Schreiber, R. D., Probert, L., Kollias, G. & McDevitt, H. O. (1997) J. Exp. Med. 185, 1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cope A., Ettinger, R. & McDevitt, H. O. (1997) Res. Immunol. 148, 307-312. [DOI] [PubMed] [Google Scholar]
- 19.Green E. A. & Flavell, R. A. (2000) Immunity 12, 459-469. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan K. C., Ruddle, N. H. & Schreiber, R. D. (1989) J. Immunol. 142, 3884-3893. [PubMed] [Google Scholar]
- 21.Thornton A. M. & Shevach, E. M. (1998) J. Exp. Med. 188, 287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabarra B. & Andrianarison, I. (1991) Int. J. Exp. Pathol. 72, 275-287. [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard N. R., Cutler, A. J., Uribe, S., Chadban, S. J., Morley, B. J. & Smith, K. G. (2000) Curr. Biol. 10, 227-230. [DOI] [PubMed] [Google Scholar]
- 24.Alegre M. L., Noel, P. J., Elsfelder, B. J., Chuang, E., Clark, M. R., Reiner, S. L. & Thompson, C. B. (1996) J. Immunol. 157, 4762-4770. [PubMed] [Google Scholar]
- 25.Kukreja A., Cost, G., Marker, J., Zhang, J., Sun, Z., Lin-Su, K., Ten, S., Sanz, M., Exley, M., Wilson, B., et al. (2002) J. Clin. Invest. 109, 131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giroir B. P., Brown, T. & Beutler, B. (1992) Proc. Natl. Acad. Sci. USA 89, 4864-4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabarra B., Dardenne, M. & Bach, J. F. (1990) J. Autoimmun. 3, 25-36. [DOI] [PubMed] [Google Scholar]
- 28.Green E. A., Choi, Y. & Flavell, R. A. (2002) Immunity 16, 183-191. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima T., Kobayashi, Y., Yamasaki, S., Kawakami, A., Eguchi, K., Sasaki, H. & Sakai, H. (2000) Biochem. Biophys. Res. Commun. 3, 768-775. [DOI] [PubMed] [Google Scholar]