Abstract
PD-1 is a receptor of the Ig superfamily that negatively regulates T cell antigen receptor signaling by interacting with the specific ligands (PD-L) and is suggested to play a role in the maintenance of self-tolerance. In the present study, we examined possible roles of the PD-1/PD-L system in tumor immunity. Transgenic expression of PD-L1, one of the PD-L, in P815 tumor cells rendered them less susceptible to the specific T cell antigen receptor-mediated lysis by cytotoxic T cells in vitro, and markedly enhanced their tumorigenesis and invasiveness in vivo in the syngeneic hosts as compared with the parental tumor cells that lacked endogenous PD-L. Both effects could be reversed by anti-PD-L1 Ab. Survey of murine tumor lines revealed that all of the myeloma cell lines examined naturally expressed PD-L1. Growth of the myeloma cells in normal syngeneic mice was inhibited significantly albeit transiently by the administration of anti-PD-L1 Ab in vivo and was suppressed completely in the syngeneic PD-1-deficient mice. These results suggest that the expression of PD-L1 can serve as a potent mechanism for potentially immunogenic tumors to escape from host immune responses and that blockade of interaction between PD-1 and PD-L may provide a promising strategy for specific tumor immunotherapy.
Antigen-induced activation and proliferation of T cells are regulated by both positive and negative costimulatory receptors belonging to the Ig superfamily. CD28 and ICOS are positive costimulatory receptors interacting with the ligands of B7 family on professional antigen-presenting cells and are essential for activation and proliferation of antigen-specific T cells (1, 2). By contrast, two distinct receptors on T cells, cytotoxic T lymphocyte (CTL)-A-4 and -PD-1, play opposite roles. CTLA-4 shares the B7 ligands with CD28 (3), and CLTA-4 gene targeted mice develop fatal polyclonal proliferation of T cells early in life (4), indicating a pivotal role in restricting T cell expansion during normal immune response. PD-1, on the other hand, interacts with distinct ligands, PD-L1 and PD-L2, which are expressed not only on antigen-presenting cells but also other cell types in nonlymphoid tissues (5, 6). Although PD-1 gene-targeted mice show only marginal lymphoproliferation (7), they develop various autoimmune diseases later in life, suggesting the involvement of PD-1 in suppressing activation and/or proliferation of autoreactive T cells (8, 9). These results suggest that the two types of negative receptors may in part control different aspects or stages of immune responses.
Accumulating evidence indicates that specific T cell immune response can be raised against many tumors in human and mouse based on recent sensitive assay systems (10, 11). It is also shown that unaltered self-antigens expressed either aberrantly or in a tissue-specific manner on tumor cells can serve as major tumor rejection antigens, suggesting that antitumor immunity may be in part autoreactive response (12). Nonetheless, tumor-specific immune responses are normally very weak, if present at all, and not sufficient enough to eradicate the tumors. Identification of negative immunoreceptors on T cells, however, has provided a novel prospect that their manipulation may lead to the enhanced tumor-specific T cell immunity in vivo. Indeed, blockade of CTLA-4 was indicated to augment the specific tumor immunity and to induce significant inhibition of tumors in vivo in a number of experimental murine models (13).
Unlike B7 molecules, PD-1 ligands (PD-Ls) are expressed on various nonlymphoid tissues in mouse such as heart, lung, liver, and kidney (5, 6). It is speculated that PD-Ls expressed on these vital tissues may function in part as “veto” molecules against the potentially autoreactive effector T cells (14). In this report, we explored the possibility for involvement of PD-1/PD-L1 system in tumor immunity by using experimental tumor models. We indicate that PD-L1 on inherently immunogenic tumor cells confers a potent escaping mechanism from the host T cell immunity and raise a possibility that blockade of PD-1–PD-L interaction may provide an effective approach for specific tumor immunotherapy.
Materials and Methods
Mice.
BALB/c, BALB/c nu/nu, DBA/2, and C57BL/6 (B6) mice were purchased from Japan Clea, Hamamatsu, Japan. PD-1-deficient mice that had been backcrossed with B6 or BALB/c mice for over 15 generations were maintained in our animal facility in a specific pathogen-free condition.
Cells and Cultures.
Mouse tumor cell lines, P815 (mastocytoma), B16 (melamoma), SP2/0, P3U1, X63, J558L, and PAI (myeloma/plasmocytomas), were maintained in the complete RPMI 1640 medium supplemented with 10% FCS, 10−5 M 2-mercaptoethanol and antibiotics. An allo (H-2Ld)-reactive cytotoxic T cell clone 2C derived from 2C-transgenic B6 mice was kindly provided by K. Udaka, Graduate School of Science, Kyoto University, and maintained in the complete RPMI 1640 medium supplemented with 5% rat ConA-conditioned medium by stimulating every 10–12 days with 100 Gy-irradiated P815 cells. To generate the syngeneic tumor-specific CTL, DBA/2 mice were injected i.p. with 100 Gy-irradiated P815 cells (5 × 106) at 2 wk apart, and the spleen cells were restimulated in vitro with irradiated P815 cells for 6 d followed by a limiting dilution in the presence of irradiated P815 cells, syngeneic splenocytes, and 25 units/ml human IL-2.
Abs and Flow-Cytometric Analysis.
Rat anti-mouse PD-1 and PD-L1 mAb were established as described (15, 16), and anti-H-2Ld was purchased from e-Bioscience (San Diego, CA). Flow cytometric analysis was done with a FACScan (Becton Dickinson) as described (7).
Gene Transfection.
A PD-L1 cDNA kindly provided by G. J. Freeman (Harvard Medical School, Boston) was digested with EcoRI and constructed into a pApuroXS expression vector. P815 cells were transfected with the pApuroXS-PD-L1 by electroporation at 360 V, 500 μF, followed by the selection with puromycin (3 μg/ml), and independent clones stably expressing PD-L1 were established.
Cytotoxicity and IFN Assay.
Cytotoxicity assay was done with a 51Cr-release assay as described (17), and IFN-γ was assayed by using an enzyme-linked immunosorbent assay kit (e-Bioscience) according to the manufacturer's protocol.
Assay of Tumor Growth and Ab Treatment.
To assess the tumor growth, tumor diameters were measured with calipers, and tumor volumes were approximated by the calculation as reported (18). For the treatment with Ab, anti-mouse PD-L1 mAb or normal rat IgG as a control was injected i.p. at 0.1 mg per mouse each time on days 1, 3, 5, and 7 after the tumor inoculation.
Histological Analysis.
DBA/2 mice inoculated with P815/PD-L1 tumor cells were killed at day 20, and the tissues were fixed in 10% formaldehyde, mounted in paraffin, and stained with hematoxylin and eosin.
Results and Discussion
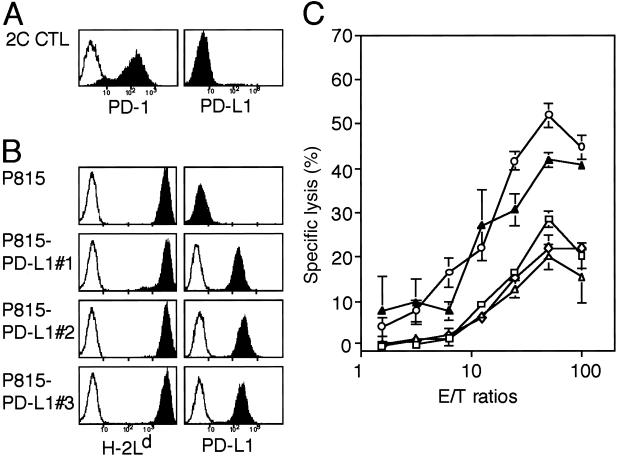
In an attempt to explore the possible roles of PD-1 receptor in the effector function of T cells, we first examined whether the engagement of PD-1 receptor affects the cytotoxic activity of a B6-derived H-2Ld-specific CTL clone, 2C (19). 2C CTL clone expressed significant PD-1 on stimulation with the specific target, P815 tumor cells (Fig. 1A). Because P815 cells expressed neither PD-L1 (Fig. 1B) nor PD-L2 (data not shown), we established three independent P815 transfectants stably expressing PD-L1. All of the P815/PD-L1 clones expressed the same level of H-2Ld antigen (Fig. 1B) and proliferated at the same rate in the culture as the parental cells. However, as shown in Fig. 1C, the P815/PD-L1 clones were less susceptible to the cytotoxic activity of 2C CTL than the parental P815 cells. The reduced susceptibility of P815/PD-L1 clone to 2C CTL was reversed almost completely by the inclusion of anti-PD-L1 mAb F(ab′)2 in the assay culture (10 μg/ml), indicating that it was not due to an inherent property of the clone. The results suggested strongly that the engagement of PD-1 of CTL with PD-L1 on the specific target cells resulted in the inhibition of T cell receptor-mediated cytotoxic activity.
Fig 1.
Inhibition of the cytotoxic activity of CTL clone by engagement of the PD-1 receptor with PD-L1 on the specific target cells. (A and B) Expression of PD-1, H-2Ld, and PD-L1 on 2C CTL clone and P815 tumor cells as well as their stable transfectant clones of PD-L1(P815/PD-L1 nos. 1–3) were analyzed by using a flow cytometry. Although not shown, none of the cells expressed PD-L2. (C) 2C cells were incubated with 51Cr-labeled P815 (○) or three independent P815/PD-L1 clones (□, ◊, ▵) in the absence or presence (▴) of 10 μg/ml rat anti-PD-L1 mAb F(ab′)2 at varying effector-to-target (E/T) ratios for 4 h, and the specific 51Cr release was determined. The means and SE of triplicate cultures are indicated.
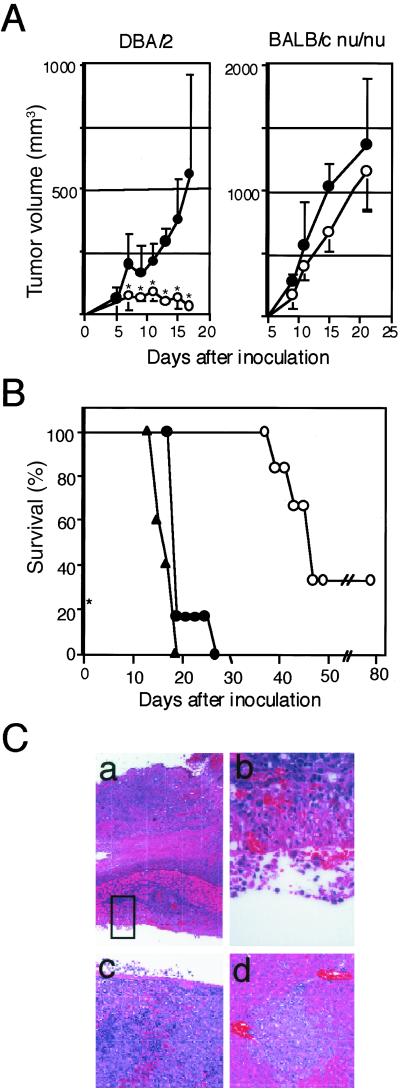
Because P815 tumor cells exhibit significant T cell immunogenicity, yet forms potentially lethal tumors in the syngeneic DBA/2 mice depending on the loading doses, it has been used as a model of tumor immunity for years. Several distinct rejection antigens recognized by the specific cytotoxic T cells in the syngeneic mice have been identified in P815 cells, including P1A antigen shared with various other tumor cells (20–22). We then compared the tumorigenesis of P815 and P815/PD-L1 cells in the syngeneic DBA/2 mice. P815 cells inoculated s.c. at 1 × 106 cells per mouse formed only transient local tumors (Fig. 2A Left), although 70% of the mice eventually died in 6–7 wk because of latent i.p. dissemination of the tumor cells (Fig. 2B). The rest 30% of mice were apparently cured of tumors. On the other hand, P815/PD-L1 cells at the same dose formed progressive local tumors (Fig. 2A Left) and killed 100% of mice in as early as 2–4 wk (Fig. 2B). Autopsy examination revealed early invasion of the s.c. P815/PD-L1 cells into the peritoneal cavity across the abdominal wall and widespread dissemination into the abdominal cavity as well as the probable bloodborne metastasis in the livers and spleens (Fig. 2C). In the H-2-identical BALB/c nu/nu mice, however, both P815 and P815/PD-L1 tumor cells formed rapidly growing tumors with only marginal, if any, difference (Fig. 2A Right) and killed them within 4 wk. The results indicated that the enhanced tumorigenicity of P815/PD-L1 cells in the normal syngeneic mice depended on the T cells.
Fig 2.
Enhanced tumorigenicity and invasiveness of P815/PD-L1 cells in the syngeneic DBA/2 mice. (A) P815 (○) or P815/PD-L1 (•) cells were inoculated s.c. at 1 × 106 cells per mouse into DBA/2 (Left) or H-2-shared BALB/c nu-nu (Right) mice, and local tumor growth was monitored. The mean tumor volumes and SE of six mice in each group are indicated. *, P < 0.01 by Student's t test. (B) DBA/2 mice (six mice per group) were inoculated with P815 (○) or P815/PD-L1 (no. 1 • and no. 2 ▴) cells as above and the survival rates were monitored. (C) DBA/2 mice inoculated s.c. with P815/PD-L1 cells were autopsied on day 18. Local s.c. tumors showing invasion across the abdominal wall into the peritoneal cavity (a and b) (×40 and ×400), spleen showing massive invasion of tumor cells (c), and liver with focal metastatic region (d).
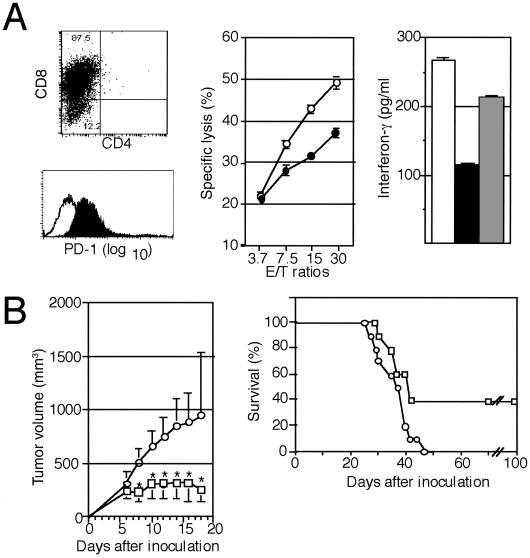
Conforming to the inherent T cell immunogenicity of P815 cells, tumor-specific CD8+ CTL could be induced from the spleen cells of DBA/2 mice immunized with P815 cells, and they expressed significant PD-1 (Fig. 3A). The syngeneic CTL again exhibited less efficient cytotoxicity as well as IFN-γ production against P815/PD-L1 than P815 cells, and the reduced IFN-γ production in response to P815/PD-L1 cells was restored by the inclusion of anti-PD-L1 mAb F(ab′)2 (Fig. 3A). The results confirmed that PD-L1 on the tumor cells significantly inhibited the activation of specific T cells in the syngeneic mice by the tumor antigens. To prove a direct role of PD-L1 in the enhanced tumorigenicity of P815/PD-L1 cells in vivo, we injected rat anti-PD-L1 mAb or normal rat IgG as a control at 0.1 mg per mouse on days 1, 3, 5, and 7 after the inoculation of P815/PD-L1 cells (3 × 106 cells per mouse). Injection with the anti-PD-L1 mAb significantly inhibited the local tumor growth, with 40% of the recipients being completely cured of the tumor. On the other hand, 100% of the mice that received control IgG showed progressive tumor growth and were killed within 6 wk (Fig. 3B).
Fig 3.
Inhibition of the tumorigenesis of P815/PD-L1 cells by the injection with anti-PD-L1 mAb in vivo. (A Left) Syngeneic P815 tumor-specific T cells were generated in DBA/2 mice as described in Materials and Methods and analyzed for the expression of CD4, CD8, and PD-1. (Center) The CD8+ T cells were incubated with 51Cr-labeled P815 (○) or P815/PD-L1 (•) cells at varying effector-to-target ratios for 4 h, and the specific cytotoxicity was determined. The means and SE of triplicated cultures are indicated. (Right) The CD8+ T cells (2 × 106) were cocultured with 5 × 106 P815 (open bar) or P815/PD-L1 cells in the absence (solid bar) or presence (shaded bar) of anti-PD-L1 mAb F(ab′)2 (10 μg/ml) for 24 h, and IFN-γ in the culture supernatants was determined by enzyme-linked immunosorbent assay. The means and SE of triplicated cultures are indicated. (B) DBA/2 mice (10 mice per group) were inoculated s.c. with 3 × 106 P815/PD-L1 cells, and normal rat IgG (○) or anti-PD-L1 mAb (□) was injected on days 1, 3, 5, and 7 at 0.1 mg per mouse each time. The mean tumor volumes and SE of 10 mice (Left) as well as their survival rates (Right) are indicated. *, P < 0.01 by Student's t test.
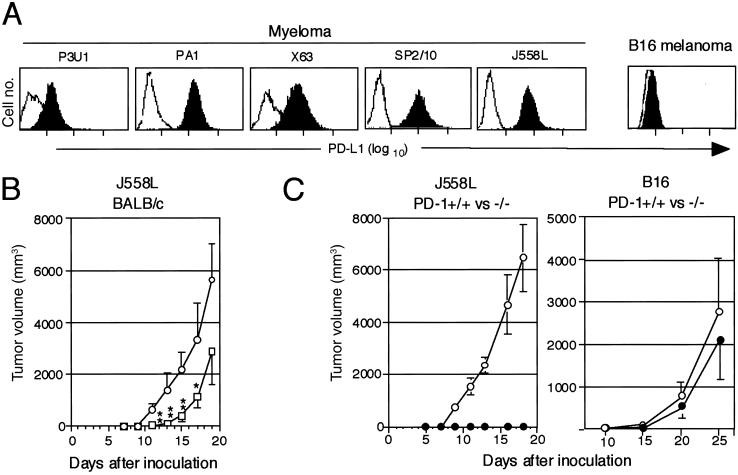
Survey of various tumor cell lines indicated that a significant proportion of murine tumor lines, most notably all of the independent myeloma lines so far examined, expressed PD-L1 (Fig. 4A). Therefore, we examined whether the PD-L1 naturally expressed on tumor cells indeed contributed to their tumorigenicity in vivo. As shown in Fig. 4B, J558L myeloma cells inoculated s.c. at 2.5 × 106 cells per mouse formed very rapidly growing tumors in the syngeneic BALB/c mice and killed them within 3 wk. Injection with the anti-PD-L1 Ab after tumor inoculation resulted in the significant suppression of the myeloma cell growth, although the tumors started to expand with delay of 1 wk or so in the most of mice (Fig. 4B). A few reasons may be considered for the only transient therapeutic effect of the anti-PD-L1 mAb on the myeloma cells as compared to that on P815/PD-L1 tumor cells, such as less T cell immunogenicity, more aggressive proliferative capacity, and possible selection for variant clones with reduced PD-L1 expression in vivo in the myeloma cells. It also should be noted that the presently available anti-PD-L1 Ab is rat origin.
Fig 4.
Inhibition of the tumorigenesis of myeloma cells endogenously expressing PD-L1 in the normal syngeneic mice treated with anti-PD-L1 Ab or in the PD-1-deficient mice. (A) Expression of endogenous PD-L1 in myeloma and B16 melanoma cells was examined with a flow cytometry. (B) J558L myeloma cells (2.5 × 105) were inoculated s.c. into the syngeneic BALB/c mice (nine mice per group) followed by the injection with normal rat IgG (○) or anti-PD-L1 mAb (□) (0.1 mg per mouse) on days 1, 3, 5, and 7. The mean tumor volumes and SE of nine mice are indicated. **, P < 0.01; *, P < 0.1 (by Student's t test). (C) J558L (Left, 2.5 × 105) or B16 (Right, 1 × 106) cells were inoculated s.c. into PD-1+/+ (○) and −/− (•) mice of BALB/c or B6 background, respectively. The mean tumor volumes and SE of four (the former) and 10 (the latter) mice are indicated.
To evaluate the potential effects of complete blockade of PD-1–PD-L1 interaction in vivo, we then examined the growth of J558L in PD-1−/− mice of BALB/c background. As indicated in Fig. 4C Left, the growth of J558L myeloma was completely suppressed in PD-1−/− BALB/c mice, whereas the tumors grew rapidly in the PD-1+/+ littermates. To exclude the possibility that PD-1−/− mice were generally resistant to any immunogenic tumors because of the lack of inhibitory signals in the cross-priming reactions, we compared the growth of B16 tumor cells, which did not express PD-L1 at all (Fig. 4A), in the PD-1+/+ and −/− mice of B6 background. As shown in Fig. 4C Right, B16 cells formed largely comparable tumors in the PD-1+/+ and −/− mice. The results suggested that a sharp distinction in the tumorigenesis of J558L cells in the syngeneic PD-1+/+ and −/− mice could be attributed primarily to the expression of PD-L1 in the tumor cells. Thus, it was suggested strongly that more persistent and efficient procedures to block PD-1 or PD-L1 in vivo, soluble PD-L1 for instance, might provide much improved therapeutic effect, and the trials are currently ongoing.
It was reported that CTLA-4 blockade in vivo led to suppression or even rejection of a number of potentially lethal transplanted tumors, in which the effectiveness apparently correlated with the inherent immunogenicity of the tumors (23). These results indicate that weak immune responses against the tumors can be converted into more potent ones effective for tumor suppression and rejection by inhibiting the effects of negative immunoreceptors. Because tumor cells per se rarely express the ligands for CTLA-4, CTLA-4 is suggested to restrict the activation and expansion of the potential effector cells by cross-presentation of the tumor antigens via host B7+ antigen-presenting cells (24). Present results, on the other hand, indicate that PD-L1 expressed on tumor cells protects them from direct immune attack by the specific CTL at the effector level at least in vitro, and enhances the tumorigenicity in vivo. Although the results imply the tumor-protective effect of PD-L1 at the level of immune effector cells such as CTL in vivo, an additional possibility remains open that the PD-L1 inhibits the T cell priming by cross-presentation of tumor antigens as well, because PD-L1 also is expressed on antigen-presenting cells (5). In any event, present results suggest strongly that effective blockade of PD-1–PD-L interaction in vivo should provide a promising strategy of immunotherapy for selected tumors expressing PD-L. It would also be interesting to examine whether there are any synergistic therapeutic effects between blockade of PD-1 and CTLA-4 on the tumors in vivo.
Acknowledgments
We thank Dr. H. Hiai for histological examination and Drs. K. Udaka and G. Freeman for providing cells and plasmids. This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Culture, Sports, and Technology (Japan).
Abbreviations
PD-L, ligands for PD-1 receptor
PD-1 −/−, PD-1 gene knock-out mice
CTL, cytotoxic T lymphocyte
References
- 1.Sperling A. I. & Bluestone, J. A. (1996) Immunol. Rev. 153, 155-182. [DOI] [PubMed] [Google Scholar]
- 2.Tafuri A., Shahinian, A., Bladt, F., Yoshinaga, S. K., Jordana, M., Wakeham, A., Boucher, L. M., Bouchard, D., Chan, V. S., Duncan, G., et al. (1996) Nature (London) 409, 105-109. [DOI] [PubMed] [Google Scholar]
- 3.Thompson C. B. & Allison, J. P. (1997) Immunity 7, 445-450. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse P., Penninger, J. M., Timms, E., Wakeham, A., Shahinian, A., Lee, K. P., Thompson, C. B., Griesser, H. & Mak, T. W. (1995) Science 270, 985-988. [DOI] [PubMed] [Google Scholar]
- 5.Freeman G. J., Long, A. J., Iwai, Y., Bourque, K., Chernova, T., Nishimura, H., Fitz, L. J., Malenkovich, N., Okazaki, T., Byrne, M. C., et al. (2000) J. Exp. Med. 192, 1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latchman Y., Wood, C. R., Chernova, T., Chaudhary, D., Borde, M., Chernova, I., Iwai, Y., Long, A. J., Brown, J. A., Nunes, R., et al. (2001) Nat. Immunol. 2, 261-268. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura H., Minato, N., Nakano, T. & Honjo, T. (1998) Int. Immunol. 10, 1563-1572. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H., Nose, M., Hiai, H., Minato, N. & Honjo, T. (1999) Immunity 11, 141-151. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H., Okazaki, T., Tanaka, Y., Nakatani, K., Hara, M., Matsumori, A., Sasayama, S., Mizoguchi, A., Hiai, H., Minato, N. & Honjo, T. (2001) Science 291, 319-322. [DOI] [PubMed] [Google Scholar]
- 10.Boon T., Cerottini, J. C., Van den Eynde, B., van der Bruggen, P. & Van Pel, A. (1994) Annu. Rev. Immunol. 12, 337-365. [DOI] [PubMed] [Google Scholar]
- 11.Tureci O., Sahin, U. & Pfreundschuh, M. (1997) Mol. Med. Today 3, 342-349. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg S. A. (1997) Immunol. Today 18, 175-182. [DOI] [PubMed] [Google Scholar]
- 13.Leach D. R., Krummel, M. F. & Allison, J. P. (1996) Science 271, 1734-1736. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H. & Honjo, T. (2001) Trends Immunol. 22, 265-268. [DOI] [PubMed] [Google Scholar]
- 15.Agata Y., Kawasaki, A., Nishimura, H., Ishida, Y., Tsubata, T., Yagita, H. & Honjo, T. (1996) Int. Immunol. 8, 765-772. [DOI] [PubMed] [Google Scholar]
- 16.Ishida M., Iwai, Y., Tanaka, Y., Okazaki, T., Freeman, G. J., Minato, N. & Honjo, T. (2002) Immunol. Lett. 84, 57-62. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y., Tanaka, Y., Miyagawa, F., Yamashita, S. & Minato, N. (2001) J. Immunol. 167, 5092-5098. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K., Nishimura, H., Matsuzaki, T., Yokokura, T., Nimura, Y. & Yoshikai, Y. (2000) Cancer Immunol. Immunother. 49, 71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udaka K., Wiesmuller, K. H., Kienle, S., Jung, G. & Walden, P. (1996) J. Immunol. 157, 670-678. [PubMed] [Google Scholar]
- 20.Brandle D., Bilsborough, J., Rulicke, T., Uyttenhove, C., Boon, T. & Van den Eynde, B. J. (1998) Eur. J. Immunol. 28, 4010-4019. [DOI] [PubMed] [Google Scholar]
- 21.Van den Eynde B., Lethe, B., Van Pel, A., De Plaen, E. & Boon, T. (1991) J. Exp. Med. 173, 1373-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramarathinam L., Sarma, S., Maric, M., Zhao, M., Yang, G., Chen, L. & Liu, Y. (1995) J. Immunol. 155, 5323-5329. [PubMed] [Google Scholar]
- 23.Chambers C. A., Kuhns, M. S., Egen, J. G. & Allison, J. P. (2001) Annu. Rev. Immunol. 19, 565-594. [DOI] [PubMed] [Google Scholar]
- 24.Huang A. Y., Golumbek, P., Ahmadzadeh, M., Jaffee, E., Pardoll, D. & Levitsky, H. (1994) Science 264, 961-965. [DOI] [PubMed] [Google Scholar]