Abstract
γ-Interferon-inducible lysosomal thiol reductase (GILT) is constitutively expressed in antigen-presenting cells. GILT facilitates unfolding of endocytosed antigens in MHC class II-containing compartments by enzymatically reducing disulfide bonds. The enzyme is synthesized as a 35-kDa precursor. Although a fraction of the precursor is secreted as a disulfide-linked dimer, the majority is directed via the mannose-6-phosphate receptor pathway to endocytic compartments where its N- and C-terminal propeptides are cleaved to generate the 30-kDa mature form. Both precursor and mature GILT reduce disulfide bonds with an acidic pH optimum. In this report, we show that the cysteine residues in the C-terminal propeptide, Cys-211 and Cys-222, serve key structural roles. Mutation of Cys-222 abolishes disulfide-linked dimerization of precursor GILT and decreases the efficiency of GILT maturation. Mutation of Cys-211 results in both impaired intracellular maturation and loss of enzymatic activity of the precursor form at an acidic pH. A similar phenotype was obtained upon mutation of Cys-200, which is retained in the mature form. Cys-200 and Cys-211 seem to form a disulfide bond that links the propeptide and the mature enzyme until reduction in the lysosome. This disulfide bridge is essential for stability of the enzyme at low pH and for its proper maturation in vivo.
Keywords: MHC class II, antigen processing, protein unfolding, disulfide bonds, dimerization
Exogenous antigens are internalized by antigen-presenting cells and delivered to MHC class II-containing compartments. During this process, they are unfolded and degraded, generating peptides that bind to MHC class II molecules. Following transport to the cell surface, MHC class II-peptide complexes stimulate CD4+ T cells (1, 2). Unfolding proteins containing disulfide bonds requires their reduction, which is facilitated by γ-interferon-inducible lysosomal thiol reductase (GILT; ref. 3). Human GILT is synthesized as a 35-kDa soluble precursor glycoprotein, and the majority is transported via the mannose 6–phosphate receptor to the endocytic pathway where the N- and C-terminal propeptides are removed. A fraction of precursor GILT is secreted and is detected largely as disulfide-linked dimers (4, 5). The significance of this dimerization as well as the role of the propeptides are unknown. Although mature GILT is present largely in late endosomes and lysosomes, precursor GILT is detected primarily in early endosomes (5). Both forms exhibit thiol reductase activity at acidic pH, and we have postulated that they may both be involved in disulfide bond reduction of antigens throughout the endocytic pathway (6).
GILT from Mus musculus has been cloned, and there are putative homologs in Danio rerio, Drosophila melanogaster, Caenorhabditis elegans, and Arabidopsis thaliana (7). Human and mouse GILTs contain 11 and 13 cysteines, respectively, and the two additional cysteines in mouse GILT are present in the N-terminal propeptide. The remaining cysteines are conserved, including those in the active-site CXXC motif that spans residues 46–49 in human GILT. Furthermore, alignment of human GILT with the other putative homologues reveals that, with the exception of the most C-terminal, all of the cysteines are conserved. Previously, we showed that although mutation of either cysteine in the active-site motif abolished thiol reductase activity, maturation and transport of GILT were unaffected (6). Here, we have used a mutagenesis approach to investigate the roles of the other cysteines in the folding, transport, function, and maturation of GILT. Surprisingly, the evidence obtained suggests that Cys-200, retained in the mature protein, and Cys-211, in the C-terminal propeptide, form a disulfide bond in the precursor form which is essential for proper maturation and stability of the GILT enzyme.
Experimental Procedures
Cells and Antibodies.
COS-7 cells were obtained from the American Type Culture Collection. The rabbit antisera R.GILT (5), R.IP30N (8), R.IP30C (8), anti-calnexin (9), and the mouse monoclonal antibody MAP.IP30 have been described (8). The anti-CD63 monoclonal antibody H5C6 was developed by J. Thomas August (Johns Hopkins University, Baltimore) and was obtained from the Developmental Studies Hybridoma Bank, maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore) and the Department of Biological Sciences, University of Iowa (Iowa City, IA).
Reagents.
Chloroquine and iodoacetamide were purchased from Sigma. N-glycosidase F was purchased from Roche Molecular Biochemicals.
GILT Purification.
Human GILT was affinity-purified from the B lymphoblastoid cell line, Raji, by using the monoclonal antibody MAP.IP30 coupled to Biogel A15m beads (Bio-Rad), as described (5). Raji cells were produced and harvested by the National Cell Culture Center (Minneapolis).
PCR-Based Mutagenesis.
GILT was subcloned from pBluescript KS+/GILT into the vector pcDNA 3.1(−) puro, and the construct was used as the template for PCR-based mutagenesis. The cysteines at residues 91, 98, 106, 122, 136, 152, 200, 211, and 222 were individually mutated to serines. For each construct, two fragments encompassing the mutation were PCR-amplified in reactions containing either GILT-5 (5′-GTCCCGGGATCCGCCACCATGGATAGTCGCCACACC-3′) and the mutagenic antisense primers or GILT-3 (5′-ATTTTATGCATCCCATGATACGTAGGATCCGA-3′) and the mutagenic sense primers. The mutagenic sense and antisense primers are as follows: C91S (sense) 5′-GGGAGTTCAAGAGCCAGCATGGAGAAGAGG-3′, C91S (antisense) 5′-CCTCTTCTCCATGCTGGCTCTTGAACTCCC-3′, C98S (sense) 5′-CCAGCATGGAGAAGAGGAGAGCAAATTCAACAAGGTGGAGG-3′, C98S (antisense) 5′-CCTCCACCTTGTTGAATTTGCTCTCCTCTTCTCCATGCTGG-3′, C106S (sense) 5′-GCAAATTCAACAAGGTGGAGGCCAGCGTGTTGGATGAACTTGACATGG-3′, C106S (antisense) 5′-CCATGTCAAGTTCATCCAACACGCTGGCCTCCACCTTGTTGAATTTGC-3′, C122S (sense) 5′-CCTTCCTGACCATTGTCAGCATGGAAGAGTTTGAGGACATGG-3′, C122S (antisense) 5′-CCATGTCCTCAAACTCTTCCATGCTGACAATGGTCAGGAAGG-3′, C136S (sense) 5′-GGAGAGAAGTCTGCCACTAAGCCTGCAGCTCTACGCTCCAGGGC-3′, C136S (antisense) 5′-GCCCTGGAGCGTAGAGCTGCAGGCTTAGTGGCAGACTTCTCTCC-3′, C152S (sense) 5′-CGCCAGACACTATCATGGAGAGCGCAATGGGGGACCGCGGC-3′, C152S (antisense) 5′-GCCGCGGTCCCCCATTGCGCTCTCCATGATAGTGTCTGGCG-3′, C200S (sense) 5′-CCCAGCTCCTTACCCTTGTCAGCCAGTTGTACCAGGGC-3′, C200S (antisense) 5′-GCCCTGGTACAACTGGCTGACAAGGGTAAGGAGCTGGG-3′, C211S (sense) 5′-GGGCAAGAAGCCGGATGTCAGCCCTTCCTCAACCAGCTCCC-3′, C211S (antisense) 5′-GGGAGCTGGTTGAGGAAGGGCTGACATCCGGCTTCTTGCCC-3′, C222S (sense) 5′-CCCTCAGGAGTGTTAGCTTCAAGTGATGGCCGG-3′, C222S (antisense) 5′-CCGGCCATCACTTGAAGCTAACACTCCTGAGGG-3′. In a second reaction, the fragments were annealed, extended, and amplified with GILT-5 and GILT-3, and the PCR products were cloned into pcDNA 3.1(−) puro.
Transient Transfection in COS-7 Cells.
COS-7 cells were seeded overnight in T75 flasks, grown to 80% confluency, and transfected with 20 μg of GILT or mutant GILT cDNAs by using CellFECTIN (Life Technologies, Rockville, MD). After 72 h, the cells were harvested and lysed on ice in 1% Triton X-100 in 150 mM NaCl/10 mM Tris, pH 6.9 whereas the supernatants were concentrated 15-fold by using Ultrafree-15 Centrifugal Filter Devices (Millipore). Mature GILT and precursor GILT were immunoprecipitated from the cell extracts and concentrated supernatants, respectively, with MAP.IP30-Biogel A15m beads. GILT was eluted with either 120 μl of 0.1% Triton X-100/100 mM NaCl/50 mM acetate, pH 3.5 or 0.1% Triton X-100/100 mM Tris, pH 11.5. The eluates were neutralized with 1 M Tris or with 1 M acetic acid, respectively.
In Vitro Assay for Thiol Reductase Activity.
The assay was described (5). Briefly, affinity-purified rabbit anti-mouse F(ab′)2 (Jackson ImmunoResearch) was iodinated by the chloramine T method (10, 11), denatured by boiling in 0.2% SDS, and diluted in 0.1% Triton X-100/0.15 M NaCl. MAP.IP30 eluates were preactivated with 25 μM DTT at room temperature for 10 min, and SDS-denatured F(ab′)2 (10,000 cpm) was added. The reaction was carried out at 37°C in a final volume of 110 μl at either pH 5 or 7 and terminated after 1 h by the addition of iodoacetamide to 5 mM. Samples were analyzed by nonreducing SDS/PAGE, and individual bands were quantitated by using a Bio-Rad GS-250 Molecular Imager.
Immunoblotting.
Immunoblotting was performed as described (12). Briefly, samples were separated by nonreducing SDS/PAGE (12% wt/vol acrylamide) and electrophoretically transferred onto an Immobilon P membrane (Millipore). The membrane was blocked for 30 min in PBS, 0.3% Tween 20, 5% dehydrated milk, and 5% bovine calf serum, washed with PBS, and probed with R.GILT (1:1,000), R.IP30N (1:1,000 or 1 μg/ml), and R.IP30C (1:500) in blocking buffer. Bands were visualized with horseradish peroxidase-conjugated goat anti-rabbit IgG [F(ab′)2 fragment-specific] (Jackson ImmunoResearch) and SuperSignal chemiluminescent substrate (Pierce).
N-Glycosidase F Digestion.
Immunoisolated precursor GILT was deglycosylated with N-glycosidase F by using the buffer recommended by the manufacturers. Digestions were performed for 16 h at 37°C. Samples were analyzed by reducing SDS/PAGE and immunoblotting.
Metabolic Radiolabeling and Immunoprecipitations.
COS-7 cells were incubated in methionine-free, cysteine-free medium for 1 h at 37°C, labeled for 30 min with 0.25 mCi (1 Ci = 37 GBq) [35S]methionine/cysteine (ICN), and chased in the presence of 15-fold excess methionine/cysteine for up to 8 h. To inhibit lysosomal degradation, chloroquine (30 μM) was added during both the label and chase. Cells were lysed in 1% Triton X-100/Tris, pH 6.9, and precleared with normal rabbit serum and protein G-Sepharose (Amersham Pharmacia); immunoprecipitations were performed with MAP.IP30 and protein G-Sepharose. Samples were analyzed by nonreducing SDS/12% PAGE followed by autoradiography, and individual bands were quantitated by using a Bio-Rad GS-250 Molecular Imager.
Crosslinking.
Supernatants from COS-7 cells expressing C222S GILT were concentrated as described above. Precursor GILT was immunoprecipitated with MAP.IP30-Biogel A15m beads, and GILT was eluted with 120 μl of 100 mM NaCl/50 mM acetate, pH 3.5. The pH was adjusted to pH 7 with 6 μl of 1 M bicine, pH 10. Disuccinimidyl tartrate (DST) was added to 5 mM to 50 μl of the eluate and incubated for 30 min at room temperature. The reaction was quenched with 20 mM Tris, and the samples were analyzed by nonreducing SDS/PAGE and immunoblotted with R.GILT.
Mass Spectrometry Analysis of Tryptic Digests.
Affinity-purified human GILT (3 μg) was either carboxyamidomethylated with 5 mM iodoacetamide at pH 8 or reduced with 25 mM DTT and treated with 500 mM iodoacetamide before SDS/PAGE analysis. The gel was stained with 0.1% Coomassie blue R250 in 10% acetic acid/50% methanol/40% H2O for 1 h and destained with 10% acetic acid/50% methanol/40% H2O for 2 h with two solvent changes. The bands were excised, and in-gel tryptic digestion and matrix assisted laser desorption ionization mass spectrometry (MALDI-MS) were performed by Howard Hughes Medical Institute Biopolymer and W. M. Keck Biotechnology Resource Laboratory at Yale University (New Haven, CT).
Results
Effect of Cysteine Mutations on Steady-State GILT Expression.
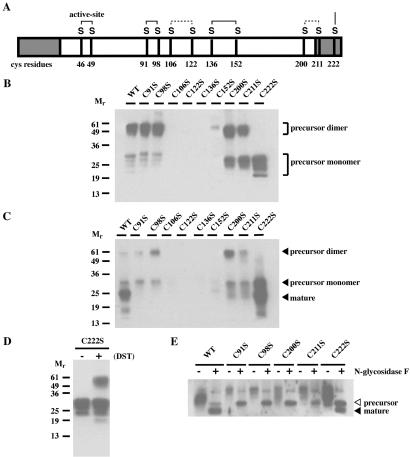
Cysteine residues 91, 98, 106, 122, 136, 152, 200, 211, and 222 in human GILT were individually changed to serine residues by PCR-based mutagenesis. Fig. 1A shows a schematic depicting the positions of the cysteines in GILT, and the disulfide bonds suggested by the data obtained in this paper are indicated. Wild-type and mutant forms of GILT were expressed in COS-7 cells, and the precursor and mature forms were immunoisolated from the supernatant and cell extract, respectively. Eluates were analyzed by nonreducing SDS/PAGE and immunoblotting with the rabbit antiserum R.GILT, which recognizes both precursor and mature forms (Figs. 1 B and C). GILT has three N-glycosylation sites and, as previously shown, the multiple species of precursor GILT detected in the supernatant result from incomplete glycosylation in COS-7 cells (6). For wild-type, C91S, C98S, C200S, and C211S GILT, the precursor form was detected as both disulfide-linked dimers and monomers in varying ratios (Fig. 1B). However, for C106S, C122S, C136S, and C152S GILT, little or no precursor GILT was detected.
Fig 1.
Steady-state expression of GILT with cysteine mutations. (A) Human GILT with its cysteines, putative disulfide bonds, and N- and C-terminal propeptides (shaded). COS-7 cells were transfected with cDNAs encoding wild-type, C91S, C98S, C106S, C122S, C136S, C152S, C200S, C211S, and C222S mutant GILT. Secreted and intracellular GILT were immunoisolated from the supernatants (B) and extracts (C). The eluates were analyzed by nonreducing SDS/PAGE and immunoblotted with the rabbit antiserum, R.GILT, which recognize both precursor and mature forms. (D) Eluate containing precursor C222S GILT was crosslinked with disuccinimidyl tartrate (DST). Samples were analyzed by nonreducing SDS/PAGE and immunoblotted with R.GILT. Crosslinking resulted in a species of approximately 60 kDa, suggesting that precursor GILT can form noncovalent dimers. (E) Wild type, C91S, C98S, C200S, and C211S GILT were immunoisolated from cell extracts and digested with N-glycosidase F. Samples were analyzed by reducing SDS/PAGE and immunoblotted with R.GILT. Deglycosylation resulted in single unglycosylated species of precursor (▹) and mature (◂) GILT.
The C222S mutant was detected only as a monomer by SDS/PAGE without reduction, suggesting that Cys-222 is responsible for the disulfide-mediated dimerization of secreted precursor GILT. To determine whether precursor GILT forms dimers independently of Cys-222, the C222S mutant precursor was crosslinked by using disuccinimidyl tartrate (DST; Fig. 1D). Analysis by SDS/PAGE and immunoblotting revealed a species of 60 kDa, indicating that at least a portion of the mutant precursor is in the form of noncovalent dimers. The data suggest that precursor GILT can dimerize independently of Cys-222.
As observed previously, the mature form of wild-type GILT was the predominant intracellular species (ref. 5; Fig. 1C). No intracellular C106S, C122S, C136S, or C152S GILT was observed. Because secreted precursor was not observed for these mutants either, it would seem that alteration of these cysteines disrupts proper folding and expression of GILT. Cell lysates and supernatants also were directly immunoblotted with R.GILT before immunoprecipitation, and little GILT was detected for these four mutants (data not shown). Thus, it is unlikely that lack of detection of these mutants results from loss of reactivity with the MAP.IP30 mAb.
For the C91S and C98S GILT mutants, small amounts of the precursor only were detected in cell extracts (Fig. 1C). For C200S and C211S, slightly more precursor and a trace of a lower molecular weight band were detected. For C222S GILT, there was high expression of both the precursor and mature forms. To ask whether any of the faster mobility species detected might result from differences in glycosylation, eluates containing intracellular wild-type, C91S, C98S, C200S, C211S, and C222S GILT were digested with N-glycosidase F to remove N-linked glycans. Immunoblotting with R.GILT showed that deglycosylation converted the precursor and mature wild-type GILT to single unglycosylated species and revealed that only the precursor was present intracellularly for all of the expressed mutants except C222S (Fig. 1E). This result was confirmed by immunoblotting with rabbit antisera R.IP30N and R.IP30C, which recognize only the N-terminal and C-terminal propeptides (data not shown). Thus, precursor forms of C91S, C98S, C200S, and C211S are secreted, but they fail to mature and accumulate intracellularly.
Effect of Cysteine Mutations on GILT Maturation.
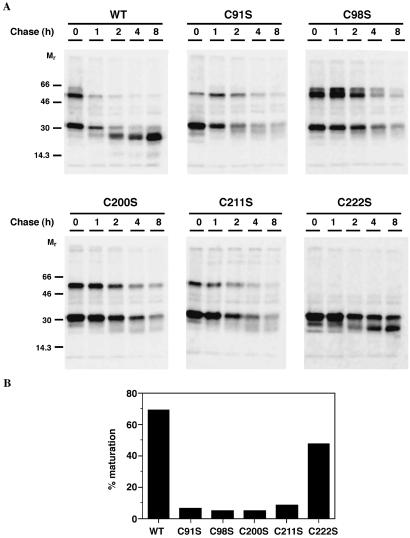
For the C91S, C98S, C200S, and C211S mutants, although expression of secreted precursor was comparable to wild-type, the intracellular expression was low. To determine whether this was caused by degradation, we examined the kinetics of GILT expression. COS-7 cells expressing wild-type, C91S, C98S, C200S, C211S, and C222S GILT were metabolically labeled with [35S]methionine for 30 min and chased with 15-fold excess cold methionine/cysteine for up to 8 h. At intervals, the supernatants were harvested, and the cells were extracted with detergent. GILT was immunoprecipitated with MAP.IP30, and the samples were analyzed by nonreducing SDS/PAGE followed by autoradiography (Figs. 2 and 3). Secreted precursor GILT was detectable and stable for wild-type, C91S, C98S, and C222S GILT, whereas the amounts of C200S and C211S precursor decreased after 4 h of chase (Fig. 2). Of the wild-type and C222S GILT, 70% and 50%, respectively, was converted to the mature form, whereas little mature GILT was detected for the other mutants (Fig. 3). Thus, mutation of Cys-200 or Cys-211 destabilized the secreted precursor form, whereas all cysteine mutations except C222S prevented normal intracellular maturation. The adverse effects of Cys-211 mutagenesis are surprising, given that this residue is in the C-terminal propeptide.
Fig 2.
Effects of cysteine mutations on secretion of precursor GILT. COS-7 cells expressing wild-type, C91S, C98S, C200S, C211S, and C222S mutant GILT were starved, metabolically labeled with [35S]methionine, and chased for up to 8 h. Secreted GILT was immunoprecipitated from the supernatants with MAP.IP30, and samples were analyzed by nonreducing SDS/12% PAGE and autoradiography. With the exception of C222S mutation, both dimeric and monomeric precursor forms were secreted by COS-7 cells.
Fig 3.
Effects of cysteine mutations on GILT maturation. COS-7 cells expressing wild-type, C91S, C98S, C200S, C211S, and C222S mutant GILT were starved, metabolically labeled with [35S]methionine, and chased for up to 8 h. (A) GILT was immunoprecipitated from cell lysates with MAP.IP30, and samples were analyzed by nonreducing SDS/12% PAGE and autoradiography. (B) Percent of mature GILT at 8 h as a percentage of precursor GILT present at time 0.
Effect of Cysteine Mutations on Thiol Reductase Activity.
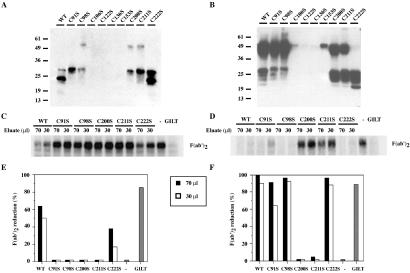
Next, we examined whether the mutations of the cysteines affected GILT enzymatic activity. We used the assay described (5) which uses 125I-F(ab′)2 as a substrate, adapted to analyze mutants expressed in COS-7 cells (6). Wild-type and mutant forms of GILT were eluted from MAP.IP30 beads at pH 3.5, and the pH was adjusted to 5. Eluates containing intracellular and secreted GILT were immunoblotted with R.GILT (Figs. 4 A and B). As described above, there was little expression of the C106S, C122S, C136S, and C152S mutants, and these eluates were not assayed for activity. Of the remaining eluates, 70 and 30 μl were adjusted to 100 μl, and DTT was added to 25 μM to preactivate GILT. The eluates then were incubated with 20,000 cpm of denatured 125I-F(ab′)2 at 37°C for 1 h at pH 5, and iodoacetamide was added to 5 mM to terminate the reaction. Reduction of F(ab′)2 into Fab′ and H′ and L chains was analyzed by nonreducing SDS/PAGE, followed by autoradiography and quantitation by image analysis (Fig. 4 C–F).
Fig 4.
Thiol reductase activity of intracellular and secreted GILT mutants. Intracellular and secreted GILT were immunoisolated from the cell extract and supernatant of COS-7 cells expressing wild-type, C91S, C98S, C200S, C211S, and C222S GILT. Eluates from the cell extract (A) and supernatant (B) were immunoblotted for GILT expression. (C and D) Eluates (70 and 30 μl) were assayed for reduction of F(ab′)2. (E and F) Subsequent quantitation. [Ctrl(−): buffer at pH 5; purified GILT: 1 μg.]
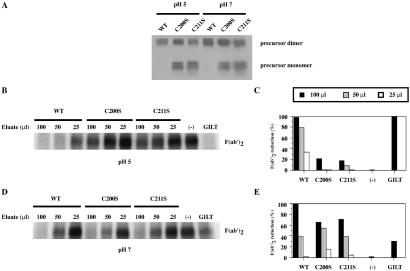
Both purified wild-type and C222S GILT from cell extracts were able to reduce F(ab′)2 (Fig. 4 C and E). Other mutants were presumably too poorly expressed intracellularly to have significant activity. Wild-type GILT and the mutant C91S, C98S, and C222S precursor forms isolated from the supernatant were also able to reduce F(ab′)2 well (Fig. 4 D and F). However, although the levels of GILT obtained from the supernatants were comparable, as detected by immunoblotting, reduction by precursor C200S and C211S GILT was poor. Their effective secretion argues that both mutants satisfy the folding criteria of the endoplasmic reticulum (ER) quality control system, indicating that they are not misfolded. An alternative explanation is that these mutants are unstable at the low pH of the assay or the lower pH of 3.5 used to elute them from the antibody-coupled beads used for purification. To examine this question, the thiol reductase activity of the C200S and C211S mutants was assayed at pH 7. Although GILT reduces disulfide bonds optimally at pH 4–5, there is detectable activity at neutral pH (5). Wild-type, C200S, or C211S GILT precursors isolated from COS-7 cell supernatants were immunoisolated with MAP.IP30–A15m beads, eluting at pH 11.5 to prevent potential denaturation of GILT by acidification. The eluates were adjusted to pH 5 or pH 7 with 1 M acetic acid and 100, 50, and 25 μl volumes were assayed for thiol reductase activity (Figs. 5 B–E). Wild-type GILT reduced F(ab′)2 better at pH 5 than pH 7, although there is significant activity at pH 7. C200S and C211S GILT exhibited weak activity at pH 5 but were clearly much more active at pH 7. Analysis of eluates by SDS/PAGE and immunoblotting with R.GILT confirmed that similar amounts were assayed (Fig. 5A).
Fig 5.
pH-dependent effect of C200S and C211S mutations on thiol reductase activity of precursor GILT. Precursor GILT was immunoisolated from the supernatants of COS-7 cells expressing wild-type, C200S, and C211S GILT. Eluates were immunoblotted for GILT expression (A) and 100, 50, and 25 μl of the eluates were assayed for reduction of F(ab′)2 at pH 5 (B) and pH 7 (D). (C and E) Subsequent quantitation. [Ctrl(−): buffer at pH 5; purified GILT: 1 μg.]
Mapping of Disulfide Bonds.
The phenotype of the C200S and C211S mutants provided evidence that the structural conformation conferred by these two cysteines is critical both for the stability and enzymatic activity of precursor GILT at an acidic pH. This instability is also likely to be responsible for degradation of the mutants in the acidic environment of the lysosome. One hypothesis to explain the phenotype is that Cys-200 and Cys-211 form an intramolecular disulfide bond, and during generation of the mature form, the disulfide bridge is reduced and the C-terminal propeptide is cleaved. The disulfide bond may confer a conformation where only the propeptide cleavage sites are accessible to proteases. If this postulate is correct, mapping of disulfide bonds in mature GILT should reveal a free cysteine at residue 200. To this end, purified mature GILT was either carboxyamidomethylated with iodoacetamide or reduced first with DTT and then treated with excess iodoacetamide. The samples were analyzed by SDS/PAGE and Coomassie blue staining. The gel slices were digested for 18 h with trypsin, which cleaves after the basic residues Arg (R) and Lys (K); extracted peptides were analyzed by MALDI-MS (at Howard Hughes Medical Institute Biopolymer and W. M. Keck Biotechnology Resource Laboratory). Peptides containing cysteines that are not involved in disulfide bonds should be detected in both the reduced and nonreduced samples, whereas peptides connected by a disulfide bond should only be detected in the nonreduced sample. In multiple trials, only two peptides, with masses of 1,060.32 and 2,808.3 Da, were specific for the nonreduced sample. Absence of other predicted masses are likely caused by poor tryptic digests.
The 1,060.32 Da and 2,792.35 Da masses correspond to the peptides 91CQHGEEE98CK and SLPL136CLQLYAPGLSPDTIME152CAMGDR with internal disulfide bonds. The predicted monoisotopic masses of these peptides are 1,060.30 and 2,792.32 Da, respectively. Predicted on mass matches alone, the assignments of these two disulfide bonds remain tentative. Nonetheless, they are consistent with the biochemical analysis, in that the C91S and C98S mutants and the C136S and C152S mutants, respectively, exhibit similar patterns of expression and activity. Given this data, Cys-106, Cys-122, and Cys-200 are all free to form a disulfide bond. Both the C106S and C122S mutants failed to express in COS-7 cells (Figs. 1 and 4), and this similarity suggests that they are likely to be involved in an intra-chain disulfide bond. We deduce that, in the mature form, Cys-200 is free, and suggest that, based on the similar expression and activity patterns of the C200S mutant and C211S mutants, it is disulfide-bonded to Cys-211 in the precursor.
Discussion
Intramolecular disulfide bonds are critical for protein folding. Hence, we expected that several of the cysteine mutations we introduced into GILT would abrogate expression. Mutations of Cys-106, -122, -136, and -152 resulted in weak or no expression of both precursor and mature GILT. We postulate that these four cysteines are involved in two disulfide bonds, and that the absence of either causes protein misfolding. Mass spectrophotometric analysis of tryptic digests of mature GILT suggests that Cys-136 and Cys-152 form a disulfide bond (Fig. 1A). Cys-106 and Cys-122 seem likely to form another. Based on mass spectrometry of peptides and the similar behavior of the C91S and C98S mutants, Cys-91 and Cys-98 also form an intramolecular disulfide bond. These mutants were expressed, and the precursor forms were both stable and active, but maturation was impeded (Figs. 1–4).
Mutation of Cys-222, located in the C-terminal propeptide, resulted in expression of monomeric precursor GILT, as analyzed by nonreducing SDS/PAGE, indicating that Cys-222 is responsible for disulfide-linked homodimerization. However, crosslinking experiments suggested that this mutant forms noncovalent dimers (Fig. 1D). Thus, it seems likely that the precursor is naturally dimeric, and that the dimer is stabilized by an intermolecular disulfide bond involving Cys-222 (Fig. 1D). As observed both by immunoblotting and pulse–chase experiments, mutation at Cys-222 also decreased the efficiency of GILT maturation (Fig. 1C, Fig. 2). A possible explanation is that this mutation may affect transport of the precursor to lysosomes, reducing the rate of processing. Alternatively, it may have a more direct effect on proteolytic removal of the propeptides.
In a similar manner to mutants Cys-91 and Cys-98, the Cys-200 and Cys-211 GILT mutants were expressed, but processing to the mature form was impaired (Figs. 1–3). Detection by immunoblotting, pulse–chase experiments, and indirect immunofluorescence (data not shown) all indicated low intracellular expression of these GILT mutants (Figs. 1, 3, and 4). Properties of the lysosomes which may account for the low expression include low pH and subsequent denaturation as well as the abundance of lysosomal proteases. Comparable yields of wild-type and mutant GILT precursors by acid elution and storage at pH 4.5 suggest that simple acidic denaturation is not sufficient to account for the loss of protein (Fig. 1). In pulse–chase experiments, addition of chloroquine, which neutralizes lysosomes and inhibits proteolytic degradation by lysosomal cathepsins, resulted in partial recovery of these precursor mutants (data not shown). These results suggest that mutations of Cys-91, -98, -200, and -211 all alter the conformation of precursor GILT such that it is no longer stable in the degradative milieu of the lysosomes, resulting in the low accumulation of mature GILT.
Cys-91 and Cys-98 form an intramolecular disulfide bond, and we postulate that Cys-200 and Cys-211 also form such a bond. The major difference in phenotype between the C200S and C211S mutants and the C91S and C98S mutants is that in the former, the enzymatic activity of precursor GILT was abolished at low pH (Fig. 4 D and F). We found this particularly striking given that Cys-211 is present in the C-terminal propeptide and is, therefore, dispensable for function in the mature form. The C200S and C211S GILT mutants were able to reduce disulfide bonds at pH 7 (Figs. 5 D and E), suggesting that at neutral pH they are able to retain the tertiary structure required for enzymatic activity. Mutation of Cys-200 or Cys-211 also resulted in a lower ratio of dimeric to monomeric secreted GILT (Fig. 5A). The mutations may induce a conformational change in the propeptide such that dimerization by means of Cys-222 is hindered. Cys-200 and Cys-211 are present in the putative GILT homologues (7), suggesting that an intramolecular disulfide bond between the propeptide and the remainder of protein may be a conserved feature of the enzyme.
Lysosomal proteins need to resist the hostile conditions of the environment in which they reside. Our data suggest that the putative disulfide bond between Cys-200 and Cys-211 of precursor GILT ensures proteolysis of only the propeptides and the subsequent accumulation of the mature enzyme. Other lysosomal proteins have propeptides with much more obvious functions. For example, the lysosomal cathepsins are synthesized as precursors, and their propeptides serve a critical function of binding to the active site, thus inhibiting activity (reviewed in ref. 13). The low pH of the lysosomes induces autocatalytic proteolysis of the propeptides and, hence, activates the cathepsins. In contrast, the C-terminal propeptide of GILT is not inhibitory. The finding that it serves a structural role instead now raises the question of why its cleavage should be necessary at all. As determined in vitro, the thiol reductase activity of precursor GILT parallels that of mature GILT, suggesting that cleavage of the C-terminal propeptide is not required for enzymatic function (6). One explanation is that the propeptide may be required for proper folding of precursor GILT upon synthesis in the ER, and that by chance the acquired conformation simply exposes proteolytic cleavage sites that are involved in the generation of mature GILT. There are other examples where lysosomal processing serves no apparent function. Ikonen et al. showed that lysosomal aspartylglucosaminidase becomes active in the ER when the 42-kDa precursor is cleaved, generating a 27-kDa pro-α chain and a 17-kDa chain. However, after transport to the lysosome, the pro-α chain is further processed to 24 kDa, although the activity of the protein is unaltered (14).
The possibility that the cleavage of the GILT propeptides is necessary and significant, and that precursor GILT might have a specific function dependent on the N- or C-terminal propeptide, cannot be excluded. Conservation of the cysteine residues in the C-terminal propeptide may argue for another function, although the lack of conservation in the N-terminal propeptide even between mouse and human does not support such an idea for this region of the molecule (7). Unfortunately, these ideas cannot be easily tested. Understanding the conformational and functional differences between the precursor and mature forms will almost certainly require the determination of their three dimensional structures. Structural characterization should reveal both the spatial orientations of the active site and propeptides and, in addition, the noncovalent interactions directing dimerization of precursor GILT.
Acknowledgments
We thank the Howard Hughes Medical Institute Biopolymer and W. M. Keck Biotechnology Resource Laboratory, Dr. Ken Williams, Kathy Stone, and Mary LoPresti for MALDI-MS analysis, and Nancy Dometios for help in preparing the manuscript. The work was supported by National Institutes of Health Grant AI23081 and the Howard Hughes Medical Institute. R.L.L. was supported by the Natural Science and Engineering Research Council, Canada.
Abbreviations
GILT, γ-interferon-inducible lysosomal thiol reductase
References
- 1.Germain R. N. & Hendrix, L. R. (1991) Nature (London) 353, 134-139. [DOI] [PubMed] [Google Scholar]
- 2.Watts C. (1997) Annu. Rev. Immunol. 15, 821-850. [DOI] [PubMed] [Google Scholar]
- 3.Maric M., Arunachalam, B., Phan, U. T., Dong, C., Garret, W. S., Cannon, K. S., Alfonso, C., Karlsson, L., Flavell, R. A. & Cresswell, P. (2001) Science 294, 1361-1365. [DOI] [PubMed] [Google Scholar]
- 4.Luster A. D., Weinshank, R. L., Feiman, R. & Ravetch, J. V. (1988) J. Biol. Chem. 263, 12036-12043. [PubMed] [Google Scholar]
- 5.Arunachalam B., Phan, U. T., Geuze, H. J. & Cresswell, P. (2000) Proc. Natl. Acad. Sci. USA 97, 745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan U. T., Arunachalam, B. & Cresswell, P. (2000) J. Biol. Chem. 275, 25907-25914. [DOI] [PubMed] [Google Scholar]
- 7.Phan U. T., Maric, M., Dick, T. P. & Cresswell, P. (2001) Immunogenetics 53, 342-346. [DOI] [PubMed] [Google Scholar]
- 8.Arunachalam B., Pan, M. & Cresswell, P. (1998) J. Immunol. 160, 5797-5806. [PubMed] [Google Scholar]
- 9.Hammond C. & Helenius, A. (1994) J. Cell Biol. 126, 41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter W. M. & Greenwood, F. C. (1964) Biochem. J. 91, 43-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche P. A. & Cresswell, P. (1990) J. Immunol. 144, 1849-1856. [PubMed] [Google Scholar]
- 12.Anderson K. S. & Cresswell, P. (1994) EMBO J. 13, 675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath M. E. (1999) Annu. Rev. Biophys. Biomol. Struct. 28, 181-204. [DOI] [PubMed] [Google Scholar]
- 14.Ikonen E., Julkunen, I., Tollersrud, O.-K., Kalkkinen, N. & Peltonen, L. (1993) EMBO J. 12, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]