Abstract
Inactivating germ-line mutations of LKB1 lead to Peutz–Jeghers syndrome (PJS). We have generated mice heterozygous for a targeted inactivating allele of Lkb1 and found that they develop severe gastrointestinal polyposis. In all cases, the polyps arising in the Lkb1+/− mice were found to be hamartomas that were histologically indistinguishable from polyps resected from PJS patients, indicating that Lkb1+/− mice model human PJS polyposis. No evidence for inactivation of the remaining wild-type Lkb1 allele in Lkb1+/−-associated polyps was observed. Moreover, polyps and other tissues in heterozygote animals exhibited reduced Lkb1 levels and activity, indicating that Lkb1 was haploinsufficient for tumor suppression. Analysis of the molecular mechanisms characterizing Lkb1+/− polyposis revealed that cyclooxygenase-2 (COX-2) was highly up-regulated in murine polyps concomitantly with activation of the extracellular signal-regulated kinases 1 and 2 (Erk1/2). Subsequent examination of a large series of human PJS polyps revealed that COX-2 was also highly up-regulated in the majority of these polyps. These findings thereby identify COX-2 as a potential target for chemoprevention in PJS patients.
Keywords: tumor suppressor, mitogen-activated protein kinase, serine-threonine kinase, hamartoma, biallelic inactivation
Peutz–Jeghers syndrome (PJS) is a rare inherited disease in which patients develop gastrointestinal hamartomatous polyps in early adulthood and exhibit mucocutaneous pigmentation on the skin and oral mucosa (1, 2). PJS polyposis is frequently associated with bleeding, intussusception, and obstruction. Consequently, a large proportion of PJS patients undergo numerous repeat laparotomies to remove symptomatic polyps beginning in early adulthood (3). PJS is also a cancer predisposition syndrome with patients exhibiting a 15-fold increased risk of developing cancers of both gastrointestinal and extraintestinal origin in later life (4–6).
Inactivating germ-line mutations in the LKB1 gene underlie the majority of PJS cases (7, 8), although linkage analysis indicates the existence of additional minor PJS loci (9). LKB1 has therefore been believed to function as a tumor suppressor. This supposition has been supported in vitro with the demonstration that LKB1 induces a G1 cell cycle arrest in tumor cell lines that have lost endogenous LKB1 expression (10).
Lkb1 encodes a serine–threonine kinase (11) with widespread expression during murine embryonic development (12). We have previously shown that mice homozygous for a targeted disruption of Lkb1 undergo embryonic lethality at midgestation as a result of defective vasculogenesis associated with a tissue-specific deregulation of vascular endothelial growth factor (VEGF) (13). The molecular mechanisms by which Lkb1 mediates its functions remain poorly characterized, and, to date, no in vivo substrates for Lkb1 have been identified. Recent reports suggest that Lkb1 might be involved in mediating p53-dependent apoptosis (14) and in Brg1-mediated growth arrest (15). Other reports suggest that Lkb1 may interact with LIP1 (16) and that Lkb1 activity may be regulated through phosphorylation by p90RSK (17).
Herein we have generated and analyzed the phenotype of mice heterozygous for a targeted inactivating mutation of Lkb1, which represent the genetic equivalent of human Peutz–Jeghers patients with germ-line LKB1 mutations.
Materials and Methods
Mice, Histology, and in Situ Hybridization.
Targeted inactivation of murine Lkb1 and genotyping have been described (13). Lkb1+/− and Lkb1+/+ littermate control mice were maintained on several heterogeneous genetic backgrounds with no observed difference in phenotype. Murine samples were dissected and fixed overnight in 4% paraformaldehyde and sectioned at 3 μm. In situ hybridization was done as described (12).
Laser Microdissection, PCR Genotyping, and Sequencing.
Paraformaldehyde-fixed polyp and control tissues were laser dissected by using a Robot-Microbeam laser microdissector (P.A.L.M. Microlaser Technologies, Munich). A total of 10–15 individual laser-dissected samples of both stroma and epithelia from each of a total of five different polyps arising in five different animals were analyzed. Real-time PCR was done with 100 ng of template DNA and 10 ng of each primer by using PCR reagents and a GeneAmp 5700 detection system (Applied Biosystems, Foster City, CA). PCR primer sequences and genotyping strategy have been described (13). DNAs for LOH and sequence analysis were extracted from polyps of varying sizes ranging from 3 mm to 2.5 cm in diameter.
Immunoblotting, Immunohistochemistry, and Kinase Assays.
Lysates were prepared in ELB lysis buffer (150 mM NaCl/50 mM Hepes, pH 7.4/5 mM EDTA/0.1% Nonidet P-40 with 5 mM DTT/12.5 mg/ml aprotinin/0.5 mM phenylmethylsulfonyl fluoride/50 mM β-glycerol phosphate/5 μg/ml leupeptin). Abs used were: Lkb1 (Upstate Biotechnology), actin (Sigma, AC-40), β-catenin (Transduction Laboratories, Lexington, KY, C19225), VEGF (Neomarkers Ab-1), COX-2 (Cayman Chemicals, Ann Arbor, MI, nos. 160116 and 160112) and phosphatase and tensin homolog deleted on chromosome ten (PTEN), phospho-Akt, phospho-GSK3α/3β, p90RSK, phospho-extracellular signal-regulated kinase (Erk1/2), Erk2, Erk1/2, phospho-p38 mitogen-activated protein kinase (MAPK), p38 MAPK (Cell Signalling, Beverly, MA, nos. 9552, 9270, 9931, 9341, 9101, 9107, 9102, 9211, and 9212, respectively). Immunohistochemistry was performed according to standard protocols after epitope unmasking by microwaving samples for 5 min in 10 mM sodium citrate buffer. Kinase assays were performed as described (10).
Results
Lkb1 Is a Tumor Suppressor in Mice.
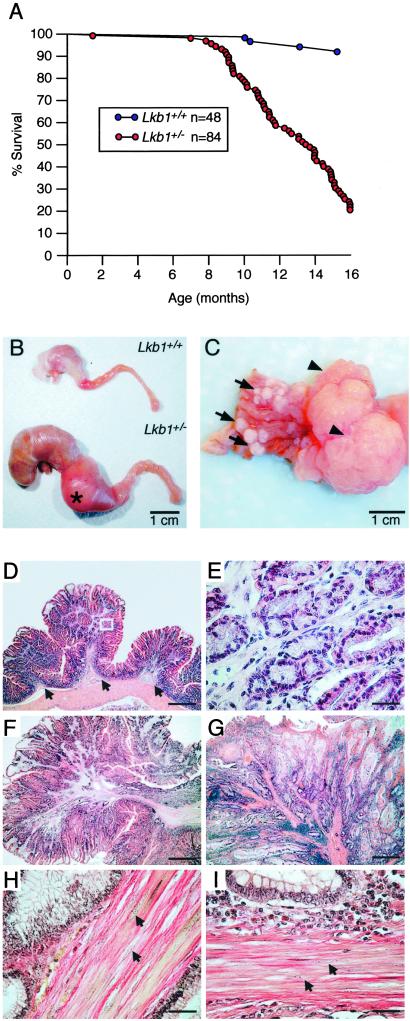
Lkb1+/− mice were found to be fertile and healthy into early adulthood. However, as they aged, they were found to have markedly reduced life spans with ≈80% dying by 16 mo of age (Fig. 1A). To investigate the cause of this increased mortality, Lkb1+/− and littermate Lkb1+/+ controls were analyzed. As the Lkb1+/− mice aged, grossly distended abdomens were noted, suggesting gastrointestinal obstruction. At necropsy, macroscopic gastrointestinal polyps were identified in 100% of the Lkb1+/− mice >6 mo of age. The vast majority of the polyps originated in the glandular stomach with approximate equal distribution in the fundus, antrum, and pylorus. A small number of polyps were found in the small intestine. All of the animals examined had multiple polyps with up to 40 polyps scored in individual animals (Fig. 1C). In the majority of animals examined, 1–3 very large polyps (20–30 mm in diameter, representing up to 25% of the total weight of the animal) originated from the pylorus (Fig. 1C, arrowheads). These large polyps protruded into the duodenum, rendering it grossly distended and resulted in obstruction (Fig. 1B). Based on these observations, the high mortality associated with Lkb1 heterozygosity was likely due in part to malnutrition resulting from gastrointestinal occlusion. The increased mortality also was due to bleeding at ulcerations of the polyps that was noted in many animals, which resulted in severe anemia.
Fig 1.
Increased mortality and polyposis modeling PJS in Lkb1+/− mice. (A) Survival curve of 48 wt (blue points) and 84 Lkb1+/− mice (red points) with points representing animals that died or were killed because of ill health. (B) Stomach, duodenum, and proximal jejunum of Lkb1+/− and Lkb1+/+ littermate. (C) Representative gastric stomach of an Lkb1+/− animal showing multiple small polyps (arrows) and large pyloric junction polyps (arrowheads). (D) Hematoxylin/eosin staining of a cross-section of glandular stomach from a Lkb1+/− mouse showing three small hamartomatous polyps. Note the smooth muscle core that is contiguous with the muscularis mucosa (arrows). (E) Higher magnification of the boxed region in D. (F and G) Hematoxylin/eosin staining of a murine polyp (F) and human PJS patient polyp (G). (H and I) Herovici's staining of a murine polyp (H) and a human PJS patient polyp (I) with smooth muscle nuclei indicated with arrows (compare to H). (Bars in D, F, and G = 0.8 mm; bars in E, H, and I = 40 μm.)
Polyposis in Lkb1+/− Mice Models Human PJS.
To further characterize the polyposis associated with Lkb1 heterozygosity, polyps were subjected to histological examination. All polyps analyzed (n = 325) revealed well differentiated glandular epithelium and normal lamina propria (Fig. 1 D and F) and were classified as hamartomas. Strikingly, all polyps had an extensive, well developed smooth muscle component, which provided a latticed framework between pockets of glandular epithelia (Fig. 1 E, F, and H). The smooth muscle originated from a large central stalk, which was contiguous with the muscularis mucosa (Fig. 1D, arrows).
Histological comparison of polyps derived from Lkb1+/− mice to polyps resected from human PJS patients revealed a striking similarity (Fig. 1, compare F and H with G and I). Particularly, the histology of the smooth muscle component, the most widely used criteria for establishing diagnosis of PJS, was indistinguishable from the human polyps (Fig. 1 F–I). These findings establish that Lkb1+/− mice model PJS polyposis. It should be noted however, that polyps in PJS patients are more evenly distributed along the full-length of the digestive tract with the highest frequency in the small intestine (18).
In addition to the characteristic hamartomas that develop in Peutz–Jeghers patients, PJS is a cancer predisposition syndrome with patients exhibiting an increased risk of developing cancers of both gastrointestinal and extraintestinal origin in later life (4–6). To examine whether Lkb1+/− mice were predisposed to cancer, we performed careful necropsy and histological examination on 59 Lkb1+/− mice and 13 Lkb1+/+ mice (ranging in age from 6 to 20 mo). In addition to the polyps observed in all of the Lkb1+/− mice, a small number of neoplasias and tumors in other organs were identified. These included a hepatocellular carcinoma, multiple liver adenomas, one endometrial carcinoma, and one hemangioma. One ovarian tumor was identified in a Lkb1+/+ control animal. These results suggest that the incidence of cancer was not markedly increased in Lkb1+/− mice compared to control animals for this age range. However, as Lkb1+/−-associated polyposis severely compromised the longevity of Lkb1+/− mice (Fig. 1A), we were therefore unable to establish whether older Lkb1+/− mice have an increased incidence of malignancy compared to littermate controls. Abnormal pigmentation of the oral mucosa in Lkb1+/− mice was not observed.
Haploinsufficiency of Lkb1 Underlies the Polyposis of Lkb1+/− Mice.
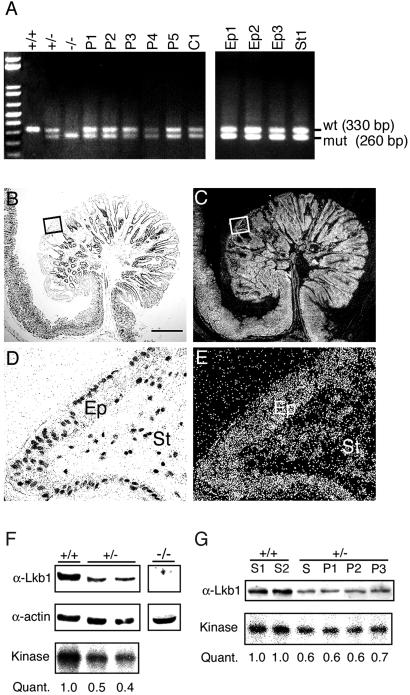
The identification of LKB1 as the tumor susceptibility locus was based in part on loss of heterozygosity (LOH) analysis (19). However, although subsequent studies have reported that LOH of LKB1 often accompanies polyp formation in Peutz–Jeghers patients (7, 20, 21), others have suggested that biallelic inactivation of LKB1 may be more rare (22, 23). Thus, the question of whether or not LOH of LKB1 is an obligate initiating event in human PJS polyposis has remained unresolved. To address this issue in the murine model, DNAs extracted from polyps (n = 41) and laser microdissected samples were genotyped as described (13). We found that both mutant and wild-type (wt) alleles were comparably amplified in all samples analyzed, suggesting that the wt allele was retained in the polyps (Fig. 2A). The copy number of the wt allele was subsequently assayed by real-time PCR on DNAs extracted from polyp (n = 12) and control tails (n = 10). The results indicated that amplification of the wt allele reached linear phase of amplification (threshold cycle) at cycle 25.96 (SD 0.47) in the polyps and at cycle 25.81 (SD 0.59) in control tails. These results demonstrate that there was no statistical difference in the copy number of the wt allele in the polyps compared to control tails. Moreover, when the remaining wt allele from polyps (n = 5) was sequenced, no evidence of Lkb1 mutation was observed within the coding regions or intron/exon boundaries.
Fig 2.
Lkb1 is haploinsufficient for tumor suppression. (A) PCR genotyping of Lkb1 from DNAs extracted from five polyps (P1–P5), one control Lkb1+/− tail (C1), and yolk sacs dissected from wt (+/+), heterozygous (+/−), and Lkb1 null (−/−) E9.5 embryos (Left), and of laser-microdissected epithelial (Ep1–Ep3) or stromal (St) cells from polyps of multiple Lkb1+/− animals (Right). A 330-bp wt band and a 260-bp mutant band are indicated. (B and C) Lkb1 in situ hybridization showing brightfield (B) and darkfield (C) images of a polyp and adjacent unaffected gastric epithelia from a Lkb1+/− animal. (D and E) Higher magnification of the brightfield (D) and darkfield (E) images of boxed regions shown in B and C demonstrating Lkb1 mRNA expression both in epithelial [Ep] and stromal [St] cell types. (F) Western blotting analysis of lysates from wt (+/+) or Lkb1 heterozygous (+/−) MEF cultures or Lkb1 null whole embryos (−/−) immunoblotted for Lkb1 (α-Lkb1) and actin (α-actin), and kinase assay showing Lkb1 autocatalytic activity with quantitation (Quant.). (G) Western blotting analysis of lysates from two independently isolated wt (+/+) stomachs (S1 and S2), and Lkb1 heterozygous (+/−) unaffected stomach (S) and three individual polyps (P1–P3) with α-Lkb1 (Upper), and Lkb1 kinase assay with quantitation (Quant.) (Lower). (Bar = 0.7 mm.)
It has been suggested that transcriptional silencing by promoter hypermethylation may be a mechanism by which LKB1 is inactivated in PJS patients that do not possess identifiable genetic second hits (24). To examine this in murine Lkb1+/− polyposis, we performed in situ hybridization analysis to assay for Lkb1 mRNA expression in a number of polyps (n = 10, Fig. 2 B–E). We found that Lkb1 mRNA levels were maintained throughout the entirety of all polyps examined at levels comparable to those observed in the adjacent unaffected gastric epithelia (Fig. 2 B and C). Higher magnification revealed that Lkb1 expression was retained in both the stromal and epithelial cells of the polyp with highest expression in the epithelia (Fig. 2 D and E).
In the course of performing the in situ hybridization experiments, it was noted that Lkb1 mRNA expression was lower in all analyzed Lkb1+/− heterozygote tissues when compared to Lkb1+/+ control mice (data not shown). To determine whether the diminished mRNA levels would lead to a corresponding decrease in protein expression, Lkb1 protein levels were investigated by Western blot analysis. Initially, the specificity of the Lkb1 Ab was verified by demonstrating the lack of the 55-kDa Lkb1 protein in whole embryo lysates of E9.5 Lkb1−/− embryos (Fig. 2F). Subsequently, Lkb1 levels and activity were assayed from Lkb1+/+ and Lkb1+/− mouse embryonic fibroblast (MEF) lysates where it was observed that Lkb1 protein expression and kinase activity were reduced to approximately one-half levels in Lkb1+/− MEFs (Fig. 2F).
To determine whether a similar decrease in Lkb1 expression and activity might accompany Lkb1+/− polyposis, Lkb1 protein levels and activity were measured from multiple polyps, adjacent unaffected stomachs, and stomachs from Lkb1+/+ animals. Similar to what had been observed in the MEF experiments, Lkb1 protein levels and activity in the unaffected stomachs of Lkb1+/− animals were lower than in wt stomachs (Fig. 2G). Consistent with our LOH, sequencing, and in situ hybridization analysis, Lkb1 levels and activity in the polyps were found to be comparable to those in adjacent unaffected stomach lysates (Fig. 2G). These observations indicate that mutations affecting protein synthesis, stability, or kinase activity do not result in biallelic inactivation of Lkb1 in polyps.
Taken together, our data indicate that LOH and epigenetic silencing of Lkb1 were not a feature of either of the major cellular components comprising the polyps. Moreover, the observation that Lkb1 heterozygosity leads to reduced Lkb1 expression and activity strongly argues that haploinsufficiency of Lkb1 underlies the polyposis arising in Lkb1+/− animals.
Characterization of Murine Lkb1+/−-Mediated Polyposis.
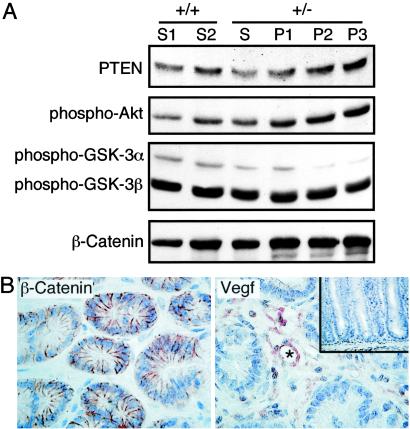
As the PTEN and Wnt-signaling pathways have been implicated in gastrointestinal tumorigenesis (25–28), we examined whether these pathways may contribute to polyp formation in our mouse model. To this end, polyp and control tissue lysates were immunoblotted for expression of several markers of these pathways including β-catenin, activated-GSK-3α/3β, PTEN, and activated-Akt. We found that although the expression levels of activated-GSK-3α/3β, PTEN, and activated-Akt were unchanged in the polyps compared to controls, a modest increase in β-catenin expression was noted in the polyps (Fig. 3A). However, examination of the subcellular localization of β-catenin indicated that it was strictly localized to the cell membrane of epithelial cells of all polyps examined (Fig. 3B). These results provide evidence that deregulation of PTEN and Wnt signaling is not characteristic of murine Lkb1+/− polyposis. We also examined the expression and cellular localization of several other molecules implicated in gastrointestinal tumorigenesis or in the regulation of Lkb1 including p53, p90RSK, and TgfβRII and found no evidence for deregulation of expression or localization (data not shown).
Fig 3.
Characterization of Lkb1+/− polyposis. (A) Western blot analysis of lysates from two independent wt (+/+) stomachs (S1 and S2), Lkb1 heterozygous (+/−) unaffected stomach (S), and three individual polyps (P1–P3) with Abs specific for PTEN, phospho-Akt (Ser-473), phospho-GSK-3α/3β (Ser-21/Ser-9), and β-catenin. (B) Immunostaining of β-catenin (Left) and VEGF (Right) of polyp from Lkb1+/− animals at ×400 magnification. (Inset) Adjacent unaffected gastric mucosa immunostained for VEGF at ×200 magnification. The blood vessel (Right) is marked by an asterisk (*).
We have reported that cultured MEFs derived from early Lkb1−/− embryos exhibited increased VEGF production compared to wt controls (13). We therefore immunostained murine polyps and unaffected gastric mucosa for VEGF expression and found that VEGF immunoreactivity was elevated in the polyps compared to unaffected gastric mucosa (Fig. 3B). The VEGF expression in the polyps was strictly restricted to the cells of the stroma while the epithelial component of the tumors was completely negative. Highest levels of VEGF expression localized to the endothelial cells surrounding the blood vessels (Fig. 3B, asterisk).
Induction of Cyclooxygenase-2 (COX-2) in Murine and PJS Polyps.
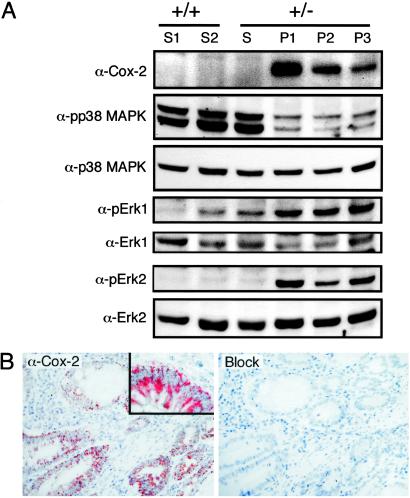
Induction of COX-2 expression has been linked to many aspects of tumorigenesis (29) and has been shown to be a characteristic feature of the gastrointestinal adenoma formation in murine models of familial adenomatous polyposis (30) where COX-2 promotes tumor development and contributes to tumor multiplicity (31). To determine whether COX-2 induction was a feature of the Lkb1+/− polyposis, whole tissue lysates prepared from polyps isolated from multiple animals, adjacent unaffected stomachs, and stomachs from Lkb1+/+ animals were immunoblotted to assay for expression of COX-2 (Fig. 4A). We observed that COX-2 was highly up-regulated in 75% (6/8) of the polyps examined indicating that COX-2 induction was a common feature of hamartoma formation in Lkb1+/− mice.
Fig 4.
COX-2 induction in murine Lkb1+/− and PJS polyposis. (A) Western blot analysis of lysates from two independent wt (+/+) stomachs (S1 and S2), Lkb1 heterozygous (+/−) unaffected stomach (S), and three individual polyps (P1–P3) with Abs specific for COX-2, activated p38 MAPK (pp38 MAPK), p38 MAPK (p38 MAPK), activated Erk1 kinase (pErk1), Erk1 kinase (Erk1), activated Erk2 kinase (pErk2), and Erk2 kinase (Erk2). (B) COX-2 immunostaining of a small intestinal polyp from patient PJ8 showing epithelial cytoplasmic localization (Left) with high magnification (Inset), and a serial section after Ab preadsorption with a COX-2 peptide (Right).
COX-2 induction is known to be regulated by several signal transduction pathways including those mediated by p38 MAPK, Akt/PKB, c-Jun N-terminal kinase (JNK), and Erk1/2 kinases (32). To examine which of these pathways might be involved in mediating COX-2 induction in the Lkb1+/− polyps, we assayed polyp and control lysates for expression of activated Akt, activated JNK kinase, activated Erk1/2 kinases, and activated p38 MAPK. Although no change in the activation of JNK kinases (data not shown) or Akt kinase (Fig. 3A) was noted in the polyps compared to the controls, a marked decrease in activated p38 MAPK was observed in the polyps (pp38 MAPK in Fig. 4A). In contrast, a marked increase in the levels of activated Erk1 and Erk2 kinases were detected in the polyp lysates (pErk1 and pErk2 in Fig. 4A). Taken together, these results suggest that induction of COX-2 in Lkb1+/− polyps is likely to be mediated by the Ras/Raf-1/MEK/Erk signal transduction pathway.
Although COX-2 expression has frequently been shown to be up-regulated in human gastrointestinal disease (33, 34), it has not been examined in PJS polyposis. We therefore wanted to determine whether the induction of COX-2 that we observed in Lkb1+/− murine polyps was also a characteristic feature of the polyposis associated with PJS patients. To this end, 23 polyps isolated from five PJS patients were assayed for COX-2 expression by immunostaining with a COX-2-specific Ab (35). Consistent with what had been observed in the Lkb1+/− mice, elevated COX-2 expression was observed in 70% (16/23) of the polyps examined (Table 1). In all cases, COX-2 expression was localized to the cytoplasm of the epithelial cells whereas the surrounding stroma was negative apart from restricted sporadic staining (Fig. 4B). These results indicate that up-regulation of COX-2 expression is a common feature of PJS polyposis.
Table 1.
COX-2 up-regulation in PJS polyposis
| Patient | LKB1 mutation | COX-2 immunoreactivity |
|---|---|---|
| SL8 | Δ188 bp, codons 307–370, stop 404 | 3/7 |
| P29 | Δnucleotide 914 (A), shift 305 stop 335 | 1/3 |
| P29Br | Δnucleotide 914 (A), shift 305 stop 335 | 1/1 |
| PJ8 | C913T, 305Gln→stop | 8/9 |
| P30 | Not identified | 3/3 |
COX-2 immunoreactivity is presented as the number of COX-2-positive polyps over the total number of polyps examined from a given patient. Polyps were scored positive if more than 10% of the polyp epithelia demonstrated significantly elevated COX-2 immunoreactivity compared to paired nonaffected mucosa.
For P30, no mutations in LKB1 were identified by sequencing exons 1–9 as described (7).
Discussion
To date, a role for LKB1 in tumor suppression has been largely based on linkage studies that have demonstrated the segregation of LKB1 mutations predicted to disable LKB1 activity, with PJS (7, 8). Indeed, many mutant alleles of LKB1 known to segregate with PJS, have been shown to encode for proteins that are deficient in kinase activity (11, 36, 37). Our observation that Lkb1+/− mice develop tumors that are histologically indistinguishable from the polyps that develop in human PJS confirms that mutation of LKB1 is the primary genetic lesion underlying hamartoma development in PJS patients and establishes Lkb1+/− mice as a model for PJS polyposis.
Establishing that a gene functions as a recessive tumor suppressor has classically relied on the demonstration that both alleles of the candidate gene are inactivated to promote tumorigenesis. This two-hit genetic criterion, originally proposed by Knudson (38), has proven accurate in affirming the tumor suppressor function of numerous genes. A second class of tumor suppressors however is emerging in which tumorigenesis does not fulfill the two-hit criterion. Instead, tumorigenesis mediated by genes such as p27 (39) and Dmp1 (40) appear to be mediated by loss of only a single allele. Our data indicates that murine Lkb1 also defies Knudson's paradigm for tumor suppression and is functionally haploinsufficient for the polyposis arising in Lkb1+/− mice. It should be noted that while this work was under review another group has confirmed our findings that biallelic inactivation of Lkb1 does not accompany polyp formation in Lkb1 heterozygous mice (41).
Studies on whether LKB1 undergoes biallelic inactivation in familial PJS polyps suggests that although LOH has been observed in some cases, it is not a consistent feature of these tumors (20–23). Interestingly, Entius et al. (23) have recently demonstrated that LOH of LKB1 in polyps was significantly higher in patients with associated carcinoma (86%) than in polyps from patients without carcinoma (29%). In light of our data from the Lkb1+/− mice, these data are consistent with the notion that biallelic inactivation of LKB1 is not required for polyp formation but rather that a second hit of LKB1 provides a further growth advantage or is involved in progression into malignancy, as has been suggested for tumor suppressors that exhibit haploinsufficiency (42).
The observation of elevated levels of VEGF in the stroma of heterozygote Lkb1+/− polyps was interesting as we have previously reported increased VEGF expression in Lkb1 null embryos and in Lkb1−/− MEF cultures (13). On the other hand, heterozygosity of Lkb1 clearly is not sufficient to deregulate VEGF, as no increased VEGF expression was noted in the adjacent unaffected tissues of the mice or in Lkb1+/− MEFs (data not shown). Thus the increased VEGF in the tumors may largely reflect the tumorigenic growth of these large polyps which require a great deal of neovascularization to satisfy metabolic needs (43). Alternatively, as stromal/epithelial collaboration is known to play a central role in VEGF induction in murine models of tumorigensis (44), it is possible that collaborative signals emanating within the epithelial/stromal milieu of the Lkb1+/− hamartomas might subvert the need for complete loss of Lkb1 activity to promote VEGF induction in the polyps.
Elevated COX-2 levels have been reported in ≈80% of adenocarcinomas and 50% of colorectal adenomas and gastric dysplasias when compared to paired nonaffected mucosa (33, 35). Mounting evidence from various in vitro and animal model systems indicates that COX-2 induction contributes to tumorigenesis by at least five mechanisms including (i) inhibition of apoptosis, (ii) increased angiogenesis, (iii) increased invasiveness, (iv) modulation of inflammation/immuno-suppression, and (v) conversion of procarcinogens to carcinogens (29). Our results demonstrating that COX-2 is up-regulated in a significant percentage of human PJS polyps suggest that COX-2 may be involved in promoting the polyposis associated with PJS.
The observation that most, but not all, polyps in the Lkb1+/− mice and PJS patients exhibit COX-2 induction coupled to the observation that Lkb1+/− MEFs do not show COX-2 deregulation (data not shown) indicates that the regulatory mechanisms eliciting COX-2 induction in the polyps are likely to be complex. The complexity of COX-2 regulation has been underscored in studies of familial adenomatous polyposis coli patients and corresponding mouse models where the mechanisms by which adenomatous polyposis coli mutations elicit COX-2 induction are still largely unknown (45). Indeed COX-2 is induced by a wide spectrum of growth factors and pro-inflammatory cytokines through several signal transduction pathways including Rac1/cdc42/MKK/p38MAPK, Ras/MEKK/SEK/JNK, PI-3K/Akt, and Ras/Raf-1/MEK/Erk (32). Analysis of the mediators of these pathways in murine Lkb1+/− polyposis revealed that only Erk1/2 was activated, thus suggesting that the Ras/Raf-1/MEK/ERK signal transduction pathway is likely to mediate COX-2 induction in murine Lkb1+/− polyposis. The significance of the observed down-regulation of p38 MAPK in the murine polyps is as yet still unclear. The identification of physiological Lkb1 substrates will surely facilitate a more comprehensive understanding on how Lkb1 activity impinges on these signaling pathways to mediate its role both in polyp formation and COX-2 induction.
Inhibition of cyclooxygenase enzymes with nonsteroidal anti-inflammatory drugs has proven effective in repressing gastrointestinal tumorigenesis both in the general population (46) and in patients with familial adenomatous polyposis coli (47). In particular, COX-2 has emerged as the central target of these therapies as shown by clinical trials by using selective COX-2 inhibitors (48). The finding that COX-2 expression is up-regulated in a significant percentage of PJS polyps identify COX-2 as a potential target for chemopreventive therapy and suggest that COX-2 inhibitors might provide a therapeutic approach in reducing the tumor burden and need for surgery in PJS patients.
Acknowledgments
We thank B. Tjäder and S. Räsänen for technical assistance and the Finnish Medical Foundation, Finnish Cancer Organization, Sigrid Juselius Foundation, and Academy of Finland for financial support. D.J.R. and A.Y. are students of the Helsinki Biomedical Graduate School. N.K. is a student of the Helsinki Graduate School in Biotechnology and Molecular Biology.
Abbreviations
COX-2, cyclooxygenase-2
PJS, Peutz–Jeghers syndrome
MAPK, mitogen-activated protein kinase
LOH, loss of heterozygosity
VEGF, vascular endothelial growth factor
wt, wild type
MEF, mouse embryonic fibroblast
JNK, c-Jun N-terminal kinase
Erk, extracellular signal-regulated kinase
PTEN, phosphatase and tensin homolog deleted on chromosome ten
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Peutz J. L. A. (1921) Ned. Maandsch. Geneeskd. 10, 134-146. [Google Scholar]
- 2.Jeghers H., McKusick, V. A. & Katz, K. H. (1949) N. Engl. J. Med. 241, 1031-1036. [DOI] [PubMed] [Google Scholar]
- 3.Vasen H. F. (2000) J. Clin. Oncol. 18, 81S-92S. [PubMed] [Google Scholar]
- 4.Giardiello F. M., Welsh, S. B., Hamilton, S. R., Offerhaus, G. J., Gittelsohn, A. M., Booker, S. V., Krush, A. J., Yardley, J. H. & Luk, G. D. (1987) N. Engl. J. Med. 316, 1511-1514. [DOI] [PubMed] [Google Scholar]
- 5.Boardman L. A., Thibodeau, S. N., Schaid, D. J., Lindor, N. M., McDonnell, S. K., Burgart, L. J., Ahlquist, D. A., Podratz, K. C., Pittelkow, M. & Hartmann, L. C. (1998) Ann. Intern. Med. 128, 896-899. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello F. M., Brensinger, J. D., Tersmette, A. C., Goodman, S. N., Petersen, G. M., Booker, S. V., Cruz-Correa, M. & Offerhaus, J. A. (2000) Gastroenterology 119, 1447-1453. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki A., Markie, D., Tomlinson, I., Avizienyte, E., Roth, S., Loukola, A., Bignell, G., Warren, W., Aminoff, M., Hoglund, P., et al. (1998) Nature (London) 391, 184-187. [DOI] [PubMed] [Google Scholar]
- 8.Jenne D. E., Reimann, H., Nezu, J., Friedel, W., Loff, S., Jeschke, R., Muller, O., Back, W. & Zimmer, M. (1998) Nat. Genet. 18, 38-43. [DOI] [PubMed] [Google Scholar]
- 9.Olschwang S., Boisson, C. & Thomas, G. (2001) J. Med. Genet. 38, 356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiainen M., Ylikorkala, A. & Mäkelä, T. P. (1999) Proc. Natl. Acad. Sci. USA 96, 9248-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ylikorkala A., Avizienyte, E., Tomlinson, I. P. M., Tiainen, M., Roth, S., Loukola, A., Hemminki, A., Johansson, M., Sistonen, P., Markie, D., et al. (1999) Hum. Mol. Genet. 8, 45-51. [DOI] [PubMed] [Google Scholar]
- 12.Luukko K., Ylikorkala, A., Tiainen, M. & Mäkelä, T. P. (1999) Mech. Dev. 83, 187-190. [DOI] [PubMed] [Google Scholar]
- 13.Ylikorkala A., Rossi, D. J., Korsisaari, N., Luukko, K., Alitalo, K., Henkemeyer, M. & Mäkelä, T. P. (2001) Science 293, 1323-1326. [DOI] [PubMed] [Google Scholar]
- 14.Karuman P., Gozani, O., Odze, R. D., Zhou, X. C., Zhu, H., Shaw, R., Brien, T. P., Bozzuto, C. D., Ooi, D., Cantley, L. C. & Yuan, J. (2001) Mol. Cell 7, 1307-1319. [DOI] [PubMed] [Google Scholar]
- 15.Marignani P. A., Kanai, F. & Carpenter, C. L. (2001) J. Biol. Chem. 276, 32415-32418. [DOI] [PubMed] [Google Scholar]
- 16.Smith D. P., Rayter, S. I., Niederlander, C., Spicer, J., Jones, C. M. & Ashworth, A. (2001) Hum. Mol. Genet. 10, 2869-2877. [DOI] [PubMed] [Google Scholar]
- 17.Sapkota G. P., Kieloch, A., Lizcano, J. M., Lain, S., Arthur, J. S., Williams, M. R., Morrice, N., Deak, M. & Alessi, D. R. (2001) J. Biol. Chem. 276, 19469-19482. [DOI] [PubMed] [Google Scholar]
- 18.Utsunomiya J., Gocho, H., Miyanaga, T., Hamaguchi, E. & Kashimure, A. (1975) Johns Hopkins Med. J. 136, 71-82. [PubMed] [Google Scholar]
- 19.Hemminki A., Tomlinson, I., Markie, D., Järvinen, H., Sistonen, P., Björkqvist, A. M., Knuutila, S., Salovaara, R., Bodmer, W., Shibata, D., et al. (1997) Nat. Genet. 15, 87-90. [DOI] [PubMed] [Google Scholar]
- 20.Gruber S. B., Entius, M. M., Petersen, G. M., Laken, S. J., Longo, P. A., Boyer, R., Levin, A. M., Mujumdar, U. J., Trent, J. M., Kinzler, K. W., et al. (1998) Cancer Res. 58, 5267-5270. [PubMed] [Google Scholar]
- 21.Miyaki M., Iijima, T., Hosono, K., Ishii, R., Yasuno, M., Mori, T., Toi, M., Hishima, T., Shitara, N., Tamura, K., et al. (2000) Cancer Res. 60, 6311-6313. [PubMed] [Google Scholar]
- 22.Wang Z. J., Ellis, I., Zauber, P., Iwama, T., Marchese, C., Talbot, I., Xue, W. H., Yan, Z. Y. & Tomlinson, I. (1999) J. Pathol. 188, 9-13. [DOI] [PubMed] [Google Scholar]
- 23.Entius M. M., Keller, J. J., Westerman, A. M., van Rees, B. P., van Velthuysen, M. L., de Goeij, A. F., Wilson, J. H., Giardiello, F. M. & Offerhaus, G. J. (2001) J. Clin. Pathol. 54, 126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteller M., Avizienyte, E., Corn, P. G., Lothe, R. A., Baylin, S. B., Aaltonen, L. A. & Herman, J. G. (2000) Oncogene 19, 164-168. [DOI] [PubMed] [Google Scholar]
- 25.Bienz M. & Clevers, H. (2000) Cell 103, 311-320. [DOI] [PubMed] [Google Scholar]
- 26.Liaw D., Marsh, D. J., Li, J., Dahia, P. L., Wang, S. I., Zheng, Z., Bose, S., Call, K. M., Tsou, H. C., Peacocke, M., et al. (1997) Nat. Genet. 16, 64-67. [DOI] [PubMed] [Google Scholar]
- 27.Marsh D. J., Dahia, P. L., Zheng, Z., Liaw, D., Parsons, R., Gorlin, R. J. & Eng, C. (1997) Nat. Genet. 16, 333-334. [DOI] [PubMed] [Google Scholar]
- 28.Olschwang S., Serova-Sinilnikova, O. M., Lenoir, G. M. & Thomas, G. (1998) Nat. Genet. 18, 12-14. [DOI] [PubMed] [Google Scholar]
- 29.Dempke W., Rie, C., Grothey, A. & Schmoll, H. J. (2001) J. Cancer Res. Clin. Oncol. 127, 411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams C. S., Luongo, C., Radhika, A., Zhang, T., Lamps, L. W., Nanney, L. B., Beauchamp, R. D. & DuBois, R. N. (1996) Gastroenterology 111, 1134-1140. [DOI] [PubMed] [Google Scholar]
- 31.Oshima M., Dinchuk, J. E., Kargman, S. L., Oshima, H., Hancock, B., Kwong, E., Trzaskos, J. M., Evans, J. F. & Taketo, M. M. (1996) Cell 87, 803-809. [DOI] [PubMed] [Google Scholar]
- 32.Stack E. & DuBois, R. N. (2001) Best Pract. Res. Clin. Gastroenterol. 15, 787-800. [DOI] [PubMed] [Google Scholar]
- 33.Eberhart C. E., Coffey, R. J., Radhika, A., Giardiello, F. M., Ferrenbach, S. & DuBois, R. N. (1994) Gastroenterology 107, 1183-1188. [DOI] [PubMed] [Google Scholar]
- 34.van Rees B. P. & Ristimäki, A. (2001) Scand. J. Gastroenterol. 36, 897-903. [PubMed] [Google Scholar]
- 35.Saukkonen K., Nieminen, O., van Rees, B., Vilkki, S., Häarkönen, M., Juhola, M., Mecklin, J. P., Sipponen, P. & Ristimäki, A. (2001) Clin. Cancer Res. 7, 1923-1931. [PubMed] [Google Scholar]
- 36.Mehenni H., Gehrig, C., Nezu, J., Oku, A., Shimane, M., Rossier, C., Guex, N., Blouin, J. L., Scott, H. S. & Antonarakis, S. E. (1998) Am. J. Hum. Genet. 63, 1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nezu J., Oku, A. & Shimane, M. (1999) Biochem. Biophys. Res. Commun. 261, 750-755. [DOI] [PubMed] [Google Scholar]
- 38.Knudson A. G. (1971) Proc. Natl. Acad. Sci. USA 68, 820-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fero M. L., Randel, E., Gurley, K. E., Roberts, J. M. & Kemp, C. J. (1998) Nature (London) 396, 177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue K., Zindy, F., Randle, D. H., Rehg, J. E. & Sherr, C. J. (2001) Genes Dev. 15, 2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi H., Nakau, M., Ishikawa To, T. O., Seldin, M. F., Oshima, M. & Taketo, M. M. (2002) Cancer Res. 62, 2261-2266. [PubMed] [Google Scholar]
- 42.Quon K. C. & Berns, A. (2001) Genes Dev. 15, 2917-2921. [DOI] [PubMed] [Google Scholar]
- 43.Folkman J. (1995) Nat. Med. 1, 27-31. [DOI] [PubMed] [Google Scholar]
- 44.Fukumura D., Xavier, R., Sugiura, T., Chen, Y., Park, E. C., Lu, N., Selig, M., Nielsen, G., Taksir, T., Jain, R. K. & Seed, B. (1998) Cell 94, 715-725. [DOI] [PubMed] [Google Scholar]
- 45.Gupta R. A. & DuBois, R. N. (2001) Nat. Rev. Cancer 1, 11-21. [DOI] [PubMed] [Google Scholar]
- 46.Thun M. J., Namboodiri, M. M. & Heath, C. W., Jr. (1991) N. Engl. J. Med. 325, 1593-1596. [DOI] [PubMed] [Google Scholar]
- 47.Giardiello F. M., Hamilton, S. R., Krush, A. J., Piantadosi, S., Hylind, L. M., Celano, P., Booker, S. V., Robinson, C. R. & Offerhaus, G. J. (1993) N. Engl. J. Med. 328, 1313-1316. [DOI] [PubMed] [Google Scholar]
- 48.Steinbach G., Lynch, P. M., Phillips, R. K., Wallace, M. H., Hawk, E., Gordon, G. B., Wakabayashi, N., Saunders, B., Shen, Y., Fujimura, T., et al. (2000) N. Engl. J. Med. 342, 1946-1952. [DOI] [PubMed] [Google Scholar]