Abstract
Sepsis, a potentially fatal clinical syndrome, is mediated by an early (e.g., tumor necrosis factor and IL-1) and late [e.g., high mobility group B-1 (HMGB1)] proinflammatory cytokine response to infection. Specifically targeting early mediators has not been effective clinically, in part because peak mediator activity often has passed before therapy can be initiated. Late-acting downstream effectors, such as HMGB1, that mediate sepsis lethality may be more relevant therapeutic targets. Ethyl pyruvate (EP) recently was identified as an experimental therapeutic that significantly protects against lethal hemorrhagic shock. Here, we report that EP attenuates lethal systemic inflammation caused by either endotoxemia or sepsis even if treatment begins after the early tumor necrosis factor response. Treatment with EP initiated 24 h after cecal puncture significantly increased survival (vehicle survival = 30% vs. EP survival = 88%, P < 0.005). EP treatment significantly reduced circulating levels of HMGB1 in animals with established endotoxemia or sepsis. In macrophage cultures, EP specifically inhibited activation of p38 mitogen-activated protein kinase and NF-κB, two signaling pathways that are critical for cytokine release. This report describes a new strategy to pharmacologically inhibit HMGB1 release with a small molecule that is effective at clinically achievable concentrations. EP now warrants further evaluation as an experimental “rescue” therapeutic for sepsis and other potentially fatal systemic inflammatory disorders.
Keywords: tumor necrosis factor, NF-κB, lipopolysaccharide
Sepsis, a lethal syndrome that develops in response to infection, occurs in 750,000 patients per year in the United States and is fatal in 20–40% of cases (1, 2). The pathological sequelae of sepsis are mediated by proinflammatory cytokines [e.g., tumor necrosis factor (TNF), IL-1, and high mobility group B-1 (HMGB1)] that are released from macrophages, neutrophils, and other cells of the innate immune system. The magnitude and duration of the systemic inflammatory response influence the development of tissue damage, hypotension, multiple organ failure, and death (3, 4). Significant advances have been made in understanding the role of proinflammatory mediators in the pathogenesis of sepsis, but effective therapies that target inflammatory mediators have not been clinically approved. A major difficulty in developing therapeutics that target cytokines (e.g., TNF and IL-1β) is that they are released early in the development of a systemic inflammatory response (3). This leaves a narrow therapeutic window for administration of antagonists, and inhibitors of TNF and IL-1β are not effective when delivered after the acute cytokine response has occurred (3). Unfortunately, in the typical clinical case, hours pass before sepsis is diagnosed and specific treatment is implemented. Thus, it is perhaps not surprising that agents directed against early proinflammatory cytokines are ineffective in large clinical trials (5, 6).
Recently, HMGB1 was implicated as a “late” mediator of lethal systemic inflammation in animal models of cytokine-mediated disease initiated by the Gram-negative bacterial product endotoxin [lipopolysaccharide (LPS)] (4). Known classically as an intracellular DNA-binding protein, HMGB1 is released by endotoxin-stimulated macrophages, but only after a delay of 12–18 h; a similar delay in HMGB1 appearance is observed in the serum of mice during endotoxemia (4). Anti-HMGB1 antibodies confer significant protection against delayed endotoxin lethality, even when antibody dosing is initiated at a time after the acute-phase cytokine responses have peaked and resolved (4). Cytokine activities of HMGB1 include stimulating the release of TNF, IL-1, and other inflammatory products from macrophages and pituicytes, inducing chemotaxis of smooth muscle cells, and mediating acute lung injury and lethality (7–9). These delayed kinetics, and the protective effects of anti-HMGB1 antibodies in vivo, indicate that HMGB1 is a late-acting mediator of lethal systemic inflammation that may provide a broader therapeutic window for treating sepsis and other systemic inflammatory disorders. To date, there has not been a report of an experimental therapeutic agent that inhibits HMGB1 release to modulate systemic inflammation.
Ethyl pyruvate (EP), a stable lipophilic pyruvate derivative identified recently by Fink and colleagues, is an experimental therapeutic that effectively protects animals from ischemia/reperfusion-induced tissue injury (10, 11). EP administration significantly improved survival in standard models of lethal hemorrhagic shock (12, 13). We reasoned that EP also might be protective in sepsis, because the pathogenesis of ischemia–reperfusion and hemorrhagic shock, like sepsis, depends on activation of early and late cytokine responses. Here, we show that EP rescued animals from lethal sepsis caused by peritonitis, even when dosing began 24 h after cecal puncture. EP inhibited the release of TNF and HMGB1 from endotoxin-stimulated RAW 264.7 murine macrophages and attenuated activation of both the p38 mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways. EP treatment of septic mice decreased circulating levels of HMGB1, indicating that delayed administration of EP protects against lethal sepsis.
Methods
Animal Experiments.
Male 6- to 8-week-old BALB/c mice (20–25 g) were purchased from Harlan–Sprague–Dawley and allowed to acclimate for 7 days housed at 25°C on a 12-h light/12-h dark cycle. Animals were grouped randomly and assigned to a specific experiment. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of North Shore University Hospital.
Endotoxin Shock.
Mice were injected with endotoxin (Escherichia coli LPS 0111:B4; Sigma) that was dissolved in sterile, pyrogen-free saline at stock concentrations of 5 mg/ml. LPS solutions were sonicated for 20 min immediately before use for each experiment. Mice received an LD75 dose of LPS (5 mg/kg, i.p.). Blood was collected at different times after LPS administration, allowed to clot for 2 h at room temperature, and then centrifuged for 20 min at 1,500 × g. Serum samples were stored at 20°C before analysis. Mortality was recorded for up to 3 weeks after injection to ensure that no additional late deaths occurred.
Cecal Ligation and Puncture.
Cecal ligation and puncture was performed as described by Wichmann et al. (14). Briefly, mice were anesthetized with ketamine (100 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.), a midline incision was performed, and the cecum was isolated. A 6-0 prolene suture ligature was placed at a level 5.0 mm from the cecal tip away from the ileocecal valve. The ligated cecal stump then was punctured once with a 22-gauge needle, and stool was extruded (1 mm) to ascertain patency of the puncture site. The cecum then was placed back into its normal intraabdominal position, and the abdomen was closed with a running suture of 6-0 prolene in two layers, peritoneum and fascia separately, to prevent leakage of fluid. All animals received an antibiotic (primaxin; 0.5 mg/kg s.c.) 12 h after surgery as a single dose. All animals received resuscitation with normal saline 24 h after surgery as a single injection (20 ml/kg of body weight). Mortality was recorded for up to 1 week after the procedure; survivors were followed for 3 weeks to ensure no late mortalities had occurred.
EP Solution.
EP was prepared in solution with sodium (130 mM), potassium (4 mM), calcium (2.7 mM), chloride (139 mM), and EP (28 mM) (pH 7.0). For injections in mice, solutions were diluted so that each injection volume was 0.4 ml per dose.
Cell Culture.
BALB/c murine macrophage-like RAW 264.7 cells, obtained from the American Type Culture Collection (ATCC TIB-71), were cultured in RPMI medium 1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated FBS (Gemini Biological Products, Calabasas, CA), 2 mM glutamine (25030-149; GIBCO/BRL), and antibiotic–antimycotic mix (15240-062; GIBCO/BRL) in a humidified incubator with 5% CO2 and 95% air. Cells were removed mechanically and resuspended in serum-free Opti-MEM I medium (Life Technologies) to perform experiments at 75% confluence.
Cytokine Measurements.
TNF concentration in mouse serum and in conditioned medium from RAW 264.7 cell cultures was measured by ELISA (minimum detectable concentration = 10 pg/ml). Recombinant mouse TNF standards were obtained from R & D Systems and dissolved in 0.1% BSA solution (low endotoxin grade from Sigma). mAb to mouse TNF was purchased from BioSource International (Camarillo, CA). Human TNF mAb, human TNF antiserum, and mouse TNF antiserum were prepared and contributed by Christine Metz (North Shore–LIJ Research Institute). Mouse serum IL-6 and IL-1β levels were measured by using ELISA kits (R & D Systems). HMGB1 was analyzed by Western blot as described by Wang et al. (4). Briefly, serum or cell culture conditioned medium first was filtered through Centricon YM-100 (Millipore) to clear the samples from cell debris and macromolecular complexes formed during clotting. Samples then were concentrated 15-fold with Centricon YM-30 and separated on 12% SDS-polyacrylamide gels. Protein was transferred to Immun-blot poly(vinylidene difluoride) membrane (Bio-Rad) and HMGB1 was analyzed by using polyclonal anti-HMGB1 antibodies and secondary anti-rabbit horseradish peroxidase (Amersham Pharmacia). Standard curves were constructed by using r-HMGB1, and the intensity of the 30-kDa band was analyzed by densitometry.
Nuclear Extract Preparation.
Cells were plated at a density of 1 × 106 per well in 6-well tissue culture plates and allowed to adhere for 24 h. After stimulation at indicated times, cells were removed from the incubator and placed on ice immediately. Cells were washed once with 2 ml PBS and then harvested in 1 ml of PBS containing 2% FBS by using a rubber scraper. The cells were transferred to a 1.5-ml tube and centrifuged at 14,000 × g for 10 s; the pellet was resuspended in 600 μl of buffer I [10 mM Tris⋅HCl, pH 7.8/10 mM KCl/1.5 mM MgCl2/0.3 M sucrose/500 μM PMSF/1.0 mM sodium orthovanadate/1 mM DTT/protease inhibitor mixture (p-8340; Sigma)] and incubated for 15 min. After 38.3 μl of 10% Nonidet P-40 was added, the tube was vortexed at full speed for 10 s. Nuclei were isolated by centrifugation at 300 × g for 3 min. The supernatant was aspirated, and the nuclear pellet was resuspended gently in 80 μl of buffer II (10 mM Tris⋅HCl, pH 7.8/420 mM KCl/1.5 mM MgCl2/20% glycerol). After a 15-min incubation period, nuclear extracts were cleared by centrifugation at 14,000 × g for 10 min. The supernatant was transferred to a new tube, and protein concentration was determined by using a commercially available Bradford assay (Bio-Rad Protein Assay). Nuclear extracts were frozen at −80°C.
Electrophoretic Mobility-Shift Assays.
The sequence of the double-stranded NF-κB oligonucleotide was as follows: sense, 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; antisense, 3′-TCA ACT CCC CTG AAA GGG TCC G-5′ (E3291; Promega). The oligonucleotides were end-labeled with adenosine [γ-32P]triphosphate (New England Nuclear) by using T4 polynucleotide kinase (Promega). Nuclear protein/reaction (3 μg) was incubated with γ-32P-labeled NF-κB probe in bandshift buffer (13 mM Hepes, pH 8.0/65 mM NaCl/1 mM DTT/0.14 mM EDTA/8% glycerol) in the presence of 1 ng of calf thymus DNA for 20 min at room temperature. For competition reactions, 100-fold molar excess of cold oligonucleotide was added simultaneously with labeled probe. Supershift assays were performed by incubating nuclear extracts with 2 μl of anti-p65 and anti-p50 (Santa Cruz Biotechnology) for 1 h before the addition of radiolabeled probe. The binding reaction mixture was electrophoresed on 4% nondenaturing PAGE gels. After PAGE, the gels were dried and exposed to XAR-5 film (Kodak) at −80°C overnight by using an intensifying screen.
Total RNA Extraction and RNase Protection Assay.
Total RNA was extracted from cultured cells by using RNAzol B in accordance with the manufacturer's instructions (Tel-Test, Friendswood, TX). The integrity of the RNA was verified by electrophoresis on 1.2% agarose/17% formaldehyde gels. The levels of TNF and cyclophilin mRNA in RAW cells were measured by using an RNase protection assay kit from PharMingen in accordance with the manufacturer's instructions. The antisense RNA probe was labeled with [α-32P]UTP (800 Ci/mmol; Amersham Pharmacia; 1 Ci = 37 GBq) by using T7 RNA polymerase. The protected transcript of TNF is 287 bp, and the control transcript (cyclophilin) is 105 bp. Molecular weight markers were prepared by using pBR-322 plasmid DNA digested with MSP I (New England Biolabs) and Klenow end-labeled (Stratagene) with [α-32P]dCTP (800 Ci/mmol; Amersham Pharmacia); mRNA levels were measured with an InstantImager (Packard).
Statistical Analysis.
Data in the figures and text are expressed as mean ± SEM; n > 20 for all animal experiments. Significance of differences between groups was determined by two-tailed Student's t test or Fisher's LSD test or the χ2 test, as indicated. Statistical analyses were performed with the assistance of the Biostatistics Unit of the North Shore–LIJ Health System.
Results
Pretreatment with EP Prevents Endotoxin Lethality and Inhibits Release of TNF and HMGB1.
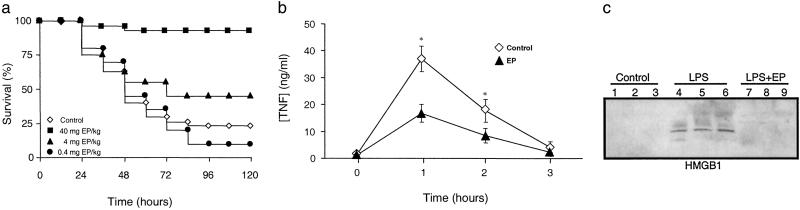
We conducted an initial evaluation of EP as a therapeutic agent for systemic inflammation in a standard model of murine endotoxemia. BALB/c mice received a single dose of EP (40 mg/kg, i.p., 4 mg/kg, i.p., or 0.4 mg/kg, i.p.), followed 30 min later by an LD75 injection of LPS (5 mg/kg LPS, i.p.). Pretreatment with a single dose of EP (40 mg/kg, i.p.) conferred significant protection from lethal endotoxemia (survival in EP-treated mice = 27/30; survival in vehicle-treated mice = 5/20; P < 0.005) (Fig. 1a). Pretreatment with this dose of EP prevented the development of clinical manifestations of endotoxin morbidity, including decreased activity, lethargy, diarrhea, piloerection, and huddling. Late deaths in EP-treated animals were not observed during the 3 weeks after endotoxin injection, indicating that EP treatment conferred a complete and lasting protection against lethal endotoxemia and did not merely delay the onset of lethal pathology. A lower dose of EP (4 mg/kg, i.p.) provided partial protection (9/20). Pretreatment of endotoxemic mice with EP (40 mg/kg, i.p.) significantly attenuated the serum levels of both TNF (Fig. 1b) and HMGB1 (Fig. 1c). Pretreatment with EP significantly decreased peak serum levels of immunoreactive IL-1β and IL-6 (data not shown), indicating that it prevented endotoxin-induced lethality by attenuating the release of early (TNF and IL-1β) and late (HMGB1) systemic mediators of lethality.
Fig 1.
EP pretreatment prevents endotoxin lethality by attenuating TNF and HMGB1 release in vivo. (a) Mice (n = 20–30 per group) were injected with a single dose of EP as indicated, followed 30 min later by a lethal infusion of endotoxin (5 mg LPS/kg, i.p.). EP conferred significant protection against lethality (P < 0.05), as measured by Fisher's exact test. (b) In a parallel group of EP-treated animals, circulating TNF levels were measured by ELISA of sera prepared at the time indicated after LPS injection (5 mg LPS/kg, i.p.). EP pretreatment (40 mg/kg, i.p.) significantly attenuated the release of serum TNF in response to LPS (* = P < 0.05, by Student's t test). (c) Circulating levels of HMGB1 20 h after LPS infusion (5 mg LPS/kg, i.p.) were measured in a parallel experimental group of endotoxemic animals by Western blot analysis. Pretreatment with EP (40 mg/kg, i.p.) attenuated serum HMGB1 levels at 20 h after LPS (5 mg LPS/kg, i.p.).
Delayed Administration of EP Prevents the Lethality of Endotoxemia and Inhibits HMGB1 Release.
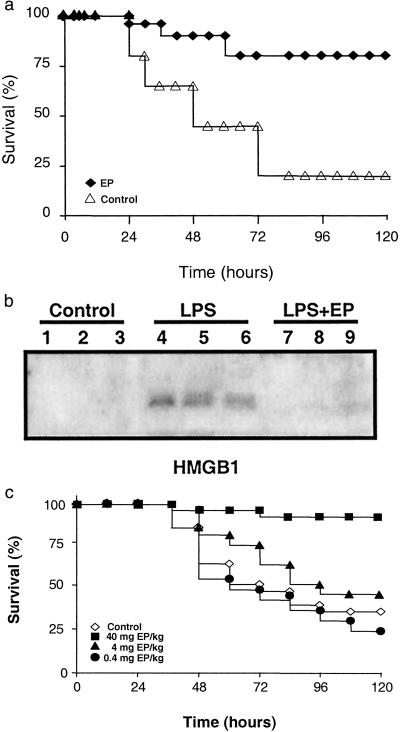
We next assessed the therapeutic efficacy of EP when first administered after the onset of endotoxemia. Treatment with EP was initiated 4 h after the onset of endotoxemia, a time at which clinical signs of LPS-induced toxicity, including diarrhea, piloerection, and depressed spontaneous activity already were evident. Notably, the first dose of EP was administered well after the early peak in serum TNF, which occurs within the first 1–2 h after the onset of endotoxemia (15). Delayed treatment with EP significantly protected mice from lethal systemic inflammation as compared with treatment with vehicle (survival with EP treatment = 24/30; survival with vehicle alone = 7/30; P < 0.005) (Fig. 2a). Because treatment with EP began after the early, acute-phase response to endotoxin (Fig. 1b), the significant protection conveyed by EP suggested that it might target late-acting mediators of lethal systemic inflammation. HMGB1 is a late mediator of endotoxin lethality (4), and treatment of endotoxemic mice with EP beginning 4 h after LPS injection significantly attenuated the systemic release of HMGB1 measured at 20 h after the onset of endotoxemia (Fig. 2b). EP treatment reversed clinical signs of morbidity, and no late deaths occurred during the subsequent 3-week period of observation, indicating that delayed treatment with the drug confers significant protection and did not merely delay the onset of death.
Fig 2.
Delayed administration of EP attenuates the lethality of endotoxemia and sepsis HMGB1. (a) Mice (n = 30 per group) received a lethal infusion of endotoxin (5 mg LPS/kg, i.p.) and were treated with EP (40 mg/kg, i.p.) 4, 8, 12, 24, and 30 h later. EP conferred significant protection against lethality (P < 0.05), as measured by Fisher's exact test. (b) In a parallel set of endotoxemic mice (n = 30 per group), HMGB1 release was analyzed by Western blot of serum collected at 20 h. LPS (5 mg/kg LPS, i.p.) induced the release of HMGB1 in vivo (lanes 4–6), and delayed treatment with EP (40 mg/kg, i.p.; lanes 7–9) inhibited the release of serum HMGB1. (c) The cecal ligation and puncture technique was used to induce intraabdominal sepsis in mice (n = 20–30 per group). Repeated administration of EP (at the doses indicated) at 24, 30, 48, and 54 h after cecal ligation and puncture significantly increased survival, as compared with vehicle-treated animals (P < 0.05), as measured by Fisher's exact test.
Delayed Administration of EP Prevents the Lethality of Sepsis.
We next tested the efficacy of EP as treatment for lethal sepsis in a standardized model of peritonitis induced by surgical perforation of the cecum (14). Mice subjected to peritonitis received either vehicle or EP beginning 24 h after the onset of peritonitis, a time at which 10% of the mice in each group already had died (Fig. 2c). Survival in vehicle-treated controls for 3 weeks was 30%, whereas treatment with EP significantly increased survival to 88% (vehicle control survival = 9/25; EP-treated survival = 22/25; P < 0.005). The protection against lethal sepsis by EP was dose-dependent, because lower doses failed to confer significant protection against death (Fig. 2c). Western blot analyses of serum prepared from blood obtained 30 h after cecal puncture revealed significantly reduced circulating HMGB1 levels (vehicle control = 167 ± 13 ng/ml; EP = 88 ± 20 ng/ml; P < 0.005).
EP Inhibits the Synthesis of TNF in Macrophage Cultures.
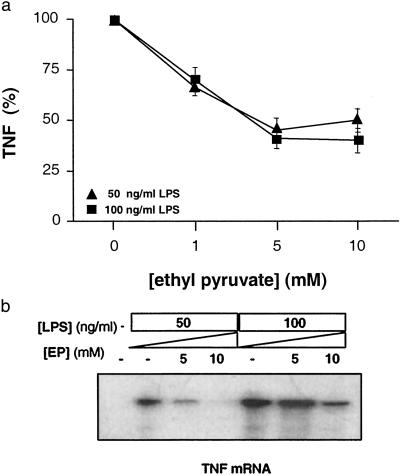
To determine the effect of EP on TNF synthesis by macrophages, murine macrophage-like RAW 264.7 cells were stimulated with endotoxin for 20 h and TNF was measured in the conditioned medium. EP significantly inhibited TNF release in macrophage cultures in concentrations (EC50 = 3.4 mM) that are clinically achievable (Fig. 3a). EP significantly reduced intracellular levels of TNF mRNA (Fig. 3b), indicating that the mechanism of EP action is to suppress TNF expression at the transcriptional level.
Fig 3.
EP prevents TNF transcription and synthesis. (a) To analyze the mechanism of EP on LPS-induced TNF release, RAW264.7 macrophage cultures were stimulated with 50 or 100 ng/ml LPS for 20 h, with or without the addition of EP in the concentrations indicated. Note that the concentration of EP that inhibited 50% of the TNF response (EP EC50) from macrophage cultures was 3.4 mM, a clinically achievable concentration. The inhibitory effect of EP on TNF release was significant at all concentrations tested (P < 0.05), as determined by Student's t test. (b) EP prevents TNF transcription, as analyzed by ribonuclease protection assay (RPA III; Ambion, Austin, TX). EP prevented LPS-induced transcription of TNF in a concentration-dependent fashion.
EP Inhibits Signal Transduction via NF-κB and p38 MAPK.
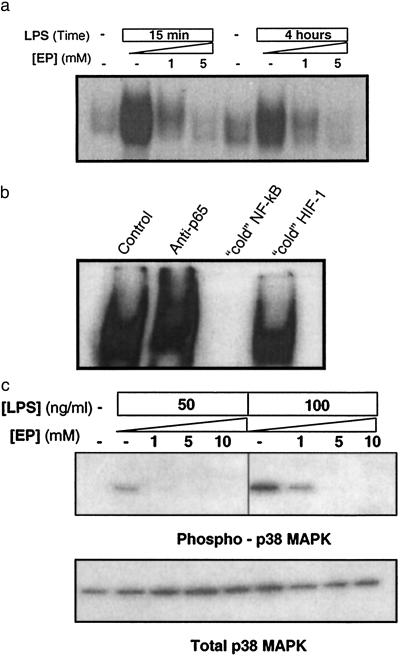
EP-mediated inhibition of TNF mRNA expression suggested that EP might modulate intracellular signaling events that coordinate the activity of proinflammatory cytokines. Signal transduction through the NF-κB and p38 MAPK pathways is a critical step in macrophage activation that leads to increased expression of TNF and other proinflammatory cytokines (16–22). To determine the effect of EP on NF-κB-responsive genes, nuclear extracts from LPS-stimulated RAW 264.7 cells were incubated with a specific 32P-labeled DNA probe containing an NF-κB-binding site and analyzed by electrophoretic mobility-shift assay. In agreement with previous studies (22–24), electrophoretic mobility-shift assay revealed increased NF-κB DNA binding at 15 min and 4 h after endotoxin exposure (Fig. 4a). LPS-induced activation of NF-κB signaling was inhibited significantly by EP for at least 4 h after LPS exposure, indicating that inhibition was not merely a result of EP-induced delay in activation of this pathway (Fig. 4a). Supershift assays using specific antibodies directed against the NF-κB subunits p50 or p65 were performed to confirm the identity of the protein–DNA complex. A supershifted band was detected only in the presence of the p65 antibody; specificity of the complex also was confirmed by incubating the nuclear extracts and labeled DNA probe with 100-fold excess unlabeled duplex oligonucleotide, which completely inhibited detection of the complex (Fig. 4b). Incubation of the nuclear extract/radiolabeled NF-κB probe with an irrelevant oligonucleotide, which contained a binding site for the transcription factor HIF-1 (25), also had no effect on complex formation (Fig. 4b).
Fig 4.
EP prevents LPS-induced activation of NF-κB and p38 MAPK pathways in macrophage cultures. (a) RAW264.7 cells were stimulated with 50 ng/ml or 100 ng/ml LPS in the presence of the indicated concentrations of EP. NF-κB activation was analyzed at 15 min and 4 h by electrophoretic mobility-shift assay by using a previously described 32P-labeled NF-κB probe. EP suppressed NF-κB activation in a concentration-dependent fashion. The results shown are representative of an experiment that was repeated three times. (b) Nuclear extracts of LPS-stimulated macrophages were incubated with antibody against p65-Rel (“anti-p65”) to induce specific supershift of NF-κB complex or 100-fold molar excess of unlabeled (cold) NF-κB or HIF-1 irrelevant duplex DNA probe for competition analysis. The results shown are representative of an experiment that was repeated three times. (c) RAW264.7 cells were stimulated with LPS (50 or 100 ng/ml) in the presence of the indicated concentration of EP. The phosphorylation of p38 MAPK was analyzed by Western blot by using antibodies against phosphorylated (Thr-180/Tyr-182) p38 MAPK, in accordance with the manufacturer's instructions (9210; New England Biolabs). EP prevented the phosphorylation of p38 MAPK in a concentration-dependent fashion (Upper), without affecting the intracellular level of total p38 MAPK (Lower). The results shown are representative of an experiment that was repeated three times.
Activation of the p38 MAPK cascade, as indicated by phosphorylation of p38 MAPK, is an important regulatory step in the release of proinflammatory cytokines from activated macrophages (16–20). As revealed by Western blot analysis with antibodies specific for the phosphorylated form of p38 MAPK, EP dose-dependently inhibited LPS-induced phosphorylation of p38 MAPK (Fig. 4c Upper). EP did not influence total cellular p38 MAPK, suggesting that treatment with the drug did not have nonspecific effects on intracellular protein levels (Fig. 4c Lower). Together, these results indicate that EP interferes with the activation of macrophages by suppressing signal transduction through NF-κB and p38 MAPK.
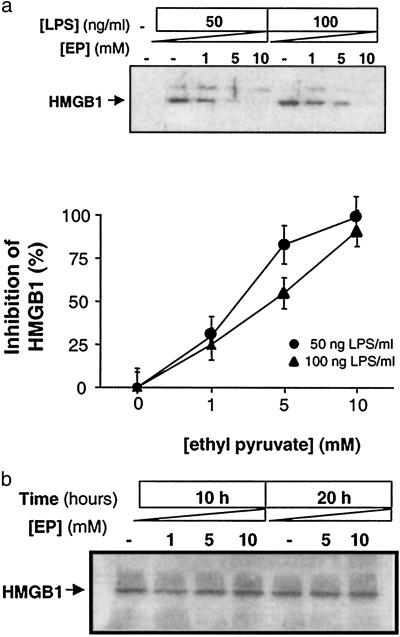
EP Attenuates the Release of HMGB1.
To determine whether EP directly inhibits macrophage HMGB1 release, HMGB1 was measured in conditioned medium of macrophage cultures incubated overnight in the presence of LPS. EP inhibited HMGB1 release in an EP concentration-dependent manner (Fig. 5a). HMGB1 exists as a preformed cell-associated protein in macrophages (ref. 4; reviewed in refs. 26 and 27). Accordingly, we sought to determine whether EP inhibited the release of preformed HMGB1 or suppressed total intracellular levels of HMGB1. Incubation with EP did not significantly influence total intracellular HMGB1 protein levels (Fig. 5b), indicating that it specifically prevented HMGB1 release without decreasing steady-state intracellular HMGB1 levels.
Fig 5.
EP inhibits HMGB1 release. (a) RAW264.7 macrophage cultures were stimulated with LPS (50 ng/ml or 100 ng/ml) for 20 h. Western blot analyses of the conditioned medium revealed that EP (1, 5, or 10 mM) significantly inhibited release of HMGB1 from stimulated macrophage cultures in a concentration-dependent manner. The results shown are representative of an experiment that was repeated three times. (Lower) HMGB1 levels were measured by densitometric analysis; the effect of EP was significant at all concentrations tested (P < 0.05 by Student's t test). (b) Analysis of the effect of EP on total cell-associated levels of HMGB1. Note that EP (1, 5, or 10 mM) did not affect the intracellular levels of HMGB1 at either 10 or 20 h after treatment. The results presented are representative an experiment that was conducted twice, in triplicate.
Discussion
These studies indicate that an experimental pharmacological agent, EP, significantly inhibits the systemic release of both early (TNF) and late (HMGB1) cytokines that mediate lethality of sepsis and systemic inflammation. EP can be administered significantly later after the acute-phase response (24 h) than any other previously described therapeutic for established sepsis. It now appears that EP can be explored further in the development of treatments for critical illness and sepsis. The molecular basis of EP action is by interfering with signal transduction through the p38 MAPK and NF-κB signal transduction pathways. Macrophage activation by endotoxin, cytokines, and products of cell injury lead to the nuclear translocation of NF-κB, a transcription factor that enhances the transcription of TNF and other products of the activated macrophage (22–24) and phosphorylation of p38 MAPK, which stabilizes TNF mRNA and increases TNF translation efficiency (16–21). EP inhibition of NF-κB and p38 MAPK signaling effectively prevented release of early (TNF) and late (HMGB1) inflammatory mediators.
HMGB1, an intracellular protein previously known only as a transcription factor (reviewed in refs. 26 and 27), now has been identified as a mediator of endotoxin lethality. Activated macrophages release HMGB1 after a significant lag of 12–18 h, and a similar, delayed kinetic is observed in the serum during lethal endotoxemia (4). The delayed kinetics of HMGB1 release provide a wider therapeutic window during which anti-HMGB1 antibodies can be administered downstream of the early TNF response (3, 4). Here, we observed that EP significantly inhibited HMGB1 release from macrophages and prevented the accumulation of serum HMGB1 levels in mice with lethal sepsis. The inhibition of serum HMGB1 significantly increased survival, giving direct evidence that HMGB1 is a late mediator of sepsis lethality. Macrophages contain large quantities of HMGB1 that are released during activation through mechanism(s) that remain unknown. EP, which inhibits signaling through both NF-κB and p38 MAPK, specifically prevented the release of HMGB1, because EP did not alter intracellular steady-state levels of HMGB1 protein. It is possible now to consider that inhibition of signaling through NF-κB and/or the p38 MAPK pathway may underlie the regulation of HMGB1 release from LPS-stimulated macrophages.
Together, these results reveal that EP may have therapeutic potential for diseases mediated by systemic release of the proinflammatory cytokines TNF and HMGB1. The molecular target of EP action is enigmatic, but it is an effective inhibitor of HMGB1 release by macrophage cultures and in an in vivo model of lethal sepsis. The doses of EP used here, which were not associated with toxicity, significantly inhibited serum HMGB1 release and conferred protection against sepsis lethality. Indeed, EP is a relatively nontoxic food additive, and the protective effects occurred in therapeutically achievable, safe doses. It will be of interest to assess the pharmacological activity of EP as an inhibitor of HMGB1 release in other models of local and systemic inflammation including arthritis and inflammatory bowel disease.
Acknowledgments
This work was supported in part by National Institutes of Health Grants ROI GM57226 and ROI GM62508 and the Defense Advanced Research Planning Agency (N65236-00-1-5434).
Abbreviations
TNF, tumor necrosis factor
HMGB1, high mobility group B-1
LPS, lipopolysaccharide
MAPK, mitogen-activated protein kinase
EP, ethyl pyruvate
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Angus D. C., Linde-Zwirble, W. T., Lidicker, J., Clermont, G., Carcillo, J. & Pinsky, M. R. (2001) Crit. Care Med. 29, 1303-1310. [DOI] [PubMed] [Google Scholar]
- 2.Marshall J. C. (2001) Crit. Care Med. 29, S99-S106. [DOI] [PubMed] [Google Scholar]
- 3.Tracey K. J., Beutler, B., Lowry, S. F., Merryweather, J., Wolpe, S., Milsark, I. W., Hariri, R. J., Fahey, T. J., III, Zentella, A., Albert, J. D., et al. (1986) Science 234, 470-474. [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Bloom, O., Zhang, M., Vishnubhakat, J. M., Ombrellino, M., Che, J., Frazier, A., Yang, H., Ivanova, S., Borovikova, L., et al. (1999) Science 285, 248-251. [DOI] [PubMed] [Google Scholar]
- 5.Abraham E., Anzueto, A., Gutierrez, G., Tessler, S., San Pedro, G., Wunderink, R., Dal Nogare, A., Nasraway, S., Berman, S., Cooney, R., et al. (1998) Lancet 28, 929-933. [PubMed] [Google Scholar]
- 6.Fisher C. J., Dhainaut, J. F., Opal, S. M., Pribble, J. P., Balk, R. A., Slotman, G. J., Iberti, T. J., Rackow, E. C., Shapiro, M. J., Greenman, R. L., et al. (1994) J. Am. Med. Assoc. 271, 1836-1843. [PubMed] [Google Scholar]
- 7.Abraham E., Arcaroli, J., Carmody, A., Wang, H. & Tracey, K. J. (2000) J. Immunol. 165, 2950-2954. [DOI] [PubMed] [Google Scholar]
- 8.Andersson U., Wang, H., Palmblad, K., Aveberger, A. C., Bloom, O., Erlandsson-Harris, H., Janson, A., Kokkola, R., Zhang, M., Yang, H., et al. (2000) J. Exp. Med. 192, 565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Vishnubhakat, J. M., Bloom, O., Zhang, M., Ombrellino, M., Sama, A. & Tracey, K. J. (1999) Surgery (St. Louis) 126, 389-392. [PubMed] [Google Scholar]
- 10.Fink M. P. (2002) Curr. Opin. Clin. Nutr. Metab. Care 5, 167-174. [DOI] [PubMed] [Google Scholar]
- 11.Sims C. A., Wattanasirichaigoon, S., Menconi, M. J., Ajami, A. M. & Fink, M. P. (2001) Crit. Care Med. 29, 1513-1518. [DOI] [PubMed] [Google Scholar]
- 12.Tawadrous Z., Delude, R. L. & Fink, M. P. (2002) Shock 17, 473-477. [DOI] [PubMed] [Google Scholar]
- 13.Yang R., Gallo, D. J., Baust, J. J., Uchiyama, T., Watkins, S. K., Delude, R. L. & Fink, M. P. (2002) Am. J. Physiol. 283, G212-G221. [DOI] [PubMed] [Google Scholar]
- 14.Wichmann M. W., Haisken, J. M., Ayala, A. & Chaudry, I. H. (1996) J. Surg. Res. 65, 109-114. [DOI] [PubMed] [Google Scholar]
- 15.Hesse D. G., Tracey, K. J., Fong, Y., Manogue, K. R., Palladino, M. A., Jr., Cerami, A., Shires, G. T. & Lowry, S. F. (1988) Surg. Gynecol. Obstet. 166, 147-153. [PubMed] [Google Scholar]
- 16.Lee J. C., Laydon, J. T., McDonnell, P. C., Gallagher, T. F., Kumar, S., Green, D., McNulty, D., Blumenthal, M. J., Heys, J. R., Landvatter, S. W., et al. (1994) Nature (London) 372, 739-746. [DOI] [PubMed] [Google Scholar]
- 17.Derijard B., Raingeaud, J., Barrett, T., Wu, I. H., Han, J., Ulevitch, R. J. & Davis, R. J. (1995) Science 267, 682-685. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C., Baumgartner, R. A., Yamada, K. & Beaven, M. A. (1997) J. Biol. Chem. 272, 13397-13402. [DOI] [PubMed] [Google Scholar]
- 19.Dong Z., Qi, X. & Fidler, I. J. (1993) J. Exp. Med. 177, 1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hambleton J., McMahon, M. & DeFranco, A. L. (1995) J. Exp. Med. 182, 147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen P. S., Nakshatri, H., Dennis, J., Caragine, T., Bianchi, M., Cerami, A. & Tracey, K. J. (1996) Proc. Natl. Acad. Sci. USA 93, 3967-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa Y., Mukaida, N., Kuno, K., Rice, N., Okamoto, S. & Matsushima, K. (1995) J. Biol. Chem. 270, 4158-4164. [PubMed] [Google Scholar]
- 23.Vincenti M. P., Burrell, T. A. & Taffet, S. M. (1992) J. Cell. Physiol. 150, 204-213. [DOI] [PubMed] [Google Scholar]
- 24.Brown M. C., Tomaras, G. D., Vincenti, M. P. & Taffet, S. M. (1997) J. Interferon Cytokine Res. 17, 295-306. [DOI] [PubMed] [Google Scholar]
- 25.Narravula S. & Colgan, S. P. (2001) J. Immunol. 166, 7543-7548. [DOI] [PubMed] [Google Scholar]
- 26.Czura C. J., Wang, H. & Tracey, K. J. (2001) J. Endotoxin Res. 7, 315-321. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Yang, H., Czura, C. J., Sama, A. E. & Tracey, K. J. (2001) Am. J. Respir. Crit. Care Med. 164, 1768-1773. [DOI] [PubMed] [Google Scholar]