Abstract
Antioxidant drugs have been reported to protect pancreatic islets from the adverse effects of chronic exposure to supraphysiological glucose concentrations. However, glucose has not been shown to increase intracellular oxidant load in islets, nor have the effects of increasing or inhibiting glutathione peroxidase (GPx) activity on islet resistance to sugar-induced oxidant stress been studied. We observed that high glucose concentrations increased intracellular peroxide levels in human islets and the pancreatic β cell line, HIT-T15. Inhibition of γ-glutamylcysteine synthetase (γGCS) by buthionine sulfoximine augmented the increase in islet peroxide and decrease in insulin mRNA levels, content, and secretion in islets and HIT-T15 cells induced by ribose. Adenoviral overexpression of GPx increased GPx activity and protected islets against adverse effects of ribose. These results demonstrate that glucose and ribose increase islet peroxide accumulation and that the adverse consequences of ribose-induced oxidative stress on insulin mRNA, content, and secretion can be augmented by a glutathione synthesis inhibitor and prevented by increasing islet GPx activity. These observations support the hypothesis that oxidative stress is one mechanism for glucose toxicity in pancreatic islets.
Type 2 diabetes mellitus is polygenic in origin and usually begins in adulthood, although specific genes for subtypes of this disease that occur earlier in life, referred to collectively as maturity-onset diabetes of the young (MODY), have been identified (1). The onset of type 2 diabetes is insidious, thus hyperglycemia develops gradually and often goes untreated for years until symptoms become clinically obvious. Consequent chronic exposure of tissues to supraphysiologic levels of blood glucose can lead to adverse intracellular outcomes, a process known as glucose toxicity (2–5). Possible mechanisms of action for glucose toxicity include the formation of advanced glycosylation end products and glucosamine, increased protein kinase C activity with c-myc induction, autooxidation of glucose, and increased levels of reactive glycolytic intermediates such as glyceraldehyde-3-phosphate or dihydroxyacetone phosphate (6–13). All these processes usually are accompanied by the formation of reactive oxygen species (ROS), setting up the potential for oxidative stress. Many weeks of exposure to high concentrations of glucose are necessary before glucotoxic effects are expressed by islet β cells in vitro and in vivo (14–20). Because isolated islets do not reliably survive in culture greater than 2 weeks, the use of short-term exposure to ribose, a sugar that generates ROS more potently than glucose, has become an accepted model for studying islet glucose toxicity (21, 22). The use of ribose as a prooxidant sugar is especially valuable in adenoviral overexpression systems, because the overexpression effect is short- lived, lasting only days.
In the pancreatic β cell, glucose and ribose toxicity cause decreased insulin mRNA levels (14–22), one mechanism for which is a marked decrease in posttranscriptional processing of PDX-1, a critical transcription factor for insulin gene expression (23). Decreased PDX-1 leads to diminished β cell insulin content and defective glucose-induced insulin secretion. The toxic effects of elevated glucose and ribose can be prevented by antioxidants in a β cell line (20) and animal models of type 2 diabetes (19, 20, 24). The beneficial effects of antioxidants may be related to the low levels of antioxidant enzyme activity that are characteristic of islets (25, 26), which render them particularly susceptible to the oxidative effects of ROS. These observations support our working hypothesis that ROS in excessive amounts may be formed during prolonged exposure of islets to supraphysiologic glucose concentrations and that this may lead to defective PDX-1-binding activity and insulin mRNA expression, decreased insulin content, and reduced insulin secretion. Nonetheless, all previously reported data must be considered indirect and associational, because there has been no previous demonstration that elevated glucose levels can increase the intracellular oxidant load in pancreatic islets or that increasing and decreasing intrinsic antioxidant activity within β cells can decrease and increase, respectively, sugar-related oxidant stress.
Consequently, in this work we designed experiments to ascertain whether (i) glucose and ribose increase intraislet peroxide levels, (ii) a reduction in glutathione synthesis worsens ribose-induced decreases in insulin gene expression, content, and insulin secretion, and (iii) adenoviral overexpression of glutathione peroxidase (GPx) in β cells prevents the adverse effects of ribose-induced oxidant stress.
Materials and Methods
HIT-T15 Cell Culture.
HIT-T15 cells were grown in 5% CO2/95% O2 at 37°C and maintained in RPMI medium 1640 supplemented with 10% FBS and containing 11.1 mM glucose as described (27).
Pancreatic Islet Isolation.
Pancreata from male Wistar rats were infused with 10 ml of a 1.5 mg/ml collagenase Type XI (Sigma)/1% FBS/2 units/ml RQ1 DNase (Promega) solution in Medium 199 (Sigma). After surgical excision, the pancreas was incubated in the collagenase solution at 37°C. Undigested tissue was removed by using a 500-μm screen, and the recovered tissue was washed twice with ice-cold Hanks' balanced salt solution (HBSS) containing 0.1% BSA followed by centrifugation at 250 × g for 4 min. Islets in the pellet were separated by using a Histopaque-1077 gradient (Sigma) and then hand-picked and cultured overnight in RPMI medium 1640 containing 10% FBS, 11.1 mM glucose, and penicillin/streptomycin/amphotericin B before experimentation. Human islets were provided by Human Islet Transplantation program (Seattle) using a similar isolation strategy.
Measurements of Intracellular Peroxides.
Oxidative activity was detected by flow-cytometric analysis using a fluorescein-labeled dye, dichlorodihydrofluorescein diacetate (H2DCFDA, Molecular Probes). The acetoxymethyl ester derivative readily permeates cell membranes and is trapped within the cell after cleavage by esterases. Oxidation by ROS converts the dye from its nonfluorescent to fluorescent form. Briefly, cells were cultured with 10 μM H2DCFDA for 30 min at 37°C. Islets were cultured with 4 μM H2DCFDA for 10 min. After incubation with the dye, cells and islets were washed with PBS to prevent measurement of any extracellular reaction between the dye and H2O2 released by the cells into the medium. Islets then were dispersed by using trypsin, and intracellular peroxide levels were measured with the EPICS XL-MCL flow cytometer controlled by SYSTEM II software (Beckman Coulter). Results were calculated as the fold difference from control untreated cells/islets.
Measurements of Insulin mRNA, Secretion, and Content.
Insulin mRNA.
Total RNA was extracted according to the method of Chomczynski and Sacchi (28). One-step reverse transcription–PCR was carried out by using the Gold reverse transcription–PCR kit from Perkin–Elmer Biosystems and an ABI Prism 7700 Sequence detector equipped with a thermocycler (Taqman technology) and a cooled charge-coupled device camera to detect fluorescence emission over a range of wavelengths (500–650 nm) as described previously (20, 29).
Islet insulin secretion and content.
Static incubation of HIT-T15 cells or islets in Krebs Ringer buffer (KRB) containing either nonstimulatory or stimulatory concentrations of glucose was performed for 1 h. Insulin levels in the KRB samples collected from the static incubations or from insulin content samples, extracted from the islets by using a 95:5 ethanol/acetic acid solution, were measured by using a sensitive rat insulin RIA kit (Linco Research Immunoassay, St. Charles, MO).
GPx Adenovirus Infection.
Adenovirus encoding human cGPx-1 along with the control adenovirus were obtained from the University of Iowa Adenoviral Core Facility (University of Iowa College of Medicine, Iowa City; ref. 30). Propagation of the adenovirus was performed as described by He et al. (31) by the Pacific Northwest Research Institute Adenoviral Core. Isolated rat islets were cultured in the presence of adenovirus [at a final concentration of 107 plaque-forming units (pfu) per islet] in RPMI medium 1640 supplemented with 10% FBS for 24 h at 37°C. After 24 h, the islets were transferred into fresh RPMI medium 1640 containing 10% FBS with or without ribose for another 72 h before experimentation. Ascertainment of adenoviral penetration into β cells within the core of the islet was performed by infection with virus encoding GFP and insulin immunostaining and detection via confocal microscopy. β cell toxicity of the adenovirus was assessed by determining glucose-induced insulin secretion after infection.
Measurement of GPx Activity.
Islets were washed once and then sonicated in 50 mM phosphate buffer. Supernatant was collected after centrifugation at 14,000 rpm in a TOMY TX-160 (TOMY Tech, Palo Alto, CA) for 1 h at 4°C and assayed for GPx activity by using the Bioxytech GPx-340 assay per manufacturer protocol (OxisResearch, Portland, OR). The assay measures cellular GPx activity based on the oxidation of NADPH to NADP+, which is accompanied by a decrease in the absorbance measured at 340 nm over 3 min. The cellular extract containing the enzyme is added to a reaction solution containing glutathione, glutathione reductase, and NADPH, and the enzyme reaction is initiated after addition of the substrate, tert-butyl hydroperoxide. One milliunit/ml of enzyme activity is defined as the rate of conversion of 1 nmol NADPH/ml/min to NADP+. Results were calculated as the fold increase over GPx activity in uninfected control islets.
Chemical Agents.
N-acetylcysteine (NAC), an antioxidant, was obtained from Sigma; buthionine sulfoximine (BSO), an inhibitor of γ-glutamylcysteine synthetase, was obtained from Sigma; and mannoheptulose, an inhibitor of glucose transport, was a gift of Michael McDaniel (Washington University, St. Louis).
Results
Intracellular Peroxide Levels.
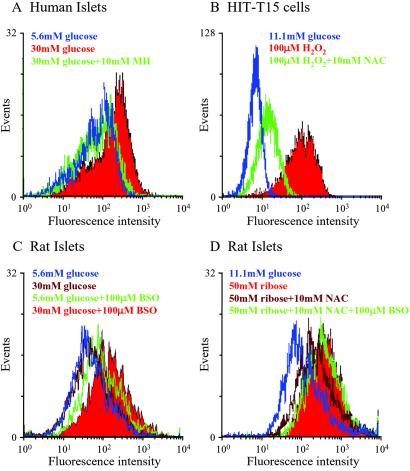
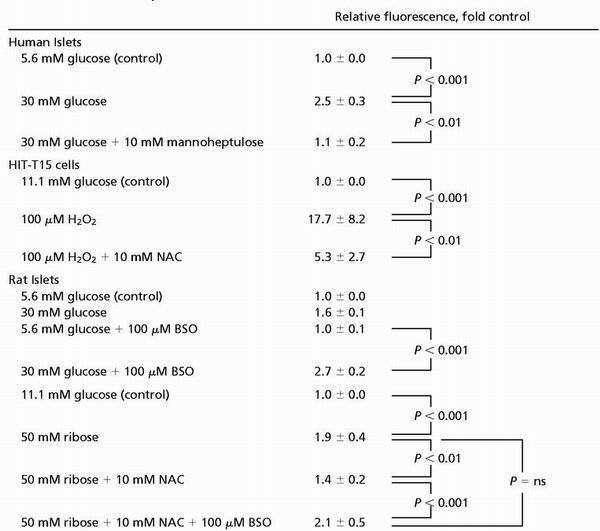
Exposure of human islets to 30 mM glucose for 72 h increased peroxide levels, an effect that was prevented by 10 mM mannoheptulose (Fig. 1A; Table 1). The antioxidant effect of 10 mM NAC against 100 μM H2O2 was demonstrated in HIT-T15 cells (Fig. 1B and Table 1). The oxidant effect of 30 mM glucose in rat islets was observed only in the presence of 100 μM BSO (Fig. 1C and Table 1). Peroxide accumulation after 72 h of exposure to 50 mM ribose was inhibited by NAC in rat islets, an effect abrogated by BSO (Fig. 1D and Table 1).
Fig 1.
Intracellular peroxide levels in human islets, HIT-T15 cells, and rat islets determined by flow cytometry. Islets or HIT-T15 cells were incubated with a peroxide-sensitive probe, carboxy dichlorodihydrofluorescein diacetate (using 4 and 10 μM, respectively) during the final 30 min of each treatment. (A) Human islets were cultured in 5.6 or 30 mM glucose with or without 10 mM mannoheptulose (MH) for 72 h. (B) HIT-T15 cells were cultured with glucose with or without 100 μM H2O2 with or without 10 mM NAC for 1 h. (C) Rat islets were cultured in 5.6 or 30 mM glucose with or without 100 μM BSO for 72 h. (D) Rat islets were cultured in ribose with or without NAC with or without BSO for 72 h. Group data from at least n = 3 experiments are shown in Table 1 as mean ± SE fold difference from untreated control cells or islets. Statistical comparisons were done by using one-way ANOVA with the Fisher's post-ANOVA test. Histograms are representatives of at least three independent experiments (see Table 1).
Table 1.
Group data from the experiments illustrated in Fig. 1 expressed as mean ± SE with relevant statistical comparisons
 |
ns, not significant.
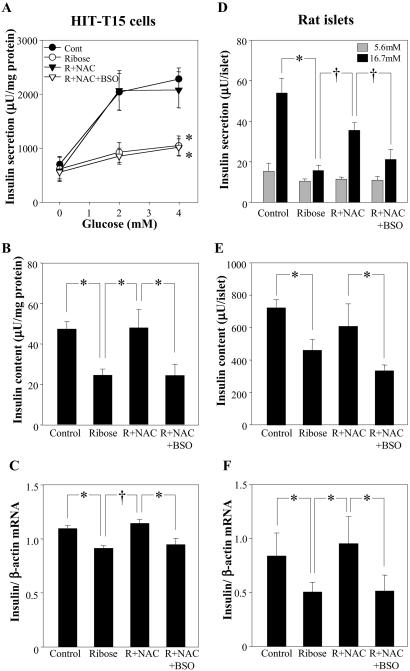
BSO Effects on Insulin Secretion, Content, and mRNA Levels in HIT-T15 Cells and Isolated Rat Islets.
In HIT-T15 cells, 50 mM ribose inhibited glucose-induced insulin secretion, decreased insulin content, and decreased insulin mRNA levels (Fig. 2 A–C). These effects were prevented completely by treatment of cells with 10 mM NAC in the incubations, an effect that was abrogated completely by coinclusion of BSO. Similar results were observed when using isolated rat islets (Fig. 2 D–F).
Fig 2.
Effects of NAC against ribose-induced decreases in glucose-induced insulin secretion, content, and mRNA and abrogation of these preventive effects of NAC by BSO in HIT-T15 cells (A–C) and rat islets (D–F). HIT-T15 cells were precultured in 30 mM ribose with or without 10 mM NAC with or without 50 μM BSO for 72 h. (A) Insulin secretion from cells incubated in KRB buffer containing increasing concentration of glucose for 2 h (*, P < 0.01 vs. control; repeated measurement ANOVA). (B) Intracellular insulin content (*, P < 0.05; one-way ANOVA). (C) Insulin mRNA level (*, P < 0.01, and †, P < 0.001; one-way ANOVA). Rat islets were precultured in 50 mM ribose with or without 10 mM NAC or 100 μM BSO for 72 h. (D) Insulin secretion from islets incubated in KRB buffer containing 5.6 or 16.7 mM glucose for 1 h (*, P < 0.001, and †, P < 0.001; one-way ANOVA). (E) Intracellular insulin content (*, P < 0.05, one-way ANOVA). (F) Insulin mRNA level (*, P < 0.05, one-way ANOVA). All data are presented as means ± SE for three or four independent experiments with duplicate or triplicate determinations.
Effect of GPx Overexpression on Ribose-Induced β Cell Dysfunction.
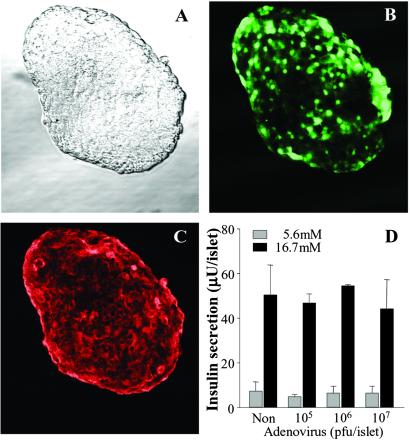
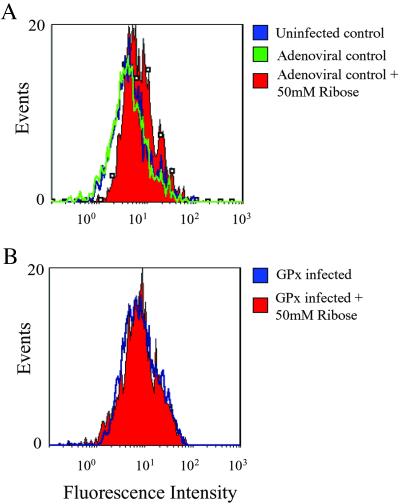
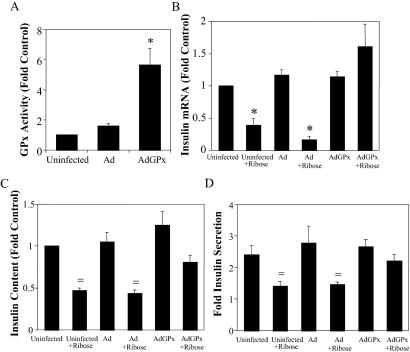
Infection of rat islets with adenovirus (107 pfu per islet) encoding GFP resulted in demonstrable penetration of virus to β cells within the core of the islet (Fig. 3 A–C). No adverse effects on glucose-induced insulin secretion were observed when islets were infected with 105–107 pfu of adenoviral concentrations (Fig. 4D). GPx overexpression prevented the increase in intracellular peroxide levels induced by 50 mM ribose (Fig. 4). Overexpression of GPx prevented the ribose-induced decreases in insulin mRNA levels, insulin content, and glucose-induced insulin secretion (Fig. 5 B–D).
Fig 3.
Infection of Wistar rat islets with adenovirus encoding GFP. Islets were infected for 24 h with virus at a concentration of 107 pfu per islet. (A) Transmitted light image. (B) GFP fluorescence after background subtraction. (C) Insulin immunostaining. (D) Insulin secretion from islets exposed in static incubation to nonstimulatory (5.6 mM) or stimulatory (16.7 mM) glucose concentrations after 24-h infection with 105–107 pfu per islet adenovirus (n = 3 experiments). A–C are from confocal microscopy sections through the center of islet.
Fig 4.
Effects of adenovirally induced GPx overexpression on ribose-dependent increases in intracellular peroxide levels. Comparisons regarding the effects of ribose on islets were calculated as fold difference from control islets not treated with ribose. (A) Intracellular peroxide levels in control infected islets were not significantly different than that in uninfected islets. Treatment with 50 mM ribose for 72 h increased peroxides 1.71 ± 0.19-fold in uninfected islets and 1.62 ± 0.26-fold in control infected islets. P < 0.05 compared with islets not treated with ribose. (B) Intracellular peroxide levels in islets overexpressing GPx after treatment with ribose did not increase significantly from untreated islets overexpressing GPx, 1.25 ± 0.19-fold from untreated control. P = not significant. Histograms are representative of raw data from n = 5 experiments.
Fig 5.
Effects of adenovirally (Ad) induced GPx overexpression in Wistar rat islets against ribose-induced decreases in insulin mRNA, content, and secretion. (A) GPx activity 72 h after infection (*, P < 0.001, one-way ANOVA). (B) Effects of GPx overexpression on ribose-dependent decreases on insulin mRNA levels normalized to mRNA levels in uninfected control islets (*, P < 0.001, one-way ANOVA). Islets were incubated in KRB buffer containing 5.6 or 16.7 mM glucose for 1 h to measure insulin secretion before extraction of insulin content. (C) Intracellular insulin content was decreased significantly by 72-h exposure to ribose in both the uninfected and control adenoviral-infected controls (†, P < 0.05, one-way ANOVA). Overexpression with the GPx adenovirus partially prevented the ribose-induced decrease in insulin content (P = not significant from control, one-way ANOVA). (D) Similar results were observed with glucose-induced insulin secretion with islets treated as described for C. All data are presented as means ± SE for 5–8 independent experiments with duplicate or triplicate determinations.
Discussion
These studies were designed to test the hypothesis that high glucose concentrations can increase the accumulation of intracellular ROS in islets and that limiting or increasing the level of β cell GPx activity, respectively, augments or prevents the induction by ribose of β cell functional defects. We observed that (i) glucose and ribose can increase the intracellular oxidant load of islets, (ii) pharmacologically decreasing glutathione provision to GPx enhances the deleterious effects of ribose, (iii) and adenoviral overexpression of GPx activity in the β cell prevents the adverse effects of ribose.
Overproduction of ROS under high glucose conditions has been shown to result in an attenuation of antioxidant mechanisms in endothelial and mesangial cells. High glucose decreased glutathione provision to GPx and involved a reduction in γ-glutamylcysteine synthetase activity (32–36) as well as a decrease in NADPH supply to the glutathione redox cycle (32). The addition of antioxidants reversed the negative effects of high glucose on extracellular matrix proteins and cell growth (34, 37, 38). The strongest previously published evidence supporting the hypothesis that glucose toxicity in β cells is mediated by ROS is indirect, i.e., demonstrations in vitro and in vivo of protective effects of antioxidants against the deterioration of insulin gene expression and β cell function associated with supraphysiologic glucose levels (19, 20, 24). The current studies provide direct evidence that glucose and ribose increase intracellular peroxide levels in isolated human and rat pancreatic islets, and that the intrinsically low levels of β cell GPx activity (25, 26) predispose them to ROS-induced damage. Our working hypothesis is that one ROS species that is likely to mediate islet oxidative damage is the hydroxyl radical. Autooxidation of glucose and/or glycolytic intermediates may initiate ROS formation by generation of superoxide followed by dismutation to hydrogen peroxide (11). Alternatively, reaction of peroxides with traces of redox-active metal ions could catalyze formation of hydroxyl radicals via Fenton reactions, and the reaction of superoxides with nitric oxide could yield peroxynitrites that all can cause DNA damage.
Our second experimental aim to determine whether decreasing glutathione synthesis would enhance the adverse effects of intracellular ROS on β cell function was approached pharmacologically. Treatment of HIT-T15 cells and islets with BSO, a drug that inhibits γ-glutamylcysteine synthetase (γGCS) and depletes cellular glutathione levels, augmented the deleterious effects of ribose on insulin mRNA level, insulin content, and glucose-induced insulin secretion. In separate experiments, we found that 72-h exposure to BSO at the final concentration used in these studies (100 μM) significantly decreased total glutathione levels in islets (data not shown).
The third aim of these studies was to increase GPx activity specifically within the β cell to levels more consistent with other rodent tissues to determine whether this maneuver would prevent the adverse effects of ROS on β cell function. Our experience is consistent with that published by Lenzen et al. (25, 26), indicating that antioxidative enzyme mRNA, mass, and activity in islets are among the lowest in mammalian tissues and as little as 20% of that found in liver. Because the islet is an organelle comprised of a core of β cells with a mantle of α cells on its outer surface, we first examined the ability of adenovirus encoding GFP to penetrate isolated islets. A 24-h incubation with 107 pfu of adenovirus per islet effectively introduced viral particles into β cells without causing toxic effects, as judged by intactness of glucose-induced insulin secretion after infection. When adenovirus encoding GPx was used for infection, we observed an 6-fold increase in GPx activity, which resulted in protection against the adverse effects of excess ROS caused by ribose. This information provided the demonstration that increasing GPx activity in β cells to levels that are more equivalent to other rat tissues completely protected the β cells from sugar-induced oxidant stress. One area for further experimentation might be to ascertain whether overexpression of catalase and/or superoxide dismutase would provide similar results.
The protective effect of antioxidant enzymes, primarily superoxide dismutase and catalase, have been examined through overexpression in islets to test their efficacy in protecting β cells against the adverse effects of streptozotocin and cytokines in experimental models of type 1 diabetes (39–42), an autoimmune disease distinct from type 2 diabetes. Our studies uniquely used an adenoviral technique to overexpress β cell GPx activity and had different goals. We focused on glucose toxicity as a secondary force in the ongoing pathogenesis of type 2 diabetes, a disease that is polygenic in origin and not initiated by autoimmunity. Our results suggest that the intrinsically low level of GPx activity in the islet sets up the β cell as an organelle particularly susceptible to oxidative stress secondary to high levels of glucose. Therapeutic strategies designed to enhance β cell antioxidant activity may be a means for preserving residual β cell function after the onset of type 2 diabetes.
Acknowledgments
We gratefully acknowledge excellent technical assistance from Catherine Gleason, Scott Holland, Eric Leroy, and Elizabeth Oseid. We thank Dr. Michael McDaniel for the mannoheptulose, Drs. John Engelhardt and Larry Oberley (University of Iowa) for discussions regarding GPx overexpression, and Jill McCuaig in the Pacific Northwest Research Institute Adenoviral Core for technical support. This work was supported by National Institutes of Health Grant RO1 DK 38325 (to R.P.R.), an American Diabetes Association Mentor-based Fellowship (to Y.T.), and a Juvenile Diabetes Research Foundation Advanced Postdoctoral Fellowship (to P.O.T.T.).
Abbreviations
ROS, reactive oxygen species
GPx, glutathione peroxidase
pfu, plaque-forming unit(s)
NAC, N-acetylcysteine
BSO, buthionine sulfoximine
References
- 1.Fajans S. S., Bell, G. I. & Polonsky, K. S. (2001) N. Engl. J. Med. 345, 971-980. [DOI] [PubMed] [Google Scholar]
- 2.Rossetti L., Giaccari, A. & DeFronzo, R. A. (1990) Diabetes Care 13, 610-630. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H. (1992) Endocr. Rev. 13, 415-431. [DOI] [PubMed] [Google Scholar]
- 4.Robertson R. P. (1989) Diabetes 38, 1501-1505. [DOI] [PubMed] [Google Scholar]
- 5.Robertson R. P., Olson, L. K. & Zhang, H. J. (1994) Diabetes 43, 1085-1089. [DOI] [PubMed] [Google Scholar]
- 6.Baynes J. W. (1991) Diabetes 40, 405-412. [DOI] [PubMed] [Google Scholar]
- 7.Wells-Knecht M. C., Lyons, T. J., McCance, D. R., Thorpe, S. R. & Baynes, J. W. (1997) J. Clin. Invest. 100, 839-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneto H., Sharma, A., Suzuma, K., Laybutt, D. R., Bonner-Weir, S. & Weir, G. C. (2002) J. Biol. Chem. 277, 12998-13006. [DOI] [PubMed] [Google Scholar]
- 9.Kaneto H., Suzuma, K., Sharma, A., Bonner-Weir, S., King, G. L. & Weir, G. C. (2002) J. Biol. Chem. 277, 3680-3685. [DOI] [PubMed] [Google Scholar]
- 10.Du X. L., Edelstein, D., Rossetti, L., Fantus, I. G., Goldberg, H., Ziyadeh, F., Wu, J. & Brownlee, M. (2000) Proc. Natl. Acad. Sci. USA 97, 12222-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff S. P. & Dean, R. T. (1987) Biochem. J. 245, 243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt J. V., Dean, R. T. & Wolff, S. P. (1988) Biochem. J. 256, 205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa T., Edelstein, D., Du, X. L., Yamagishi, S., Matsumura, T., Kaneda, Y., Yorek, M. A., Beebe, D., Oates, P. J., Hammes, H. P., et al. (2000) Nature (London) 404, 787-790. [DOI] [PubMed] [Google Scholar]
- 14.Robertson R. P., Zhang, H. J., Pyzdrowski, K. L. & Walseth, T. F. (1992) J. Clin. Invest. 90, 320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson L. K., Redmon, J. B., Towle, H. C. & Robertson, R. P. (1993) J. Clin. Invest. 92, 514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poitout V., Olson, L. K. & Robertson, R. P. (1996) J. Clin. Invest. 97, 1041-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eizirik D. L., Korbutt, G. S. & Hellerstrom, C. (1992) J. Clin. Invest. 90, 1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seufert J., Weir, G. C. & Habener, J. F. (1998) J. Clin. Invest. 101, 2528-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneto H., Kajimoto, Y., Miyagawa, J., Matsuoka, T., Fujitani, Y., Umayahara, Y., Hanafusa, T., Matsuzawa, Y., Yamasaki, Y. & Hori, M. (1999) Diabetes 48, 2398-2406. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y., Gleason, C. E., Tran, P. O., Harmon, J. S. & Robertson, R. P. (1999) Proc. Natl. Acad. Sci. USA 96, 10857-10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneto H., Fujii, J., Myint, T., Miyazawa, N., Islam, K. N., Kawasaki, Y., Suzuki, K., Nakamura, M., Tatsumi, H., Yamasaki, Y. & Taniguchi, N. (1996) Biochem. J. 320, 855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka T., Kajimoto, Y., Watada, H., Kaneto, H., Kishimoto, M., Umayahara, Y., Fujitani, Y., Kamada, T., Kawamori, R. & Yamasaki, Y. (1997) J. Clin. Invest. 99, 144-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson L. K., Sharma, A., Peshavaria, M., Wright, C. V., Towle, H. C., Rodertson, R. P. & Stein, R. (1995) Proc. Natl. Acad. Sci. USA 92, 9127-9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihara Y., Yamada, Y., Toyokuni, S., Miyawaki, K., Ban, N., Adachi, T., Kuroe, A., Iwakura, T., Kubota, A., Hiai, H. & Seino, Y. (2000) FEBS Lett. 473, 24-26. [DOI] [PubMed] [Google Scholar]
- 25.Lenzen S., Drinkgern, J. & Tiedge, M. (1996) Free Radical Biol. Med. 20, 463-466. [DOI] [PubMed] [Google Scholar]
- 26.Tiedge M., Lortz, S., Drinkgern, J. & Lenzen, S. (1997) Diabetes 46, 1733-1742. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H. J., Walseth, T. F. & Robertson, R. P. (1989) Diabetes 38, 44-48. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P. & Sacchi, N. (1987) Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- 29.Tran P. O., Gleason, C. E., Poitout, V. & Robertson, R. P. (1999) J. Biol. Chem. 274, 31245-31248. [DOI] [PubMed] [Google Scholar]
- 30.Li Q., Sanlioglu, S., Li, S., Ritchie, T., Oberley, L. & Engelhardt, J. F. (2001) Antioxid. Redox. Signal. 3, 415-432. [DOI] [PubMed] [Google Scholar]
- 31.He T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashiwagi A., Nishio, Y., Asahina, T., Ikebuchi, M., Harada, N., Tanaka, Y., Takahara, N., Taki, H., Obata, T., Hidaka, H., et al. (1995) Diabetes 46, 2088-2095. [DOI] [PubMed] [Google Scholar]
- 33.Urata Y., Yamamoto, H., Goto, S., Tsushima, H., Akazawa, S., Yamashita, S., Nagataki, S. & Kondo, T. (1996) J. Biol. Chem. 271, 15146-15152. [DOI] [PubMed] [Google Scholar]
- 34.Paget C., Lecomte, M., Ruggiero, D., Wiernsperger, N. & Lagarde, M. (1998) Free Radical Biol. Med. 25, 121-129. [DOI] [PubMed] [Google Scholar]
- 35.Ha H. & Lee, H. B. (2000) Kidney Int. 58, S19-S25. [DOI] [PubMed] [Google Scholar]
- 36.Catherwood M. A., Powell, L. A., Anderson, P., McMaster, D., Sharpe, P. C. & Trimble, E. R. (2002) Kidney Int. 61, 599-608. [DOI] [PubMed] [Google Scholar]
- 37.Ceriello A., dello Russo, P., Amstad, P. & Cerutti, P. (1996) Diabetes 45, 471-477. [DOI] [PubMed] [Google Scholar]
- 38.Clarkson M. R., Murphy, M., Gupta, S., Lambe, T., Mackenzie, H. S., Godson, C., Martin, F. & Brady, H. R. (2002) J. Biol. Chem. 277, 9707-9712. [DOI] [PubMed] [Google Scholar]
- 39.Kubisch H. M., Wang, J., Luche, R., Carlson, E., Bray, T. M., Epstein, C. J. & Phillips, J. P. (1994) Proc. Natl. Acad. Sci. USA 91, 9956-9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubisch H. M., Wang, J., Bray, T. M. & Phillips, J. P. (1997) Diabetes 46, 1563-1566. [DOI] [PubMed] [Google Scholar]
- 41.Benhamou P. Y., Moriscot, C., Richard, M. J., Beatrix, O., Badet, L., Pattou, F., Kerr-Conte, J., Chroboczek, J., Lemarchand, P. & Halimi, S. (1998) Diabetologia 41, 1093-1100. [DOI] [PubMed] [Google Scholar]
- 42.Xu B., Moritz, J. T. & Epstein, P. N. (1999) Free Radical Biol. Med. 27, 830-837. [DOI] [PubMed] [Google Scholar]