Abstract
Phenotypic knockout of nerve growth factor (NGF) activity in transgenic anti-NGF mice (AD11 mice) results in a progressive neurodegenerative phenotype resembling Alzheimer's disease. In this article, we examine whether and how the progressive neurodegenerative phenotype of AD11 mice could be prevented or ameliorated by pharmacological treatments with NGF or the cholinergic agonist galantamine, at a relatively early phase of Alzheimer's disease-like neurodegeneration. We demonstrate that the neurodegeneration induced by the expression of anti-NGF antibodies in AD11 mice can be largely reversed by NGF delivery through an olfactory route.
A loss of basal forebrain cholinergic neurons (BFCNs) underlies the behavioral and cognitive deficits observed in Alzheimer's disease (AD) (1). The cholinergic phenotype of BFCNs is influenced by nerve growth factor (NGF) (2–4), which promotes the survival and differentiation of BFCNs during development and adulthood (5–10). Consequently, it has been suggested that a decrease in NGF function could contribute to the onset of AD. In AD brains, the levels of NGF mRNA are unchanged (11, 12), whereas increased levels of NGF protein can be detected in the cortex and hippocampus (13–16), associated to a decreased amount of NGF in the BF (17). A deficit in NGF activity has been obtained in a transgenic model (AD11 mice) (18), in which a recombinant anti-NGF antibody is secreted by neuronal and glial cells and neutralizes the activity of NGF in the extracellular space. Aged AD11 mice display a neurodegenerative phenotype characterized by behavioral deficits linked to cholinergic atrophy, neuronal loss, tau hyperphosphorylation and insolubility, abnormalities of the neuronal cytoskeleton reminiscent of tangles (19), β-amyloid plaques [from the endogenous amyloid precursor protein (APP) gene] (20), and deficits in cortical synaptic plasticity (21). AD11 mice recapitulate many of the neurodegenerative markers that characterize AD and therefore represent a comprehensive model for sporadic AD.
To gain further insights into the mechanisms whereby blocking NGF activity with an antibody leads to an AD-like neurodegeneration and to further validate AD11 mice as a model for human sporadic AD, we investigated the extent to which the NGF deficit and the ensuing cholinergic deficit are causally linked to the observed neurodegeneration. We examined whether and how the neurodegenerative phenotype of AD11 mice could be prevented or ameliorated by pharmacological treatments with NGF or cholinergic agonists, at a relatively early phase of AD-like neurodegeneration.
Methods
Anti-NGF AD11 Mice.
AD11 anti-NGF mice were produced as described (18). Double transgenic mice expressing functional anti-NGF antibodies were obtained by crossing single transgenic mice expressing only the light chain (CMV-VK αD11) with single transgenic mice expressing only the heavy chain (CMV-VH αD11) (18).
Pharmacological Treatments.
LT4 (l-thyroxine) was administered according to the schedule and dosages shown to produce the maximal increase of NGF expression (22). LT4 was administered i.p. (10 μg in 0.1 ml of 0.1 mM sodium carbonate in PBS) daily from 1.5 months until 2 months age, from 4 months until 6 months of age, or from 6 months until 6.5 months of age.
Recombinant human NGF (rhNGF) (Alomone Laboratories, Jerusalem) was delivered intra-nasally every 2 days according to a procedure modified from Frey et al. (23). Mice were anesthetized with i.p. 2,2,2-tribromoethanol (400 mg/kg), and rhNGF (0.01–10 μM in 40 mM PBS, pH 7.4, total volume of 48 μl) was given in 3-μl drops to each naris over 30 min, alternating drops every 2 min between the left and right naris. rhNGF was administered to the following groups of AD11 and control mice: from 1.5 to 2 months of age, from 4 to 6 months of age, and from 6 to 6.5 months of age.
Galantamine hydrobromide (GAL; 3.5 mg/kg) (Tocris Cookson, Bristol, U.K.) was injected i.p. daily for 15 days from 1.5 months and 6 months of age and daily from 4 to 6 months of age.
Immunohistochemistry.
Analysis was performed as described (18, 19). The following primary antibodies were used: anti-choline acetyl transferase (ChAT, 1:500, Chemicon), antiphosphorylated tau (clone AT8, Innogenetics, Zwijnaarde, Belgium), anti-APP (clone 2.F2.19B4, reacting with intact full-length Alzheimer precursor protein and selectively with the cytoplasmic carboxyl fragment of APP 643–695; Chemicon), and antibodies raised against Aβ17–24 (mAb 4G8, Senetek, Maryland Heights, MO) and against the NH2 terminus of Aβ (R3660, kindly provided by G. Schettini and C. Russo, University of Genova, Genova, Italy; ref. 24).
Determination of Free NGF.
The levels of free NGF (i.e., NGF not bound to the transgenic antibodies) were determined by two-site ELISA as described (18, 25).
Quantitative Stereology.
The number of ChAT- positive neurons in the BF was determined as described (18). The same stereological technique was applied to count Aβ-positive clusters of cells in the hippocampus. Statistical analysis was performed by using a two-tailed t test. The amyloid burden was quantified by image analysis on anti-APP stained sections as described (26), using the OPTIMAS 6.1 video image analysis system (Optimas, Bothell, WA) linked to a Zeiss Axiovert microscope through a charge-coupled device video camera.
Results
Time Course of Neuronal Degeneration in AD11 Mice.
In this study we assessed the ability of NGF and a cholinergic agonist to reverse the early phases of the progressive neurodegenerative phenotype of AD11 mice (18–21).
The end points of the rescue study were chosen on the basis of the time course of neurodegeneration in AD11 mice. Early stages of neurodegeneration are characterized by a cholinergic deficit in the BF (Fig. 1 A and B; ref. 18) starting from 2 months of age and increasing at 6 months of age (Fig. 1 C and D). In the entorhinal cortex of 2-month-old AD11 mice, an abnormal phosphorylation of the microtubule-associated protein tau is observed in neuronal somata (Fig. 1E). At 6 months of age, the number of neurons expressing phosphotau in AD11 mice increases and the labeling extends to dendrites (Fig. 1G). Phosphotau is absent in the corresponding sections of 2- and 6-month-old control mice (Fig. 1 F and H).
Fig 1.
(A) Two-month-old AD11 mice show a decreased number of ChAT-positive neurons in the BF. (B) Control mice. (C) Increased cholinergic deficit in 6-month-old AD11 mice. (D) Age-matched control. Entorhinal cortex: (E) Abnormal localization of phosphotau in 2-month-old AD11 mice, (F) absent in age-matched controls. (G) Higher number of phosphotau-positive neurons in 6-month-old AD11 mice with respect to (H) control mice. (I) APP in vessels (arrows) of 2-month-old AD11 mice. (J) No labeling in control mice. (K) APP extracellular deposits in 6-month-old AD11 mice. (L) Age-matched control mice. (M) Aβ-positive clusters of cells in 6-month-old AD11 mice. (N) Age-matched control mice. (O) β-amyloid plaques in 15-month-old AD11 mice. These plaques are not revealed in control mice (P). (Scale bars: A–J, M, and N, 200 μm; K, L, O, and P, 50 μm.)
Two-month-old AD11 mice show an increased APP staining in the wall of some cerebral vessels (Fig. 1I, compared with a section from control mice in Fig. 1J). At 6 months of age, the number and intensity of APP-labeled vessels increases in AD11 mice, and extracellular deposits of APP start becoming visible (Fig. 1K).
In the brain of 6-month-old AD11 mice, Aβ-positive clusters of cells are observed in the hippocampus (Fig. 1 M and N, AD11 and control mice, respectively). At 15 months of age, extracellular deposition of Aβ-reactive material, forming amyloid plaques, is observed in correspondence of these Aβ-positive cells (Fig. 1O). Aβ-positive amyloid plaques are not observed in 6-month-old brains.
Pharmacological Increase of the Levels of Endogenous NGF Ameliorates Cholinergic Deficit and Reduces Tau Hyperphosphorylation.
The thyroid hormone LT4 is known to increase the expression of endogenous NGF (22). LT4 was administered daily to AD11 mice, at a very early stage of neurodegeneration (n = 12), to determine whether increasing endogenous NGF levels could prevent the loss of ChAT-positive neurons in the BF or the increase of tau hyperphosphorylation in the entorhinal cortex. The treatment increased the levels of free endogenous NGF both in peripheral organs and the brain (Fig. 2A). LT4 was effective in preventing the cholinergic deficit in the BF (Fig. 2 B, C, and E) and the increased hyperphosphorylation of tau in the soma of entorhinal cortical neurons (Fig. 2 F–H). On the other hand, LT4 treatment did not influence the increased APP expression in cerebral vessels of AD11 mice (Fig. 2 I–K).
Fig 2.
(A) Analysis of free NGF levels in the brain, blood serum, and salivary gland from control mice, AD11 mice, and AD11 mice treated with LT4 or vehicle. (B) Total number of ChAT-positive neurons in control mice, AD11 mice, and AD11 mice treated with different concentrations of LT4. Immunohistochemical study: ChAT-positive neurons in (C) 2-month-old control mice, (D) AD11 untreated mice, and (E) LT4-treated AD11 mice. Hyperphosphorylated tau in (F) 2-month-old control mice, (G) untreated AD11 mice, and (H) LT4-treated AD11 mice. APP expression in (I) 2-month-old control mice, (J) untreated AD11 mice, and (K) LT4-treated AD11 mice. Arrows point to vessels heavily stained by the anti-APP antibody. (Scale bar = 200 μm.)
A delayed start of the treatment (4 or 6 months of age instead of 1.5), even if more prolonged (from 4 to 6 months), did not affect the neurodegenerative phenotype (see Table 1).
Table 1.
Summary of the effects of NGF and GAL treatment on different markers of neurodegeneration in AD11 mice
| Treatment
|
Age of treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5–2 months | 4–6 months | 6–6.5 months | ||||||||||
| ChAT | TAU | APP | Aβ | ChAT | TAU | APP | Aβ | ChAT | TAU | APP | Aβ | |
| LT4 | ↑ | ↑ | = | NR | = | = | = | NR | = | = | = | NR |
| rhNGF | ↑ | ↓ | = | = | ↑ | ↓/= | = | ↓ | ↑ | = | = | ↓ |
| GAL | ↑ | = | ↓ | = | ↑ | = | ↓ | ↓ | ↑ | = | ↓/= | ↓ |
All comparisons were done with respect to vehicle-treated AD11 mice. NR, not reported. ↑, Increased expression after treatment. ↓, Decreased expression after treatment. ↓/=, Partial decreased expression after treatment. =, No changes with respect to control.
Rescue of the Cholinergic Loss in the BF by NGF and GAL.
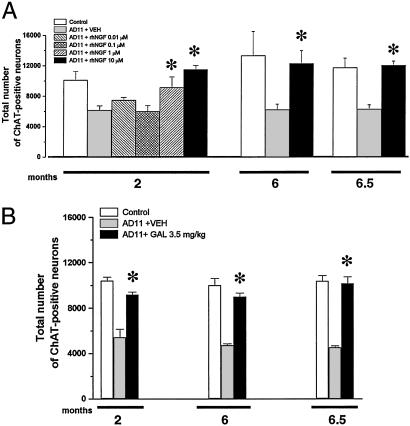
LT4 could have pleiotropic effects independent from the increase of endogenous NGF levels. Thus, we performed a direct delivery of exogenous rhNGF, by an intranasal route (23). Preliminary experiments aimed at determining the effective dose of NGF revealed that 1 μM (n = 3) and 10 μM (n = 10) (concentration in the injection solution) of rhNGF, administered every other day or every 2 days, was able to prevent the reduction of BFCNs (Fig. 3A) at 2 months of age. This finding was associated to a 2.5-fold increase of the levels of free NGF in the brain of AD11 mice (20.0 ± 0.1 ng/mg tissue versus 8.0 ng ± 0.2 ng/mg tissue in vehicle-treated AD11 mice), confirming that the intranasally delivered NGF does reach the brain effectively. The efficacy of rhNGF in preventing or rescuing the loss of BF ChAT-positive neurons was then tested at an age (6 months) in which their number in AD11 mice is 50% with respect to control mouse. rhNGF was delivered for 2 months, starting from 4 months of age (n = 5). This process results in a significant increase of the number of ChAT-positive BFCNs with respect to AD11 vehicle-treated mice (Fig. 3A). This treatment increased the body weight of rhNGF-treated animals and gave no signs of toxicity as revealed by gross histological examination (data not shown). The administration of rhNGF for 15 days starting from 6 months of age (n = 4) rescued the cholinergic deficit by reducing the loss of ChAT-positive neurons in the BF (Fig. 3A).
Fig 3.
(A) Total number of ChAT-positive neurons in control mice, AD11 mice, and AD11 mice treated with different concentrations of rhNGF. (B) Total number of ChAT-positive neurons in control mice, AD11 mice, and AD11 mice treated with GAL.
The effects of NGF were compared with those of GAL, a cholinesterase inhibitor that allosterically modulates nicotinic receptors (27, 28). GAL was administered to AD11 transgenic mice daily for 15 days starting from 1.5 months of age (n = 5). This treatment restored the number of ChAT-positive BFNs in AD11 mice (Fig. 3B). The same dose of GAL given for 2 months starting from 4 months of age (n = 6), or for 15 days starting from 6 months (n = 4), was effective in reversing the reduction of ChAT-positive neurons in the BF (Fig. 3B).
NGF, but Not GAL, Prevents the Increase of Tau Hyperphosphorylation in the Entorhinal Cortex.
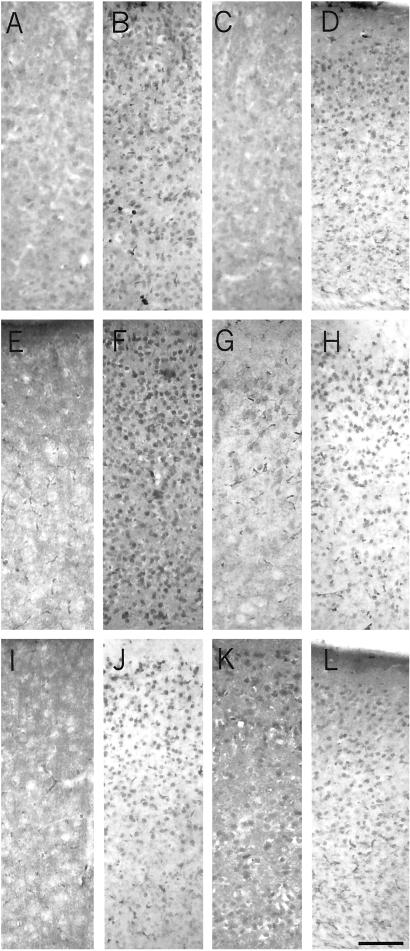
Experiments were performed to determine whether rhNGF and GAL were able to prevent or revert the increase in tau hyperphosphorylation in the cortex. Immunohistochemistry was performed on sections from rhNGF-, GAL-, and vehicle-treated AD11 mice. At 2 months of age, when control mice do not show hyperphosphorylated tau in the somatodedendritic cellular compartment (Fig. 4A), AD11 mice show an intense labeling in the neuronal body (Fig. 5B). Treatment with 10 μM rhNGF led to a significant reduction of tau hyperphosphorylation in the entorhinal cortex (n = 10) (Fig. 4C), whereas GAL had no effect on phosphotau expression (n = 5) (Fig. 4D).
Fig 4.
Hyperphosphorylated tau in 2-month-old mice is localized in the soma of neurons in the entorhinal cortex of AD11 mice (B) with respect to control mice (A). (C) At 2 months of age, the treatment with rhNGF decreases the expression in the soma. (D) The treatment with GAL does not decrease the expression in the soma. At 6 months of age, after a treatment for 2 months, rhNGF partially prevents the abnormal localization of phosphotau in the soma (G) with respect to control (E) and untreated AD11 mice (F). (H) GAL does not prevent the abnormal localization of phosphotau in the soma. At 6.5 months of age, phosphotau is not affected by rhNGF (K) or GAL (L). (J) Phosphotau distribution is comparable to those observed in untreated mice. (I) Control mice do not display labeling for phosphotau in neuronal soma. (Scale bar = 200 μm.)
Fig 5.
At 2 months of age, APP is localized in the cerebral vessel of AD11 mice (B) with respect to control mice (A). (C) At this age APP increased expression in vessels in AD11 mice is not affected by rhNGF administration. (D) At this age APP increased expression in vessels in AD11 mice is reduced by GAL administration, and staining is comparable to that observed in control mice. Arrows point to APP-positive cerebral vessels. At 6 months of age, APP is extracellularly localized in AD11 mice (F) with respect to age-matched controls (E). After a treatment of 2 months, rhNGF does not influence APP extracellular deposition (G), whereas GAL is effective in decreasing APP extracellular deposition (H). At 6.5 months of age, control mice do not display APP extracellular deposits (I), whereas AD11 mice show them (J). After a treatment for 15 days, rhNGF is unable to rescue APP deposition (K), whereas GAL partially restores APP distribution (L). Arrows point to extracellular deposits of APP. (Scale bar = 200 μm.)
NGF was also able to prevent the overall increase in tau hyperphoshorylation, when administered from 4 months to 6 months of age (n = 5), even if some neurons in layer II still showed phosphotau immunoreactivity (Fig. 4 G compared with E and F). Even after such a prolonged administration, GAL was still not effective in rescuing tau hyperphosphorylation (n = 4) (Fig. 4 H compared with E and F).
rhNGF delivered from 6 months of age for 15 days (n = 4) had no effect on the expression of phosphotau in cortical neurons of AD11 mice (Fig. 4 K compared with I and J), showing that at this age the abnormal phosphorylation process has lost the ability of being regulated by NGF.
Also GAL (n = 4) had no effect on phosphotau, when administered at 6 months of age (Fig. 4 L compared with I and J).
GAL, but Not NGF, Reduces APP Deposition in AD11 Mice.
At 2 months of age, APP expression in cerebral vessels is absent in control mice (Fig. 5A) but starts appearing in AD11 mice (Fig. 5B). rhNGF was unable to decrease cerebrovascular deposition of APP (n = 10) (Fig. 5 C compared with A and B). On the contrary, APP vascular deposition was markedly reduced after the administration of GAL for 15 days at 2 months of age (n = 5) (Fig. 5D). The treatment with rhNGF for 2 months, starting at 4 months, before the onset of any visible APP extracellular deposition (n = 5) (Fig. 5 G compared with E and F) or its administration for 15 days at 6 months of age, after the beginning of amyloid deposition (n = 4) (Fig. 5 K compared with I and J) proved to be ineffective in preventing or reverting APP-linked neuropathology. APP burden in 6-month-old AD11 mice was 3.5 ± 0.1, versus 0.5 ± 0.8 in control mice and 3.6 ± 0.9 in NGF-treated 6-month-old AD11 mice.
The treatment with GAL for 2 months, starting from 4 months of age, markedly reduced APP extracellular deposition in AD11 mice (n = 5) (Fig. 5 H compared with E and F), leading to an APP burden of 0.62 ± 0.2 (P < 0.01).
Treatment with GAL for 15 days, starting from 6 months of age, partially improved APP deposition [n = 4; Fig. 6L, APP burden of 2.62 ± 0.3 (P < 0.05)], when compared with AD11 vehicle-treated mice (Fig. 5J, APP burden of 3.8 ± 0.5) and control mice (Fig. 5I, APP burden of 0.5 ± 0.4). rhNGF was ineffective also at this age, leaving an APP burden of 3.26 ± 0.035 (Fig. 5K).
Fig 6.
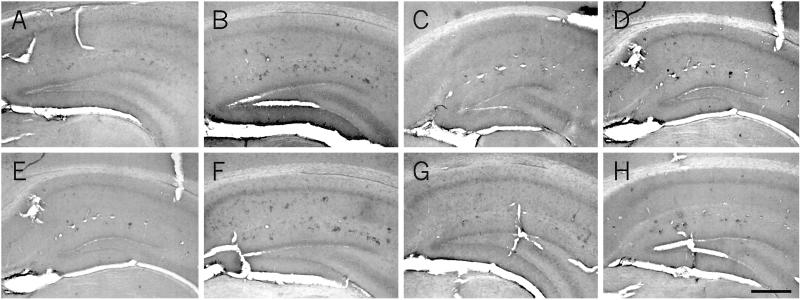
At 6 months of age, Aβ is localized in the soma of cells forming clusters in the hippocampus of AD11 mice (B) and is absent in control mice (A). At this age, treatment with rhNGF (C) and GAL (D) decreases the number of Aβ-positive clusters of cells. A more prolonged treatment, started at 4 months of age, further decreases the number of clusters in AD11-treated mice (G) compared with control mice (E) and untreated mice (F). (H) Treatment with GAL does not result in a further decrease of the number of Aβ-positive clusters of cells. (Scale bar = 100 μm.)
rhNGF and GAL Rescue the Intracellular Deposition of Aβ in AD11 Mice.
At 6 months of age AD11 mice show the presence of clusters of cells, intensely labeled by anti-Aβ antibodies (Fig. 6 B and F), whereas control mice show no intracellular or extracellular labeling for Aβ (Fig. 6 A and E). These clusters of Aβ-positive cells appear to be the sites were the extracellular deposits of Aβ develop, in the aged AD11 brain (refs. 19 and 20 and unpublished data). Stereological counts on hippocampal sections revealed that the mean number of clusters observed in 6-month-old AD11 mice is 21 ± 2 versus 0 ± 0.01 in age-matched control mice. The treatment with rhNGF (Fig. 6C) and GAL (Fig. 6D) for 15 days, starting from 6 months of age, markedly decreased the number of Aβ-positive clusters in AD11 mice (6 ± 1 with NGF and 7 ± 2 with GAL). The administration rhNGF earlier on (4 months of age) and for a more prolonged period (2 months) resulted in a more pronounced reduction in the number of clusters (1 ± 0.01) (Fig. 6G). This effect was not observed with GAL (6 ± 2) (Fig. 6H).
The overall results are summarized in Table 1.
Discussion
Multiple factors can be part of the cascade of events leading to the common core of neuronal degeneration in AD (29, 30). Early-onset familial AD involves more than 100 different rare, highly penetrant, autosomal-dominantly transmitted mutations in three genes (31). On the other hand, in the much more frequent cases of sporadic AD (more than 90% of AD cases), the causal links between the different risk factors involved and their role in shaping possible pathways leading to the central core of AD pathology have remained elusive, and a unitary theory that can account for all of the clinical and neuropathological features has failed to emerge (30). The difficulty in generating a comprehensive mouse model for human AD, based on the expression of one or combinations of human mutant genes for familial AD, represents a significant gap toward our understanding of the mechanisms leading to AD (32), and this is particularly true for mechanisms that link the amyloid, tau, and cholinergic sides of the disease (33).
For this reason, it is particularly significant that AD11 mice expressing anti-NGF antibodies (18) develop an age-dependent neurodegeneration that is highly reminiscent of AD (19–21). This finding strengthens the previously suggested connection between NGF and AD (34, 35) and shows that reducing the availability of NGF to its target cells (14, 19, 36, 37) can represent one significant pathway leading to sporadic AD.
In this study we assessed the ability of pharmacological treatments of AD11 mice with NGF or cholinergic agonists to reverse the early phases of the progressive neurodegenerative phenotype, before the onset of the full-blown neurodegeneration that is observed in aged mice.
To facilitate the delivery and the access of NGF to the brain, we adopted a noninvasive olfactory route (23) and demonstrated an uptake of NGF from its receptors in the olfactory epithelium to the BF and other brain regions.
NGF exerts a significant protection against loss of BFCNs, increase of tau hyperphosphorylation in the cortex, and intracellular accumulation of Aβ in the hippocampus. These results offer an important and conclusive additional validation to the concept that the neurodegeneration observed in AD11 mice results from the inhibition of NGF activity by the transgenic antibody. It is noteworthy that, at all ages tested, rhNGF was unable to reduce the increase of cerebrovascular APP expression or to decrease the number of extracellular deposits of APP (Table 1).
The results obtained with GAL are somewhat complementary to the NGF results. GAL has the same protective effect on the BF cholinergic deficit. However, GAL had no effect on tau hyperphosphorylation, whereas it interfered successfully with the formation of extracellular deposition of APP.
Both NGF and GAL were effective in reducing the number of Aβ-positive clusters of cells in the hippocampus, with NGF inducing a more complete protection than GAL. The characterization of these cells will be important to learn more about how interfering with the NGF signaling leads to an altered processing of APP, to the formation of intracellular Aβ in these cells, and eventually, in the older animals (19, 20), to the formation of extracellular deposits around these cells.
Thus, intranasally delivered NGF is able to fully revert all of the phenotypic markers of neurodegeneration tested, with the exception of the increase of cerebrovascular APP and the extracellular APP deposits. This finding might have different explanations. First, the increase in cerebrovascular APP expression, and the ensuing formation of extracellular APP deposition, might be a peripheral effect, linked to effects by the anti-NGF antibodies present in the blood stream (18) on the sympathetic innervation of blood vessels and/or on vascular smooth muscle cells expressing TrkA receptors (38). Accordingly, the NGF delivered intranasally does not have access to the peripheral pool of transgenic anti-NGF antibodies, but only to the pool present in the central nervous system and therefore would not be able to revert any effect exerted by the anti-NGF antibodies outside of the central nervous system.
Second, the increase in cerebrovascular APP might lose very rapidly its initial dependence on the inducing causes, i.e., a reduced NGF activity caused by the anti-NGF antibodies, and therefore would become insensitive to NGF itself.
Third, the onset of this particular subset of APP-linked phenotype might be related not only to the neutralization of NGF activity per se, but also to the presence of NGF–anti-NGF complexes in the central nervous system, thus explaining why the delivery of NGF does not result in an effective reversion of this particular marker.
Specific experiments addressing these well defined possibilities will help in the understanding of the mechanisms of anti-NGF-induced neurodegeneration. The reasons the increase of cerebrovascular APP and APP extracellular deposition can be rescued by GAL and not by NGF remain to be determined and may be linked to the relative strength of NGF and cholinergic influences on APP metabolism (39).
Another major conclusion from this study is that the complex neurodegeneration induced by anti-NGF antibodies in AD11 mice might be a multisite process, involving the neutralization of NGF actions at different levels, including BFCNs, cortical cells expressing TrkA and p75 receptors, and the cerebral vascular system (38). Thus, a double stream emerges in the development of AD11 neurodegeneration, one encompassing the cholinergic system and the regulation of tau phosphorylation (S.C. and A.C., unpublished data), and the second one involving, again, the cholinergic system and some aspects of the regulation of APP processing (39).
The rescue of the cholinergic phenotype by NGF treatment is not surprising, given the known actions of NGF on these cells (5–7, 40). This rescue involves not only the phenotypic expression of ChAT, but also the increase in TrkA-positive viable cells in the BF (data not shown). The rescue of the (presynaptic) cholinergic deficit in AD11 mice by GAL is noteworthy, in light of its dual activity, being both an AChE inhibitor (27) and an allosteric potentiating ligand on the nicotinic response by acetylcholine and competitive agonists (28, 41). These effects by GAL can be interpreted in the framework of the suggested reciprocal interactions between cholinergic afferents and NGF-producing cells in the hippocampus and the cortex (42, 43), according to which there would be a positive reinforcing loop whereby acetylcholine release elevates NGF production and then NGF affects acetylcholine neurons to provide trophic support and further enhance acetylcholine synthesis and release (44).
In line with this conclusion, we can exclude that amelioration of AD-like phenotype in AD11 mice by GAL injections is caused by AChE inhibition, but rather to its nicotinic agonist activity, also because the administration of the powerful AChE inhibitors tacrine and physostigmine to AD11 mice was shown to be ineffective (S.C. and A.C., unpublished observation). Thus, our results allow us to correlate the effects obtained on the AD11 model with the therapeutic value of pharmacological agents.
The hyperphosphorylation of tau in the cortex appears to be directly linked to NGF deprivation, rather than to the ensuing cholinergic deficit, because it is rescued by NGF, but cannot be rescued by GAL. Indeed, there are precedents showing that NGF deprivation in PC12 cells determines an increase in the level of hyperphosphorylated tau (45). Thus, an altered signaling by the NGF/TrkA/p75 system in cortical cells might disrupt the phosphorylation balance of tau, triggering more generalized effects on the neuronal cytoskeleton downstream. The effects of NGF may be mediated by changes either in protein phosphatase/kinase activities or in the accessibility of tau to these enzymatic activities. Further experiments are needed to clarify these aspects of NGF interaction with tau protein.
It remains to be seen whether a combined treatment with NGF and GAL could complete the rescue obtained with rhNGF on BFNCs, tau hyperphosphorylation, and Aβ intracellular accumulation with the reduction of APP deposition (as achieved by GAL).
The ability of rhNGF or GAL to exert their rescuing effects in AD11 mice differs significantly in relation to the age of treated mice. Our study suggests that in AD11 mice a critical time window exists in which some aspects of the AD-like neurodegeneration can be reverted before the full neurodegeneration becomes irreversible.
In conclusion, we showed that the neurodegeneration induced by anti-NGF antibodies in AD11 mice can be largely reversed by NGF delivery. The pharmacological experiments revealed the existence of a double stream, leading to the final AD-like neurodegeneration observed in aged mice. Thus, the AD11 mice promise to become an important experimental system, not only for understanding the molecular mechanisms leading to neurodegeneration per se but, also, for predicting the outcome of clinical trials of new potential therapeutic agents.
Acknowledgments
We thank Dr. Marco Stebel and the animal house staff at the University of Trieste for management of the mouse colony. We are grateful to Prof. Gennaro Schettini and Dr. Claudio Russo for providing the anti-NH2 terminus antibody. This work was partially supported by Telethon Grants E.1044 and D.122.
Abbreviations
AD, Alzheimer's disease
APP, amyloid precursor protein
BFCN, basal forebrain cholinergic neuron
ChAT, choline acetyl transferase
GAL, galantamine hydrobromide
NGF, nerve growth factor
rhNGF, recombinant human NGF
References
- 1.Bartus R. T. (2000) Exp. Neurol. 163, 495-529. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Montalcini R. (1952) Ann. N.Y. Acad. Sci. 55, 330-343. [DOI] [PubMed] [Google Scholar]
- 3.Levi-Montalcini R. (1987) Science 237, 1154-1162. [DOI] [PubMed] [Google Scholar]
- 4.Sofroniew M. V., Howe, C. L. & Mobley, W. C. (2001) Annu. Rev. Neurosci. 24, 1217-1281. [DOI] [PubMed] [Google Scholar]
- 5.Mobley W. C., Rutkwoski, J. L., Tennekoon, G. I., Gemski, J., Buchanan, K. & Johnston, M. V. (1986) Mol. Brain Res. 1, 53-52. [DOI] [PubMed] [Google Scholar]
- 6.Hefti F. (1986) J. Neurosci. 6, 2155-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer W., Wictorin, K., Bjorklund, A., Williams, L. R., Varon, S. & Gage, F. H. (1987) Nature (London) 329, 65-68. [DOI] [PubMed] [Google Scholar]
- 8.Tuszynski M. H., Sang, H., Yoshida, K. & Gage, F. H. (1991) Ann. Neurol. 30, 625-636. [DOI] [PubMed] [Google Scholar]
- 9.Smith D. E., Roberts, J., Gage, F. H. & Tuszynski, M. H. (1999) Proc. Natl. Acad. Sci. USA 96, 10893-10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowska A. L., Koliatsos, V. E., Breckler, S. J., Price, D. & Olton, D. S. (1994) J. Neurosci. 14, 4815-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jette N., Cole, M. S. & Fahnestock, M. (1994) Mol. Brain Res. 25, 242-250. [DOI] [PubMed] [Google Scholar]
- 12.Goedert M., Fine, A., Hunt, S. P. & Ullrich, A. (1986) Brain Res. 387, 85-92. [DOI] [PubMed] [Google Scholar]
- 13.Crutcher K. A., Scott, S. A., Liang, S., Everson, W. V. & Weingartner, J. (1993) J. Neurosci. 13, 2540-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mufson E. J., Conner, J. M. & Kordower, J. H. (1995) NeuroReport 6, 1063-1066. [DOI] [PubMed] [Google Scholar]
- 15.Scott S. A., Mufson, E. J., Weingartner, J. A., Skau, K. A. & Crutcher, K. A. (1995) J. Neurosci. 15, 6213-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahnestock M., Scott, S. A., Jette, N., Weingartner, J. A. & Crutcher, K. A. (1996) Mol. Brain Res. 42, 175-178. [DOI] [PubMed] [Google Scholar]
- 17.Mufson E. J., Kroin, J. S., Sendera, T. J. & Sobreviela, T. (1999) Prog. Neurobiol. 57, 451-484. [DOI] [PubMed] [Google Scholar]
- 18.Ruberti F., Capsoni, S., Comparini, A., Di Daniel, E., Franzot, J., Gonfloni, S., Rossi, G., Berardi, N. & Cattaneo, A. (2000) J. Neurosci. 20, 2589-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capsoni S., Ugolini, G., Comparini, A., Ruberti, F., Berardi, N. & Cattaneo, A. (2000) Proc. Natl. Acad. Sci. USA 97, 6826-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capsoni, S., Giannotta, S. & Cattaneo, A. (2002) Mol. Cell. Neurosci., in press. [DOI] [PubMed]
- 21.Pesavento E., Capsoni, S., Domenici, L. & Cattaneo, A. (2002) Eur. J. Neurosci. 15, 1-9. [DOI] [PubMed] [Google Scholar]
- 22.Watson A. Y., Radie, K., McCarthy, M., Reed Larsen, P. & Murphy, R. A. (1982) Endocrinology 110, 1392-1401. [DOI] [PubMed] [Google Scholar]
- 23.Frey W. H., Liu, J., Chen, X., Thorne, R. G., Fawcett, J. R., Ala, T. A. & Rahman, Y. E. (1997) Drug Delivery 4, 87-92. [Google Scholar]
- 24.Russo C., Salis, S., Dolcini, V., Venezia, V., Song, X. H., Teller, J. K. & Schettini, G. (2001) Neurobiol. Dis. 8, 173-180. [DOI] [PubMed] [Google Scholar]
- 25.Gonfloni S., (1995) Ph.D. thesis (International School for Advanced Studies, Trieste, Italy).
- 26.Schenk D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., et al. (1999) Nature (London) 400, 173-177. [DOI] [PubMed] [Google Scholar]
- 27.Bores G. M., Huger, F. P., Petko, W., Mutlib, A. E., Camacho, F., Rush, D. K., Selk, D. E., Wolf, V., Kosley, R. W., Jr., Davis, L., et al. (1996) J. Pharmacol. Exp. Ther. 277, 728-738. [PubMed] [Google Scholar]
- 28.Schrattenholz A., Pereira, E. F., Roth, U., Weber, K. H., Albuquerque, E. X. & Maelicke, A. (1996) Mol. Pharmacol. 49, 1-6. [PubMed] [Google Scholar]
- 29.Iqbal K. & Grundke-Iqbal, I. (2000) Neurobiol. Aging 21, 901-902. [DOI] [PubMed] [Google Scholar]
- 30.Mesulam M. M. (1999) Neuron 24, 521-529. [DOI] [PubMed] [Google Scholar]
- 31.Tanzi R. E. & Bertram, L. (2001) Neuron 32, 181-184. [DOI] [PubMed] [Google Scholar]
- 32.Janus C. & Westaway, D. (2001) Physiol. Behav. 73, 873-886. [DOI] [PubMed] [Google Scholar]
- 33.Mudher A. & Lovestone, S. (2002) Trends Neurosci. 25, 22-26. [DOI] [PubMed] [Google Scholar]
- 34.Hefti F. & Weiner, W. J. (1986) Ann. Neurol. 20, 275-281. [DOI] [PubMed] [Google Scholar]
- 35.Scott S. A. & Crutcher, K. A. (1994) Rev. Neurosci. 5, 179-211. [DOI] [PubMed] [Google Scholar]
- 36.Fahnestock M., Michalski, B., Xu, B. & Coughlin, M. D. (2001) Mol. Cell. Neurosci. 18, 210-220. [DOI] [PubMed] [Google Scholar]
- 37.Lee R., Kermani, P., Teng, K. K. & Hempstead, B. L. (2001) Science 294, 1945-1948. [DOI] [PubMed] [Google Scholar]
- 38.Donovan M. J., Miranda, R. C., Kraemer, R., McCaffrey, T. A., Tessarollo, L., Mahadeo, D., Sharif, S., Kaplan, D. R., Tsoulfas, P., Parada, L., et al. (1995) Am. J. Pathol. 147, 309-324. [PMC free article] [PubMed] [Google Scholar]
- 39.Rossner S., Ueberham, U., Schliebs, R., Perez-Polo, J. R. & Bigl, V. (1998) Prog. Neurobiol. 56, 541-569. [DOI] [PubMed] [Google Scholar]
- 40.Molnar M., Tongiorgi, E., Avignone, E., Gonfloni, S., Ruberti, F., Domenici, L. & Cattaneo, A. (1998) Eur. J. Neurosci. 10, 3127-3140. [DOI] [PubMed] [Google Scholar]
- 41.Maelicke A. & Albuquerque, E. X. (2000) Eur. J. Pharmacol. 393, 165-170. [DOI] [PubMed] [Google Scholar]
- 42.Knipper M., da Penha Berzaghi, M., Blochl, A., Thoenen, H. & Lindholm, D. (1994) Eur. J. Neurosci. 4, 668-671. [DOI] [PubMed] [Google Scholar]
- 43.Yu J., Pizzo, D. P., Hutton, L. A. & Perez-Polo, J. R. (1995) Brain Res. 705, 247-252. [DOI] [PubMed] [Google Scholar]
- 44.French S. J., Humby, T., Horner, C. H., Sofroniew, M. V. & Rattray, M. (1999) Brain Res. Mol. Brain Res. 67, 124-136. [DOI] [PubMed] [Google Scholar]
- 45.Nuydens R. (1997) Biochem. Biophys. Res. Commun. 240, 687-691. [DOI] [PubMed] [Google Scholar]