Abstract
Single painful stimuli evoke two successive and qualitatively distinct sensations referred to as first and second pain sensation. Peripherally, the neural basis of this phenomenon is a dual pathway for pain with Aδ and C fibers mediating first and second pain, respectively. Yet, the differential cortical correlates of both sensations are largely unknown. We therefore used magnetoencephalography to record and directly compare first and second pain-related cortical responses to cutaneous laser stimuli in humans. Our results show that brief painful stimuli evoke sustained cortical activity corresponding to sustained pain perception comprising early first pain-related and late second pain-related components. Cortical activity was located in primary (S1) and secondary (S2) somatosensory cortices and anterior cingulate cortex. Time courses of activations disclosed that first pain was particularly related to activation of S1 whereas second pain was closely related to anterior cingulate cortex activation. Both sensations were associated with S2 activation. These results correspond to the different perceptual characteristics of both sensations and probably reflect different biological functions of first and second pain. First pain signals threat and provides precise sensory information for an immediate withdrawal, whereas second pain attracts longer-lasting attention and motivates behavioral responses to limit further injury and optimize recovery.
It is a unique perceptual phenomenon that single painful stimuli yield two successive and qualitatively distinct sensations referred to as first and second pain sensation (1–4). First pain is brief, pricking, and well localized, whereas second pain is longer-lasting, burning, and less well localized. Peripherally, the neural basis of this phenomenon is a dual pathway for pain with Aδ and C fibers mediating first and second pain, respectively (2, 3). Different conduction velocities of both fiber types of about 10–20 and 1 m/s (5, 6) account for the temporal sequence of both sensations with reaction times to first pain of 400–500 ms and to second pain of about 1,000 ms after application of painful stimuli to the hand (1, 2, 4, 7).
The biological functions and the differential cortical correlates of first and second pain are less well known. Anatomical, physiological, and lesion studies in humans and animals have revealed an extensive cortical network associated with sensory, cognitive, and affective aspects of pain (for review, see ref. 8). This network consistently includes primary (S1) and secondary (S2) somatosensory cortices, insular cortex, and anterior cingulate cortex (ACC). However, only a few studies disentangled Aδ and C fiber activations and, thus, first and second pain. Neurophysiological recordings in humans revealed early Aδ fiber-mediated activations in S1, S2, and ACC (for review, see ref. 8), whereas C fiber-mediated cortical responses at latencies of about 1,000 ms have been shown in scalp recordings (9–13) but have not yet been consistently localized. Conversely, functional imaging studies using tonic C fiber stimuli demonstrated activation of S1, S2, Insula, and ACC (14–17) but did not provide temporal information. Thus, the temporal sequence and a differential involvement of Aδ and C fiber-mediated cortical activations related to first and second pain remains to be demonstrated.
Here, we used magnetoencephalography (MEG) to record and compare early Aδ fiber- mediated and late C fiber-mediated cortical responses to single painful cutaneous laser stimuli in healthy human subjects. We directly demonstrate the differential cortical correlates of first and second pain. These findings probably reflect the different perceptual characteristics and biological functions of first and second pain.
Methods
Ten healthy male subjects with a mean age of 31 years (range, 22–38 years) participated in the study. Informed consent was obtained from all subjects before participation. The study was approved by the local ethics committee and conducted in conformity with the declaration of Helsinki. Forty painful cutaneous laser stimuli, which have been shown to activate selectively nociceptive Aδ and C afferents (18), were delivered to the dorsum of the right hand. The laser device was a Tm:YAG-laser (Carl Baasel Lasertechnik, Starnberg, Germany) with a wavelength of 2,000 nm, a pulse duration of 1 ms, and a spot diameter of 6 mm. The laser beam was led through an optical fiber from outside into the recording room. Stimulation site was slightly changed within an area of 4 × 3 cm after each stimulus. Interstimulus intervals were randomly varied between 10 and 14 s. Applied stimulus intensity was 600 mJ evoking moderately painful sensations.
Continuous pain ratings were obtained in four subjects. These measurements were done separately from the MEG recordings to prevent confounding effects of motor- and stimulus-related activation. Subjects were instructed to rate continuously stimulus intensity with the finger span of thumb and index of the left hand while stimuli were applied to the right hand. Minimal finger span was defined as no pain and maximal finger span as worst tolerable pain. Positions of fingertips of thumb and index were tracked by an ultrasound-based motion analysis system (Zebris Medizintechnik, Tübingen, Germany) with a sampling rate of 50 Hz. Euclidean distance of fingertips as a function of time was normalized to individual maximal finger span and averaged with respect to laser stimuli. To familiarize subjects with the rating procedure at least 10 stimuli were applied before the recordings started.
Cortical activity was recorded with a Neuromag-122 whole-head neuromagnetometer containing 122 planar SQUID gradiometers (19) in a magnetically shielded room. Signals were digitized at 483 Hz, high-pass filtered at 0.03 Hz, and low-pass filtered at 20 Hz. Neuromagnetic activity was averaged time-locked to application of laser stimuli. Vertical and horizontal electrooculograms were used to reject epochs contaminated with blink artifacts and eye movements. An epoch comprising 1,000 ms prestimulus baseline and 3,000 ms after stimulation was analyzed. In each subject, global stimulus-evoked neuromagnetic activity was calculated as mean rectified signal of all 122 sensors. Cortical activity was localized during two time windows reflecting Aδ fiber-mediated first pain-related and C fiber-mediated second pain-related activity, respectively. The early time window had a duration of 100 ms and was individually centered around first peak of global stimulus-evoked activity resulting in time windows between 100–200 and 150–250 ms. The late time window had a duration of 1,000 ms and uniformly ranged from 500 to 1,500 ms. For both time windows covariance matrices across all sensors were calculated. From these covariance matrices pain-evoked activity was localized by using a spatial filtering algorithm (20). The spatial filter was used with a realistic head model to estimate power in the whole brain resulting in individual tomographic power maps with voxel sizes of 6 × 6 × 6 mm. This approach is a time-domain variant of the frequency-based dynamic imaging of coherent sources method, which was recently introduced to the investigation of oscillatory activity (21). Further processing of tomographic power maps was performed by using SPM99 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, www.fil.ion.ucl.ac.uk/spm). Individual maps were spatially normalized to Talairach space by using parameters derived from normalization of individual T1-weighted magnetic resonance images (22). Mean group normalized power maps were calculated for both time windows. From these mean power maps locations of activations defined as local cortical power maxima exceeding 80% of the global maximum were determined. Time courses of activations were individually determined from a spatiotemporal source model with fixed locations and orientations where activation strengths were allowed to vary over time to provide the best fit for the recorded data (23). For source models, locations of activations were individual power maxima within the cortical areas defined previously from the mean group maps. On the basis of resulting activation strengths as a function of time group mean time courses of activations were calculated. For each area 95% confidence intervals of activation were calculated from the 1,000-ms prestimulus baseline. Mean amplitudes of activations were determined in both time windows and an activation ratio early/late was calculated. Friedman's analysis of variance and two-tailed Wilcoxon signed rank tests were used for statistical comparison of activation ratios.
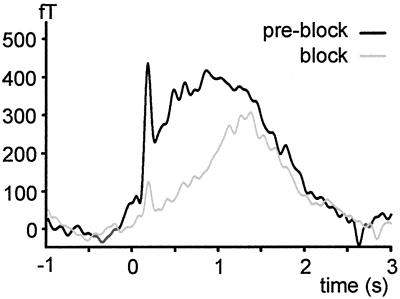
In two subjects, mediation of early and late cortical activity by Aδ and C fibers, respectively, was verified by a selective pressure block of myelinated A fibers. In this procedure compression of the superficial branch of the radial nerve is exerted by a band across the forearm loaded with a weight. By using microneurographic recordings in humans this procedure has been shown to yield a preferential block of myelinated A fibers (24, 25; see ref. 26 for details of the procedure). Conduction block of A fibers was monitored by tactile v.Frey-hair stimuli and nociceptive cutaneous laser stimuli. MEG measurements were started when tactile perception and first pain sensation was abolished but second pain was preserved. This continuation was confirmed by verbal report and an increase in mean reaction time from 378 to 1,148 ms. A fiber block differentially affected early and late activity. Early activity was substantially more attenuated than late activity, as indicated by a decrease in activation ratio early/late from 0.93 to 0.50 (Fig. 1). Thus, Aδ and C fibers most likely mediate early and late activity, respectively.
Fig 1.
Effect of A fiber pressure block on global stimulus-evoked neuromagnetic activity calculated as mean rectified signal of all sensors corrected to baseline in a single subject.
Results
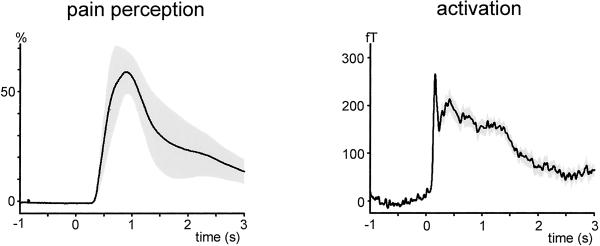
Group mean time courses of pain perception (Left) and of stimulus-evoked global neuromagnetic activity (Right) are shown in Fig. 2. Painful laser stimuli evoked sustained pain perception and sustained neuromagnetic activity. Both parameters show an initial peak within the first 1,000 and 500 ms after stimulus application, respectively, and a longer-lasting later slowly decreasing component. Mean peak intensity of pain was nearly 60% of maximal intensity defined as worst tolerable pain.
Fig 2.
Time courses of stimulus perception and global stimulus-evoked neuromagnetic activity. Time course of stimulus perception was continuously rated by thumb–index finger span of the left hand while the right hand was stimulated. One hundred percent is defined as maximal distance between fingers corresponding to worst tolerable pain. Time course of stimulus-evoked neuromagnetic activity was calculated as mean rectified signal of all sensors corrected to baseline. Rating and MEG signals were recorded separately under the same experimental conditions, except that during the MEG recordings no rating was required. Data were averaged across 4 and 10 subjects, respectively. Shaded areas indicate SEM.
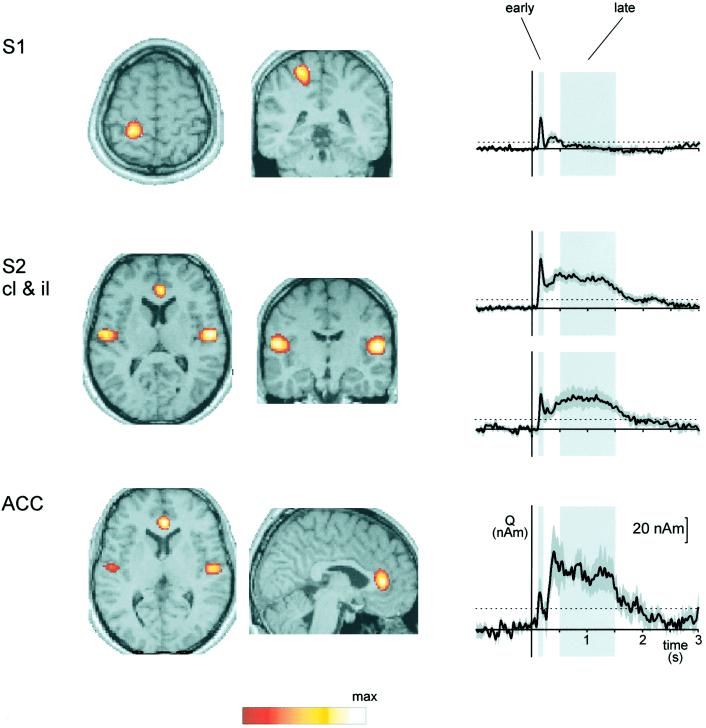
Fig. 3 summarizes locations and time courses of pain-evoked cortical activations. Activations were located in the contralateral postcentral gyrus (S1), in the upper banks of the Sylvian fissures bilaterally (S2), and in the ACC. Location of S1 activation was obtained from the early time window, and locations of ACC and bilateral S2 activations from the late time window. Coordinates of activations in Talairach space are given in Table 1. Coordinates of S1 and S2 activations correspond well to results from our previous investigations in early cortical responses to painful laser stimuli (27, 28).
Fig 3.
Locations and time courses of pain-evoked activations. Locations of activations are maxima of mean normalized power maps superposed on a normalized structural T1 weighted magnetic resonance image. Power was normalized to the local power maximum coded in white. For the sake of clarity scaling is different for each area. In the time course profiles light-shaded areas depict early and late time windows with predominantly Aδ fiber-mediated first pain-related and predominantly C fiber-mediated second pain-related activity, respectively. Dark-shaded areas indicate SEM. The dotted lines show 95% confidence intervals of activation for each area. In S1, ipsilateral S2, and ACC in one, two, and four subjects, respectively, no individual power maxima corresponding to the mean power maxima were identified. Thus, calculation of group mean activation time courses in S1, ipsilateral S2, and ACC was based on nine, eight, and six subjects, respectively. S1, primary somatosensory cortex; S2, secondary somatosensory cortex; ACC, anterior cingulate cortex; cl, contralateral; il, ipsilateral.
Table 1.
Locations and latencies of cortical activations to painful laser stimulation
| Region
|
Location x, y, z, mm
|
Latency, ms | |
|---|---|---|---|
| Early | Late | ||
| S1 | −24, −40, 62 | 164 ± 8 | |
| S2 cl | −54, −14, 14 | 161 ± 6 | 874 ± 104 |
| S2 il | 54, −14, 10 | 169 ± 4 | 1,057 ± 103 |
| ACC | 4, 34, 16 | 188 ± 20 | 782 ± 156 |
Locations are coordinates in Talairach space. Latencies are mean peak latencies ± SEM. S1, primary somatosensory cortex; S2, secondary somatosensory cortex; ACC, anterior cingulate cortex; cl, contralateral; il, ipsilateral.
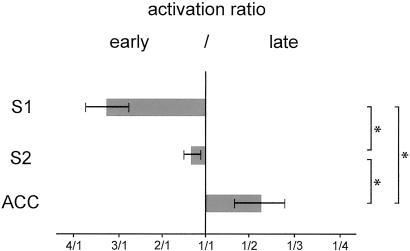
In the early time window, the time courses of activations show significant activation of S1, bilateral S2, and ACC reflecting Aδ fiber-mediated and first pain-related activation of these areas. In the late time window bilateral S2 and ACC show strong activations, whereas no significant activation is seen in S1 indicating C fiber-mediated and second pain-related activation of bilateral S2 and ACC but not of S1. Fig. 4 summarizes the relationships between early and late activations in S1, S2, and ACC. Activation ratios differ significantly between all areas (P < 0.05). S1 shows stronger early than late activation, S2 has a balanced activation pattern, and ACC shows stronger late than early activation. Peak latencies of activations in both time windows are given in Table 1.
Fig 4.
Activation ratio early/late of each area. Ratios were calculated from mean amplitudes in early and late time windows shown in Fig. 3. Error bars represent SEM. Friedman's analysis of variance showed a significant effect of area on activation ratio (P < 0.05). Brackets indicate statistical comparisons between activation ratios with two-tailed Wilcoxon signed rank tests. *, P < 0.05.
Discussion
In the present study, we investigated the cortical representation of first and second pain sensation to single painful stimuli in humans. By using a continuous pain-rating procedure and magnetoencephalography our results demonstrate that brief painful laser stimuli evoke sustained pain perception and sustained cortical activity comprising Aδ fiber-mediated first pain and C fiber-mediated second pain. Localization of activity revealed activation of contralateral S1, bilateral S2, and ACC. Time courses of activations disclosed differential temporal activation patterns of these areas. S1 showed a strong predominance of first pain-related activation whereas ACC displayed a strong predominance of second pain-related activation. S2 was about equally activated during first and second pain. These differences in cortical representation probably reflect perceptual and functional differences between first and second pain.
Mediation of early and late pain-evoked activations by Aδ and C fibers is in accordance with conduction velocities of both fiber types of about 10–20 and 1 m/s, respectively (5, 6). In addition, our early and late time windows correspond well to latencies of Aδ and C fiber-mediated cortical responses in previous electroencephalographic studies (9–13) and to reaction times to first and second pain of about 400–500 and 1,000 ms, respectively (1, 2, 4, 7). Taken together, these points strongly suggest that early and late responses reflect perception of first and second pain, respectively. This suggestion is corroborated by the results of the pressure block condition and the correspondence between time courses of pain perception and cortical activation. Thus, a contribution of Aδ fiber-mediated responses to late activations seems very unlikely, although it cannot ultimately be ruled out.
Our finding of participation of S1, bilateral S2, and ACC in human pain processing is in accordance with results from experimental animal studies and neurophysiological, functional imaging and lesion studies in humans (8). However, studies differentially investigating projections of nociceptive Aδ and C fibers are scarce and do not provide consistent evidence on the cortical representation of first and second pain. In humans, neurophysiological recordings revealed Aδ fiber-mediated responses in S1, bilateral S2, and ACC (8), whereas C fiber-mediated responses have not yet been consistently localized. Conversely, neurophysiological investigations in rats revealed C fiber-mediated responses in S1 (29–31), but Aδ fiber-mediated responses in S1 were successfully recorded in only one of these studies (29, 30). So far, the limited temporal resolution of functional imaging does not allow for direct investigation of the temporal sequence of first and second pain-related activations. In a few studies activations to selective C fiber stimulation were investigated. These studies demonstrated activations of S1 and ACC, whereas activation of S2 was inconsistently observed (14–17). However, in these studies tonic painful stimuli were applied which most probably yield activations that reflect a mixture of bottom-up and top-down processes and comprise complex pain-coping strategies and perceptual and physiological phenomena like temporal summation (4) and wind-up (32). Thus, these results probably reflect neural mechanisms distinct from the sequential first and second pain-related activations to single painful stimuli in the present study. Conversely, because cutaneous laser stimulation selectively activates nociceptors responding to heat, the present findings do not necessarily apply to all nociceptive fiber types.
Converging evidence from experimental animal studies and neurophysiological, functional imaging and lesion studies in humans indicate an essential role of S1 in the sensory-discriminative aspects of pain (for reviews, see refs. 8 and 33; for most recent studies, see refs. 34–36). Thus, our finding of strong first pain-related but a virtual lack of second pain-related activation of S1 probably reflects the different perceptual characteristics of first and second pain. First pain is of short duration, sharp and well localized, whereas second pain is longer-lasting, diffuse, and ill localized (1–4). The lack of significant second pain-related activation in S1 in the present study might also contribute to the understanding of divergent results from functional imaging studies concerning participation of S1 in human pain processing (for review, see ref. 37). Partial failure to detect S1 activation was taken as evidence against participation of S1 in pain processing by some investigators and attributed to cognitive modulation of S1 activity and to inhibitory effects within S1 by others (37). The present results add another argument. The strong but short S1 activation is less likely to be detected by single photon emission computed tomography, positron-emission tomography, or functional MRI than the longer-lasting activation of S2 and ACC.
On the basis of response characteristics, anatomical connections, and lesion studies, S2 has been suggested to be involved in cognitive–evaluative components of pain perception like recognition, learning, and memory of painful events (for reviews, see refs. 8 and 38). Our result of about equal first and second pain-related activation of S2 suggests that the recognition of the painful nature of the stimulus and pain-related learning and memory are relevant to both first and second pain. In the present study, as in other MEG studies, activation of insular cortex that has also been shown to participate in pain processing has not been detected most probably because of a mainly radial orientation of insular currents not detected by MEG and a cancellation of currents in the opposite walls of the insula. However, although pain-evoked insular activations have been shown to be located more anteriorly than S2, in principle, a small contribution of insular activation to the S2 signals cannot be ruled out.
A close association between ACC as a part of the limbic system and affective-motivational components of pain perception has been indicated by experimental animal and human lesion, functional imaging, and opioid-binding studies (for reviews, see refs. 8 and 39; for more recent evidence, see refs. 40 and 41). Thus, our finding of particular strong second pain-related activation of ACC supports an association between second pain and pain affect. However, ACC has been shown to participate in a variety of tasks involving cognitive, attention-related, and motor control processes (for reviews, see refs. 42 and 43). Thus, ACC may have a role in interrelating pain affect, attention, and motor responses (44). This role might be reflected by results from functional imaging studies showing more than one pain-evoked activation focus with different stimulus–response functions within ACC (45–49). These activation foci are located in anterior as well as in posterior regions of the ACC. The location of the ACC focus in the present study corresponds to the most anterior locations.
An association between Aδ fiber-mediated first pain, the sensory component of pain and S1 on the one hand and C fiber-mediated second pain, affective aspects of pain and ACC on the other hand is supported by a recent case report of a patient with a lesion comprising S1 and S2 but sparing of ACC (50). This patient had a selective loss of first pain sensation and sensory aspects of pain, whereas second pain sensation and pain affect were preserved. This possibility to dissociate different perceptual components of pain has recently been experimentally confirmed (51). These findings indicate a parallel mode of pain processing most probably subserved by parallel thalamocortical projections to S1, S2, insula, and ACC (8).
The distinct cortical representations of first and second pain are likely to reflect distinct biological functions of both sensations. First pain signals the noxious nature of a stimulus and provides precise sensory information for an appropriate and rapid motor response, i.e., for an immediate withdrawal. Thus, first pain aims at achieving relative safety from the source of injury. Second pain with its strong affective component attracts longer-lasting attention and initiates behavioral responses to limit further injury and optimize recovery. Thus, second pain may subserve the recuperative healing mechanism of pain (52).
Acknowledgments
We thank Frank Schmitz for programming analysis software and Christoph Ploner for helpful comments on the manuscript. This study was supported by the Volkswagen-Stiftung (I/73240).
Abbreviations
ACC, anterior cingulate cortex
MEG, magnetoencephalography
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gad J. & Goldscheider, A. (1892) Z. Klin. Med. 20, 339-373. [Google Scholar]
- 2.Lewis T. & Pochin, E. E. (1937) Clin. Sci. 3, 67-76. [Google Scholar]
- 3.Bishop G. H. & Landau, W. M. (1958) Science 128, 712-713. [DOI] [PubMed] [Google Scholar]
- 4.Price D. D., Hu, J. W., Dubner, R. & Gracely, R. H. (1977) Pain 3, 57-68. [DOI] [PubMed] [Google Scholar]
- 5.Adriaensen H., Gybels, J., Handwerker, H. O. & Van Hees, J. (1983) J. Neurophysiol. 49, 111-122. [DOI] [PubMed] [Google Scholar]
- 6.Van Hees J. & Gybels, J. (1981) J. Neurol. Neurosurg. Psychiatry 44, 600-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell J. N. & LaMotte, R. H. (1983) Brain Res. 266, 203-208. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler A. & Ploner, M. (2000) J. Clin. Neurophysiol. 17, 592-603. [DOI] [PubMed] [Google Scholar]
- 9.Bromm B. & Treede, R. D. (1987) Exp. Brain Res. 67, 153-162. [DOI] [PubMed] [Google Scholar]
- 10.Arendt-Nielsen L. (1990) J. Neurol. Neurosurg. Psychiatry 53, 405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Towell A. D., Purves, A. M. & Boyd, S. G. (1996) Pain 66, 79-86. [DOI] [PubMed] [Google Scholar]
- 12.Magerl W., Ali, Z., Ellrich, J., Meyer, R. A. & Treede, R. D. (1999) Pain 82, 127-137. [DOI] [PubMed] [Google Scholar]
- 13.Opsommer E., Weiss, T., Plaghki, L. & Miltner, W. H. (2001) Neurosci. Lett. 298, 41-44. [DOI] [PubMed] [Google Scholar]
- 14.Di Piero V., Ferracuti, S., Sabatini, U., Pantano, P., Cruccu, G. & Lenzi, G. L. (1994) Pain 56, 167-173. [DOI] [PubMed] [Google Scholar]
- 15.Andersson J. L., Lilja, A., Hartvig, P., Langstrom, B., Gordh, T., Handwerker, H. & Torebjork, E. (1997) Exp. Brain Res. 117, 192-199. [DOI] [PubMed] [Google Scholar]
- 16.Iadarola M. J., Berman, K. F., Zeffiro, T. A., Byas-Smith, M. G., Gracely, R. H., Max, M. B. & Bennett, G. J. (1998) Brain 121, 931-947. [DOI] [PubMed] [Google Scholar]
- 17.Petrovic P., Petersson, K. M., Ghatan, P. H., Stone-Elander, S. & Ingvar, M. (2000) Pain 85, 19-30. [DOI] [PubMed] [Google Scholar]
- 18.Bromm B. & Treede, R. D. (1984) Hum. Neurobiol. 3, 33-40. [PubMed] [Google Scholar]
- 19.Ahonen A. I., Hämäläinen, M. S., Kajola, M. J., Knuutila, J. E. T., Laine, P. P., Lounasmaa, O. V., Parkkonen, L. T., Simola, J. T. & Tesche, C. D. (1993) Physica Scripta T49, 198-205. [Google Scholar]
- 20.Van Veen B. D., van Drongelen, W., Yuchtman, M. & Suzuki, A. (1997) IEEE Trans. Biomed. Eng. 44, 867-880. [DOI] [PubMed] [Google Scholar]
- 21.Gross J., Kujala, J., Hamalainen, M., Timmermann, L., Schnitzler, A. & Salmelin, R. (2001) Proc. Natl. Acad. Sci. USA 98, 694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friston K. J., Ashburner, J., Frith, C. D., Poline, J.-B., Heather, J. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 1-25. [Google Scholar]
- 23.Hämäläinen M., Hari, R., Ilmoniemi, R. J., Knuutila, J. & Lounasmaa, O. V. (1993) Rev. Mod. Phys. 65, 413-497. [Google Scholar]
- 24.Mackenzie R. A., Burke, D., Skuse, N. F. & Lethlean, A. K. (1975) J. Neurol. Neurosurg. Psychiatry 38, 865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torebjork H. E. & Hallin, R. G. (1973) Exp. Brain Res. 16, 321-332. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler E. A., Magerl, W., Meyer, R. A. & Treede, R. D. (1999) Brain 122, 2245-2257. [DOI] [PubMed] [Google Scholar]
- 27.Ploner M., Schmitz, F., Freund, H. J. & Schnitzler, A. (1999) J. Neurophysiol. 81, 3100-3104. [DOI] [PubMed] [Google Scholar]
- 28.Ploner M., Schmitz, F., Freund, H. J. & Schnitzler, A. (2000) J. Neurophysiol. 83, 1770-1776. [DOI] [PubMed] [Google Scholar]
- 29.Schouenborg J., Kalliomaki, J., Gustavsson, P. & Rosen, I. (1986) Brain Res. 397, 86-92. [DOI] [PubMed] [Google Scholar]
- 30.Kalliomaki J., Weng, H. R., Nilsson, H. J. & Schouenborg, J. (1993) Brain Res. 622, 262-270. [DOI] [PubMed] [Google Scholar]
- 31.Shaw F. Z., Chen, R. F., Tsao, H. W. & Yen, C. T. (1999) Brain Res. 824, 183-196. [DOI] [PubMed] [Google Scholar]
- 32.Mendell L. & Wall, P. (1965) Nature (London) 206, 97-99. [DOI] [PubMed] [Google Scholar]
- 33.Kenshalo D. R. & Willis, W. D. (1991) in Cerebral Cortex, eds. Peters, A. & Jones, E. G. (Plenum, New York), Vol. 9, pp. 153–212. [Google Scholar]
- 34.Kenshalo D. R., Iwata, K., Sholas, M. & Thomas, D. A. (2000) J. Neurophysiol. 84, 719-729. [DOI] [PubMed] [Google Scholar]
- 35.Hofbauer R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. (2001) J. Neurophysiol. 86, 402-411. [DOI] [PubMed] [Google Scholar]
- 36.Timmermann L., Ploner, M., Haucke, K., Schmitz, F., Baltissen, R. & Schnitzler, A. (2001) J. Neurophysiol. 86, 1499-1503. [DOI] [PubMed] [Google Scholar]
- 37.Bushnell M. C., Duncan, G. H., Hofbauer, R. K., Ha, B., Chen, J. & Carrier, B. (1999) Proc. Natl. Acad. Sci. USA 96, 7705-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenz F. A., Gracely, R. H., Zirh, A. T., Romanoski, A. J. & Dougherty, P. M. (1997) Pain Forum 6, 22-31. [Google Scholar]
- 39.Vogt B. A. & Sikes, R. W. (2000) Prog. Brain Res. 122, 223-235. [DOI] [PubMed] [Google Scholar]
- 40.Petrovic P., Kolso, E., Petersson, K. N. & Ingvar, N. (2002) Science 295, 1737-1740. [DOI] [PubMed] [Google Scholar]
- 41.Johansen J. P., Fields, H. L. & Manning, B. H. (2001) Proc. Natl. Acad. Sci. USA 98, 8077-8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bush G., Luu, P. & Posner, M. I. (2000) Trends Cognit. Sci. 4, 215-222. [DOI] [PubMed] [Google Scholar]
- 43.Paus T. (2001) Nat. Rev. Neurosci. 2, 417-424. [DOI] [PubMed] [Google Scholar]
- 44.Devinsky O., Morrell, M. J. & Vogt, B. A. (1995) Brain 118, 279-306. [DOI] [PubMed] [Google Scholar]
- 45.Tolle T. R., Kaufmann, T., Siessmeier, T., Lautenbacher, S., Berthele, A., Munz, F., Zieglgansberger, W., Willoch, F., Schwaiger, M., Conrad, B. & Bartenstein, P. (1999) Ann. Neurol. 45, 40-47. [DOI] [PubMed] [Google Scholar]
- 46.Vogt B. A., Derbyshire, S. & Jones, A. K. (1996) Eur. J. Neurosci. 8, 1461-1473. [DOI] [PubMed] [Google Scholar]
- 47.Derbyshire S. W., Vogt, B. A. & Jones, A. K. (1998) Exp. Brain Res. 118, 52-60. [DOI] [PubMed] [Google Scholar]
- 48.Kwan C. L., Crawley, A. P., Mikulis, D. J. & Davis, K. D. (2000) Pain 85, 359-374. [DOI] [PubMed] [Google Scholar]
- 49.Buchel C., Bornhovd, K., Quante, M., Glauche, V., Bromm, B. & Weiller, C. (2002) J. Neurosci. 22, 970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ploner M., Freund, H. J. & Schnitzler, A. (1999) Pain 81, 211-214. [DOI] [PubMed] [Google Scholar]
- 51.Rainville P., Carrier, B., Hofbauer, R. K., Bushnell, M. C. & Duncan, G. H. (1999) Pain 82, 159-171. [DOI] [PubMed] [Google Scholar]
- 52.Wall P. D. (1979) Pain 6, 253-264. [DOI] [PubMed] [Google Scholar]