Abstract
Unintended block of HERG K+ channels is a side effect of many common medications and is the most common cause of acquired long QT syndrome associated with increased risk of life-threatening arrhythmias. The molecular mechanism of high-affinity HERG block by structurally diverse compounds has been attributed to π-stacking and cation-π interactions of a drug (e.g., cisapride) with specific aromatic amino acid residues (Tyr-652 and Phe-656) in the S6 α-helical domain that face the central cavity of the channel. It also has been proposed that strong C-type inactivation of HERG facilitates or is the primary determinant of high-affinity drug binding. The structurally related, but noninactivating eag channel is insensitive to HERG blockers unless inactivation is induced by specific amino acid mutations [Ficker, E., Jarolimek, W. & Brown, A. M. (2001) Mol. Pharmacol. 60, 1343–1348]. Here we examine the relative importance of inactivation vs. positioning of S6 aromatic residues in determining sensitivity of HERG and eag channels to block by cisapride. The repositioning of Tyr-652 or Phe-656 along the S6 α-helical domain of HERG reduced sensitivity of channels to block by cisapride. Moreover, independent of inactivation, repositioning of the equivalent aromatic residues in Drosophila eag channels induced sensitivity to block by cisapride. These findings suggest that positioning of S6 aromatic residues relative to the central cavity of the channel, not inactivation per se determines drug block of HERG or eag channels.
Keywords: voltage clamp, delayed rectifier, Xenopus oocyte
HERG encodes α-subunits that coassemble to form channels that conduct the rapid delayed rectifier K+ current (IKr) in the heart (1, 2). IKr is activated by membrane depolarization and is a key determinant of action potential duration in the ventricle. Mutations in HERG that lead to a partial or complete loss of function are a major cause of dominantly inherited long QT syndrome (LQTS) (3). The hallmark arrhythmia associated with LQTS is torsades de pointes. This arrhythmia can spontaneously revert to normal sinus rhythm or degenerate into ventricular fibrillation and cause sudden death (4). Inherited LQTS also can be caused by gain of function mutations in the cardiac Na+ channel gene SCN5A or loss of function mutations in other cardiac K+ channel genes, including KCNQ1, KCNE1, and KCNJ2 (5).
Acquired LQTS is more common than inherited LQTS and is usually caused by preferential block of IKr by common medications. This undesirable side effect has prompted removal of several drugs from the market and is now recognized as a significant hurdle in the development of new and safer drugs. For example, cisapride is a prokinetic agent that was widely used for the treatment of gastrointestinal motility disorders until it was discovered that on rare occasions this drug was associated with arrhythmia and sudden death. Soon afterward it was demonstrated that cisapride is a potent HERG channel blocker (6). Elucidating the molecular mechanisms of HERG by cisapride and other drugs will facilitate the rational drug design of new pharmaceutical compounds devoid of this unwanted side effect.
The key residues that determine high-affinity block of HERG channels by several chemically unrelated compounds, including cisapride, terfenadine, and the antiarrhythmic agents quinidine, dofetilide, and MK-499, were recently described (7, 8). These residues were localized to the S6 domain and the bottom of pore helix, and homology modeling predicted they faced the central cavity of the HERG channel. Most important were the aromatic residues Tyr-652 and Phe-656, located one helical turn away from one another in the S6 domain. Mutation of either residue to an Ala drastically reduced the potency of channel block by cisapride, terfenadine, MK-499 (8), chloroquine (9), or vesnarinone (10). These findings suggest that Tyr-652 and Phe-656 are the most important determinants of binding to HERG by structurally diverse drugs.
HERG belongs to the eag family of potassium channels. The amino acid sequence of the S6 domain of eag is 50% identical to HERG, and Tyr-652 and Phe-656 of HERG is conserved in eag. However, eag channels do not inactivate and are relatively insensitive to block by drugs that inhibit HERG. Differential drug sensitivity of channels has been explained by the presence (HERG) or absence (eag) of inactivation gating and several studies have demonstrated a positive correlation between inactivation and block. First, the S620T mutation in HERG channels caused a loss of inactivation and dramatically reduced the sensitivity of mutant channels to block by dofetilide (11). Second, eag-HERG chimeric channels that contain part of the pore and S6 domains from HERG inactivated and were sensitive to block by E-4031 (12). Third, introduction of three point mutations into the pore of bovine eag channels were shown to induce inactivation and confer sensitivity to block by dofetilide (13). However, the link between inactivation and drug sensitivity has been confused by other findings. Specifically, some mutations in HERG removed inactivation, but the mutant channels retained relatively high sensitivity to block by methanesulfonanilides (8, 14). Moreover, other mutations increased inactivation compared to wild-type (wt) HERG channels, but greatly reduced drug sensitivity (8). These inconsistent findings indicate that the molecular mechanisms of HERG channel block, especially the role of inactivation, is not well understood.
The aim of the present study was to reconcile the disparate findings concerning the role of inactivation as a critical determinant for potent drug block of HERG. We hypothesized that gating of HERG channels includes a twisting of S6 and repositioning of Tyr-652 and Phe-656 in an orientation that is associated with C-type inactivation and optimal for drug binding, and that activation-associated gating of eag is insufficient to reposition these residues in the equivalent orientation. To test this hypothesis, we used site-directed mutagenesis to relocate the Tyr or Phe in the S6 domain of HERG or Drosophila eag and tested the sensitivity of the resultant mutant channels to block by cisapride. Similar to most drugs that block HERG, cisapride strongly interacts with Tyr-652 and Phe-656 of the S6 domain, yet unlike the methanesulfonanilides has minimal or no interaction with the pore helix. By shifting the position of Tyr and Phe and altering inactivation properties, we demonstrate that the position of aromatic residues along the S6 α helix and not inactivation per se is the main determinant of HERG and eag channel sensitivity to block by cisapride.
Materials and Methods
Molecular Biology.
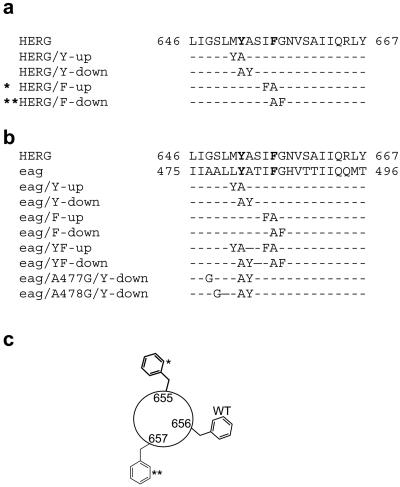
Site-directed mutagenesis was used to reposition Tyr and Phe residues located in the S6 domain of HERG and Drosophila eag channels by one position, either toward the N or C terminus as shown in Fig. 1. In each case an aromatic residue (e.g., Phe-656 of HERG) was replaced with an Ala and a neighboring residue (e.g., Ile-655) mutated to the aromatic residue that was mutated to Ala. The resulting mutant channel was named according to whether the aromatic residue was transferred in the N-terminal (“up”) or C-terminal (“down”) direction (Fig. 1c). For example, I655F/F656A HERG was named HERG/F-up. A similar approach was used to construct and name several mutant HERG channels (Fig. 1a) and eag channels (Fig. 1b). In addition, mutant eag channels were constructed that contained a shift in both aromatic residues, either both in the N-terminal direction or C-terminal direction as illustrated in Fig. 1b.
Fig 1.
Sequences of S6 domain for HERG and Drosophila eag channels and the location of introduced point mutations. (a) Sequence of wt and mutant HERG S6 domains with Tyr-652 and Phe-656 highlighted in bold type. (b) Sequence of wt and mutant eag S6 domains with Tyr-451 and Phe-455 highlighted in bold type. (c) Diagram showing position of Phe residue in wt and repositioning in mutant HERG channels (*, HERG/F-up; **, HERG/F-down).
Drosophila eag cDNA was cloned in pGH19, kindly provided by Jeff Warmke (Merck Research Labs), and HERG cDNAs were ligated into the pSP64 vector. Mutations were introduced as described (15). The constructs were linearized, then transcribed with T7 (eag) or SP6 (HERG) polymerase by using CapScribe (Roche) to make cRNA that was injected into Xenopus laevis oocytes as described (16).
Electrophysiology.
Standard two-microelectrode voltage clamp techniques (17) were used to record currents in oocytes 1–3 d after injection of cRNA. For voltage clamp experiments, oocytes were bathed in a solution that contained (in mM): 96 Na MES [2-(morpholino)ethane sulfonic acid], 2 KMES, 2 CaMES2, 5 Hepes, and 1 MgCl2; adjusted to pH 7.6 with methane sulfonic acid. The effect of cisapride on wt and mutant channels was determined by using repetitive 2.5-s pulses to 0 mV applied at a frequency of 0.33 Hz from a holding potential of −90 mV. The endogenous, nearly instantaneous current at 0 mV was determined for uninjected cells in each batch of oocytes. This current averaged 50 nA and was subtracted from wt and mutant channel currents. Digitized data were analyzed off-line by using PCLAMP 8 (Axon Instruments, Foster City, CA) and origin (Origin Lab, Northampton, MA) software.
Cisapride was purchased from Research Diagnostics (Flanders, NJ), and MK-499 was supplied by Merck. Drugs were prepared daily by dilution of a DMSO stock solution kept at −20°C. Drugs were applied with a switching device as described (18).
Results
Position of Aromatic Residues Is Critical for Block of HERG.
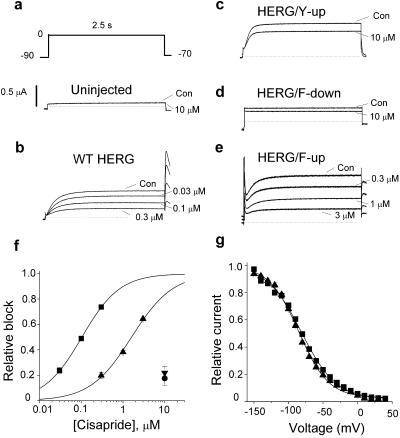
The effect of cisapride on wt and mutant HERG channels was determined by using repetitive voltage clamp pulses to 0 mV. A high concentration of cisapride (10 μM) had no effect on the small endogenous currents expressed in oocytes (Fig. 2a). As we reported (8), wt HERG was blocked in a concentration-dependent manner as illustrated for 30–300 nM of drug in Fig. 2b. The IC50 for block of wt HERG by cisapride was 102 ± 1 nM (n = 4; Fig. 2f, ▪).
Fig 2.
Effect of repositioning S6 aromatic residues in HERG on biophysical properties and sensitivity to bock by cisapride. (a) Voltage pulse protocol and endogenous currents in an uninjected oocyte. (b) Block of wt HERG channel current by 30 nM, 100 nM, and 0.3 μM cisapride. (c and d) HERG/Y-up and HERG/F-down channels are only partially blocked by 10 μM cisapride. Note that HERG/F-down channel current is time-independent. (e) Effect of 0.3, 1, and 3 μM cisapride on HERG/F-up channel current. (f) Concentration–effect relationship for block of wt (▪), Y-up (•), F-down (▾), and F-up (▴) HERG channels by cisapride. The IC50 was 0.102 ± 0.001 μM and 1.60 ± 0.11 μM for wt HERG and HERG/Y-up (n = 4). (g) Voltage dependence of channel availability for wt HERG and HERG/F-up channels, determined with a triple pulse protocol. Data were fitted with a Boltzmann function (curve) to estimate one-half point (V1/2) and slope factor (k): wt-HERG (▪, V1/2 = −81.2 ± 1.4 mV, k = 25.0 ± 1.3 mV, n = 7) and HERG/F-up (▴, V1/2 = −85.3 ± 1.3 mV, k = 21.5 ± 1.2 mV, n = 8).
We hypothesized that the positions of Tyr-652 and Phe-656 in the S6 domain of HERG are optimal for high-affinity block. To test this idea, we shifted the position of an aromatic residue in either the N-terminal (“up”) or C-terminal (“down”) direction (Fig. 1a) and tested the sensitivity of the resultant mutant channels to block by cisapride. M651Y/Y652A channels (HERG/Y-up, Fig. 2c) deactivated faster than wt HERG but were relatively resistant to 10 μM cisapride as current was only reduced 17.6% ± 5.4% (n = 4). Y652A/A653Y (HERG/Y-down) subunits did not functionally express. Channels formed by F656A/G657F subunits (HERG/F-down) expressed poorly, were constitutively open, and relatively insensitive to block (Fig. 2d), with only a 22.0% ± 4.8% decrease at 10 μM cisapride (n = 4). I655F/F656A (HERG/F-up) channels deactivated slower than wt HERG and were less sensitive to cisapride (Fig. 2e). The slow deactivation prevented complete closure of channels during the interpulse interval, observed as a transient outward current at the beginning of each pulse to 0 mV because of inactivation of open channels. The IC50 for block of HERG/F-up channels by cisapride was 1.60 ± 0.11 μM (n = 4), ≈16-fold less than wt HERG (Fig. 2f). This altered sensitivity to cisapride was not caused by altered voltage dependence of inactivation (Fig. 2g). These data suggest that position of the two aromatic residues in S6 affect the sensitivity of HERG to block by cisapride. The finding that HERG/F-up channels were less sensitive to block but had similar inactivation properties compared to wt HERG indicates that inactivation is not the only determinant of high-affinity block. However, because HERG/Y-down did not express and HERG/Y-up and HERG/F-down channels had dramatically altered gating properties, we could not make definitive conclusions regarding the importance of Tyr and Phe positioning in S6 and block of HERG channels by cisapride. To explore the relative roles of inactivation and aromatic residue positioning, we performed similar experiments on the eag channel.
Alteration of eag Sensitivity to Cisapride by Repositioning of Aromatic Residues in S6.
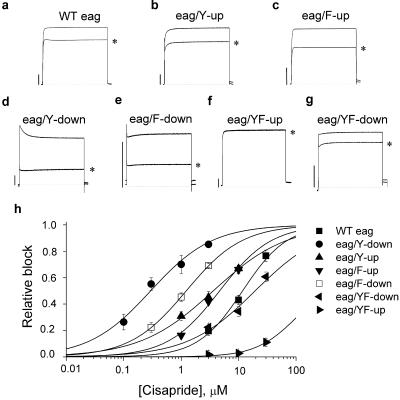
The S1–S6 domains of eag channels share ≈50% overall amino acid identity with HERG. Bovine and mouse eag channels are resistant to HERG blockers such as dofetilide and E-4031 (11–13). Drosophila eag is 50% identical and 77% homologous to HERG in the S6 domain (Fig. 1b); however, 3 μM cisapride caused >90% block of HERG but only a 20% reduction in eag current (Fig. 3a). The wt eag channel current was 120-fold less sensitive to block by cisapride (IC50 = 11.9 ± 1.2 μM, n = 4) compared to wt HERG. Tyr-481 and Phe-485 of eag are homologous to Tyr-652 and Phe-656 of HERG (Fig. 1b). As was done for HERG, we shifted the location of each aromatic residue of the eag S6 domain in either the N-terminal (“up”) or C-terminal (“down”) direction and tested the sensitivity of the resultant mutant channels to block by 3 μM cisapride. Transfer of either Tyr-481 or Phe-485 in the N-terminal direction (Y-up or F-up) only slightly increased drug block (Fig. 3 b and c). In contrast, transfer of either residue in the C-terminal direction (Y-down, F-down) induced inactivation and had a more dramatic effect on sensitivity to cisapride (Fig. 3 d and e). The IC50 for eag/Y-down and eag/F-down channels was decreased by 43- and 10-fold, respectively (Fig. 3h). We next determined whether shifting the position of both residues, either up or down the α-helix of S6 influenced channel block by cisapride. eag/YF-up channels (Fig. 1b) gated normally but current was unaffected by 3 μM (Fig. 3f) and only reduced 11% by 30 μM cisapride. eag/YF-down channels (Fig. 3g) were as sensitive as wt eag channels to cisapride. The concentration-dependent block of wt and mutant eag channels is summarized in Fig. 3h. In summary, shifting Tyr-481 to the 482 position greatly increased drug sensitivity whereas the additional mutations used to transfer Phe-485 to the -486 position greatly reduced drug sensitivity of eag channels.
Fig 3.
Effect of cisapride on wt and mutant eag channel currents. (a–g) wt and mutant eag currents recorded during a 2.5-s pulse to 0 mV before and after steady-state effects of 3 μM cisapride (*). (h) Concentration-effect relationships for wt and mutant eag channels. The IC50 was 11.9 ± 1.2 μM for wt eag, 3.7 ± 0.3 μM for eag/Y-up, 0.28 ± 0.05 μM for eag/Y-down, 4.6 ± 0.4 μM for eag/F-up, 1.3 ± 0.1 μM for eag/F-down, and 18.9 ± 0.3 μM for eag/YF-down (n = 4–6). The 30 μM cisapride blocked eag/YF-up current by 11% ± 3% (n = 5).
Position of Aromatic Residues Versus Inactivation in eag Channels.
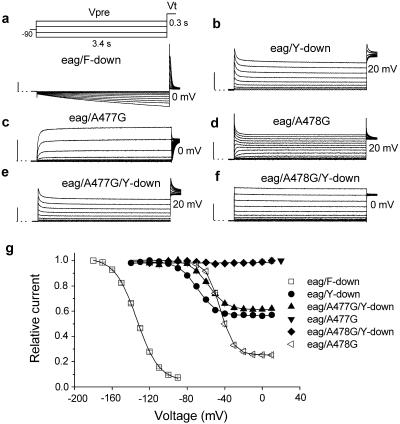
The wt eag channels do not inactivate and are only poorly blocked by cisapride and dofetilide. Ficker et al. (13) demonstrated that mutation-induced inactivation of eag channels greatly enhanced drug block by dofetilide. The increased sensitivity of eag/Y-down and eag/F-down channels to cisapride could be caused by repositioning of the aromatic residues, or to preferential binding of drug to inactivated channels. Therefore, the voltage dependence of inactivation for each mutant channel was determined with a two-pulse protocol from a holding potential of −80 mV. A 3.4-s prepulse was applied in 10-mV increments, followed by a test pulse to either 0 or +20 mV (Fig. 4a). Because most F-down channels were inactivated at a holding potential of −80 mV, the prepulse potential (Vpre) was varied from −180 to −90 mV. Membrane hyperpolarization elicited a slow inward current as channels recovered from inactivation. Subsequent depolarization to a test potential (Vt) of 0 mV induced inactivation of channels that were opened during the hyperpolarizing prepulse (Fig. 4a). The one-half point for inactivation (V1/2) determined with this pulse protocol was −134 mV (Fig. 4g, □), and >95% of channels were inactivated at −90 mV. eag/Y-down channels exhibited less pronounced inactivation. To estimate the voltage dependence of inactivation for eag/Y-down channels, the prepulse was varied from −140 to +10 mV and the test pulse was applied to +20 mV (Fig. 4b). The V1/2 for eag/Y-down channel inactivation was −69 mV, and minimal channel availability was 0.56 (Fig. 4g, •). Contrary to the predicted correlation between inactivation and drug block, eag/F-down channels exhibited more inactivation but were less sensitive to block by cisapride (IC50 = 1.23 μM) compared to eag/Y-down channels (IC50 = 0.28 μM).
Fig 4.
Voltage dependence for inactivation of mutant eag channels. (a–f) Voltage pulse protocol and currents used to assess inactivation properties of the indicated mutant channel. Prepulses (Vpre) were applied from −180 to −90 mV for eag/F-down (a) and from −140 to +10 mV for all other mutant channels. The test pulse (Vt) was applied to 0 or +20 mV as indicated. (g) Voltage dependence of channel availability of eag mutant channels. Peak or extrapolated peak currents recorded at Vt were normalized and plotted vs. Vpre. Data were fitted with a Boltzmann function to obtain the one-half point (V1/2) and slope factor (k) for the relationship. The one-half point and slope factor were −113.6 ± 0.3 mV and 11.7 ± 0.3 mV for eag/F-down (□); −68.6 ± 0.3 mV, 8.9 ± 0.3 mV for eag/Y-down (•); −57.4.3 ± 0.3 mV, 9.3 ± 0.3 mV for eag/A477G/Y-down (▴); and −45.1 ± 0.2 mV, 11.7 ± 0.2 mV for eag/A478G (◃). eag/A478G/Y-down (♦) and eag/A477G (▾) channels did not inactivate within the test range (n = 7–11).
Other point mutations were introduced into the S6 domain to further analyze the relationship between inactivation and drug block of eag channels. Ala-477 and Ala-478 are located approximately one helical turn above Tyr-481 in the S6 domain of eag. The mutation of Ala-477 to Gly slowed the rate of channel activation but did not induce inactivation (Fig. 4c). In contrast, mutation of Ala-478 to Gly introduced substantial voltage-dependent inactivation (Fig. 4d) with a V1/2 of −45 mV and a minimal channel availability of 0.25 (Fig. 4g). Introduction of the A477G or A478G mutation into eag/Y-down channels further modulated the properties of inactivation. eag/A477G/Y-down channels inactivated similar to Y-down (Fig. 4 e and g), whereas eag/A478G/Y-down channels did not inactivate (Fig. 4f).
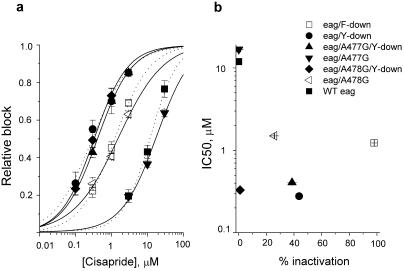
The concentration-dependent block by cisapride of the mutant channels shown in Fig. 4 is summarized in Fig. 5a. Despite differences in inactivation properties, the IC50 for block by cisapride of Y-down, A477G/Y-down and A478G/Y-down eag channels was similar and all were more sensitive to the drug compared to wt eag. The IC50 values for wt eag and the six mutant channels were plotted as a function of percentage inactivation in Fig. 5b. There was no correlation between drug block and inactivation. This finding suggests that positioning of the aromatic residues in the α-helix of the S6 domain of eag channels is more important than inactivation in determining sensitivity to block by cisapride. Thus, although inactivation can facilitate block of HERG or eag channels, it is not sufficient or required for block.
Fig 5.
Potency for block of mutant eag channels is not correlated with extent of inactivation. (a) The IC50 for block by cisapride was 0.28 ± 0.05 μM for eag/Y-down, 0.41 ± 0.04 μM for eag/A477G/Y-down, 0.33 ± 0.03 μM for A478G/Y-down, 16.8 ± 1.5 μM for eag/A477G, and 1.5 ± 0.2 μM for eag/A478G (n = 4–6). (b) IC50 for block by cisapride plotted as a function of the percentage of channels inactivated at 0 mV.
Discussion
Drug-induced LQTS was described long ago (reviewed in refs. 19 and 20) but only recently commonly attributed to block of HERG K+ channels. This recognition has prompted preclinical testing of drugs in development for potential block of heterologously expressed HERG channels or IKr in isolated cardiac myocytes (21). Understanding the molecular determinants of drug binding to HERG channels is important for design of new drugs devoid of this unwanted side effect. Toward this goal, previous studies defined the putative drug-binding site by using a site-directed mutagenesis approach (7, 8). Unlike other voltage-gated K+ channels, the S6 domain of HERG subunits has two aromatic residues (Tyr-652 and Phe-656) that face the central cavity of the channel. Mutation of either residue greatly reduced the affinity for block by MK-499, dofetilide, cisapride, terfenadine, quinidine, and chloroquine (7–9), suggesting that these aromatic residues comprise crucial components of the binding site for structurally diverse drugs.
Block of HERG also seems to be state-dependent. For most of the compounds investigated, block only occurs after the channel has opened. Moreover, an obligatory link between HERG channel inactivation and high-affinity drug binding has been suggested (11–14, 22). The most compelling evidence was the observation that noninactivating eag channels were insensitive to dofetilide despite having a Tyr and Phe located in positions equivalent to Tyr-652 and Phe-656 of HERG. Second, a combination of point mutations that induced inactivation of eag (T432S/A443S/A453S) also induced sensitivity to blockers such as dofetilide (13). Third, reduction of steady-state inactivation caused by removal of extracellular [Na+] or addition of Cd2+ also reduced drug block of HERG (22). However, several exceptions to the correlation between inactivation and potency of block have been noted. Some mutations of HERG (e.g., G648A, T623A, and F656A) enhanced inactivation but reduced block by MK-499 (8). These mutations might directly or allosterically reduce the affinity of the drug-binding site. Furthermore, removal of inactivation by mutagenesis does not always result in lowered drug potency. For example, G628C/S631C HERG channels do not inactivate (23), yet compared to wt HERG are only ≈10-fold less sensitive to block by MK-499 (24) and equally sensitive to disopyramide (25). V625A HERG channels do not appreciably inactivate but are blocked by terfenadine and cisapride at concentrations similar to that required to block wt HERG channels (8). Some, but certainly not all of the discrepancies between inactivation and block of mutant HERG channels might be explained by differences in voltage pulse protocols or because specific mutations might alter the drug-binding site in addition to enhancing inactivation. In this study, we engineered mutations into HERG and eag channels to determine whether enhanced drug sensitivity could be accounted for by a repositioning of aromatic residues in the S6 domain rather than the gating associated with inactivation per se.
Voltage-dependent rearrangements of the S6 domain appear to mediate opening of Kv channels. Recent studies of bacterial KcsA and MthK channels suggest that the inner helices, equivalent to the S6 α-helical domains of Kv channels, either rotate and translate outward (26, 27), or bend ≈30° away from the central cavity at a kink in the α-helix located at a conserved Gly residue (28, 29). Either opening mechanism would widen the diameter of the narrow region formed by crisscrossing of the S6 domains near their C-terminal ends of channels in the closed state. This movement would enable large drugs like cisapride to gain access to its binding site within the inner pore of HERG. Less is known about the protein rearrangements that accompany C-type inactivation. Mutations of many residues in the pore helix and S6 domain either enhance or reduce inactivation of Kv channels (30–32), suggesting these domains participate in C-type inactivation. Rotation or other movements of S6 may accompany or induce inactivation of HERG, resulting in a reorientation of the residues that line the central cavity of the open or closed sate. An inactivation-associated reorientation of Tyr-652 and Phe-656 to positions that favor drug binding could explain the commonly observed link between inactivation and drug sensitivity of HERG channels (13).
To test the importance of positioning of S6 domain aromatic residues in HERG channels, we mutated Tyr-652 to Ala and one of the neighboring residues to Tyr. A similar mutation scheme was used to move Phe-656 of HERG or Tyr-481 and Phe-485 of eag by one position up or down the S6 α-helix. The mutant HERG channels were less sensitive to block by cisapride, but altered gating properties prevent clear interpretation of the findings. In contrast, repositioning of Tyr-481 or Phe-485 of eag channels enhanced sensitivity to cisapride. eag/Y-down channels were 43-fold more sensitive and F-down channels were 10-fold more sensitive than wt eag channels. Like the HERG mutants, interpretation of these results was complicated because these mutations also altered gating properties. However, eag/F-down channels inactivated >95% at 0 mV, whereas eag/Y-down channels were <50% inactivated at this potential. These data suggest that repositioning of the aromatic residues was a more important determinant of cisapride block than inactivation. Most revealing was the finding that eag/A478G/Y-down channels did not inactivate but retained the same sensitivity to cisapride as the inactivating eag/Y-down channel. HERG/F-down, eag/F-down, and eag/YF-down channels were constitutively open. Repositioning the bulky Phe down (but not up) one position in the α-helix of S6 of HERG or eag apparently disrupted closure of the activation gate. The inability to close and trap the drug within the central cavity could explain the reduced drug sensitivity of HERG/F-down and eag/YF-down channels. Taken together, our findings indicate that inactivation facilitates drug binding to eag, but this gating is neither sufficient nor required for high-affinity channel block.
There are several limitations of our study. First, repositioning of a single aromatic residue in HERG or eag required two mutations per subunit, resulting in eight mutations per tetrameric channel. Most mutations altered gating properties as well as sensitivity to cisapride, making it difficult to determine the difference between a direct and an allosteric effect on drug interaction. Second, mutations in eag that induce sensitivity to cisapride may not confer sensitivity to other HERG blockers. For example, we found that sensitivity to MK-499 was not induced by the A478G/Y-down mutations in eag (data not shown), even though MK-499, like cisapride, interacts with Tyr-652 and Phe-656 (REF). However, unlike cisapride, MK-499 also interacts with residues located at the base of the pore helix (Thr-623, Ser-624, and Val-625) and the nearby Gly-648 located in the S6 domain of HERG. Thus, although we were able to enhance the sensitivity of eag channels to cisapride by transferring Tyr from position 451 to 452, such a repositioning would likely disturb the interaction of MK-499 with residues near the pore helix. Our findings suggest that orientation of S6 aromatic residues with respect to the central cavity of HERG channels differs from eag channels. The positioning of Tyr-652 and Phe-656 in open or inactivated HERG channels may facilitate high-affinity interaction with many drugs, whereas Tyr-451 and Phe-455 of open eag channels do not.
Based on our mutant channel analyses, the Tyr and Phe of open wt eag channels may be located above (toward the N-terminal end) the optimal position for cisapride binding. The opening of K+ channels is facilitated by a gating hinge in the S6 domain formed by a conserved Gly. Five residues C-terminal to this Gly is a conserved Ala or Gly that faces the pore in KcsA and MthK channels. It was proposed that a large side chain at this residue would plug the pore and interfere with ion conductance (29). Consistent with this model, we found that mutation of this Ala to a Tyr in HERG (HERG/Y-down) caused loss of function. However, eag/Y-down channels with the equivalent mutations retained the ability to conduct K+. The different functional consequences of the Ala to Tyr mutations in eag and HERG provide further circumstantial evidence that equivalent residues based on sequence alignment can have dissimilar orientation of their side groups relative to the central cavity. The finding that rotating the position of Tyr-451 on the S6 α-helix by 100° induces drug sensitivity suggests, albeit indirectly, that gating-associated rotation of S6 differs between eag and HERG, or that the residues in S6 are not positioned relative to the central cavity in an identical manner as predicted simply by sequence alignment. Our experimental approach cannot distinguish between these two possibilities. We conclude that reduced drug affinity of noninactivating HERG mutant channels and the increased drug affinity of inactivating eag channels are not due to inactivation, per se, but to inactivation gating-associated reorientation of residues in the S6 domain that comprise a high-affinity drug-binding site. Analogous gating-associated reorientation of residues in the S6 domain might explain enhanced binding and block of Na+ or Ca2+ channels by many drugs (33, 34).
Acknowledgments
We thank Peter Westenskow and Meng San Pun for technical assistance. The study was supported by National Heart, Lung, and Blood Institute/National Institutes of Health Grant HL55236 (to M.C.S.).
Abbreviations
HERG, human ether-a-go-go-related gene
eag, Drosophila ether-a-go-go
wt, wild type
IKr, rectifier K+ current
LQTS, long QT syndrome
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sanguinetti M. C., Jiang, C., Curran, M. E. & Keating, M. T. (1995) Cell 81, 299-307. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau M., Warmke, J. W., Ganetzky, B. & Robertson, G. A. (1995) Science 269, 92-95. [DOI] [PubMed] [Google Scholar]
- 3.Curran M. E., Splawski, I., Timothy, K. W., Vincent, G. M., Green, E. D. & Keating, M. T. (1995) Cell 80, 795-803. [DOI] [PubMed] [Google Scholar]
- 4.Roden D. M., Lazzara, R., Rosen, M., Schwartz, P. J., Towbin, J. & Vincent, G. M. (1996) Circulation 94, 1996-2012. [DOI] [PubMed] [Google Scholar]
- 5.Keating M. T. & Sanguinetti, M. C. (2001) Cell 104, 569-580. [DOI] [PubMed] [Google Scholar]
- 6.Mohammad S., Zhou, Z., Gong, Q. & January, C. T. (1997) Am. J. Physiol. 42, H2534-H2538. [DOI] [PubMed] [Google Scholar]
- 7.Lees-Miller J. P., Duan, Y., Teng, G. Q. & Duff, H. J. (2000) Mol. Pharmacol. 57, 367-374. [PubMed] [Google Scholar]
- 8.Mitcheson J. S., Chen, J., Lin, M., Culberson, C. & Sanguinetti, M. C. (2000) Proc. Natl. Acad. Sci. USA 97, 12329-12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Chapula, J. A., Navarro-Polanco, R. A., Culberson, C., Chen, J. & Sanguinetti, M. C. (2002) J. Biol. Chem., in press. [DOI] [PubMed]
- 10.Kamiya K., Mitcheson, J. S., Yasui, K., Kodama, I. & Sanguinetti, M. C. (2001) Mol. Pharmacol. 60, 244-253. [DOI] [PubMed] [Google Scholar]
- 11.Ficker E., Jarolimek, W., Kiehn, J., Baumann, A. & Brown, A. M. (1998) Circ. Res. 82, 386-395. [DOI] [PubMed] [Google Scholar]
- 12.Herzberg I. M., Trudeau, M. C. & Robertson, G. A. (1998) J. Physiol. (London) 511, 3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ficker E., Jarolimek, W. & Brown, A. M. (2001) Mol. Pharmacol. 60, 1343-1348. [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Morales, M. J., Liu, S., Strauss, H. C. & Rasmusson, R. L. (1997) FEBS Lett. 417, 43-47. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar G. & Sommer, S. S. (1990) BioTechniques 8, 404-407. [PubMed] [Google Scholar]
- 16.Goldin A. L. & Sumikawa, K. (1992) Methods Enzymol. 207, 279-296. [DOI] [PubMed] [Google Scholar]
- 17.Stuhmer W. (1992) Methods Enzymol. 207, 319-339. [DOI] [PubMed] [Google Scholar]
- 18.Mitcheson J. S., Chen, J. & Sanguinetti, M. C. (2000) J. Gen. Physiol. 115, 229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipes D. P. (1987) Am. J. Cardiol. 59, 26E-31E. [DOI] [PubMed] [Google Scholar]
- 20.Zehender M., Hohnloser, S. & Just, H. (1991) Cardiovasc. Drugs Ther. 5, 515-530. [DOI] [PubMed] [Google Scholar]
- 21.Cavero I., Mestre, M., Guillon, J. M. & Crumb, W. (2000) Exp. Opin. Pharmacother. 1, 947-973. [DOI] [PubMed] [Google Scholar]
- 22.Numaguchi H., Mullins, F. M., Johnson, J. P., Jr., Johns, D. C., Po, S. S., Yang, I. C., Tomaselli, G. F. & Balser, J. R. (2000) Circ. Res. 87, 1012-1018. [DOI] [PubMed] [Google Scholar]
- 23.Smith P. L., Baukrowitz, T. & Yellen, G. (1996) Nature (London) 379, 833-836. [DOI] [PubMed] [Google Scholar]
- 24.Mitcheson J. S., Chen, J. & Sanguinetti, M. C. (2000) Jpn. J. Electrocardiol. Suppl. 3, 67-70. [Google Scholar]
- 25.Paul A. A., Witchel, H. J. & Hancox, J. C. (2001) Biochem. Biophys. Res. Commun. 280, 1243-1250. [DOI] [PubMed] [Google Scholar]
- 26.Perozo E., Cortes, D. M. & Cuello, L. G. (1999) Science 285, 73-78. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y. S., Sompornpisut, P. & Perozo, E. (2001) Nat. Struct. Biol. 8, 883-887. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y., Lee, A., Chen, J., Cadene, M., Chait, B. T. & MacKinnon, R. (2002) Nature (London) 417, 515-522. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y., Lee, A., Cadene, M., Chait, B. T. & MacKinnon, R. (2002) Nature (London) 417, 523-526. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Barneo J., Hoshi, T., Heinemann, S. H. & Aldrich, R. W. (1993) Recept. Channels 1, 61-71. [PubMed] [Google Scholar]
- 31.Molina A., Castellano, A. G. & Lopez-Barneo, J. (1997) J. Physiol. (London) 499, 361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiss L. & Korn, S. J. (1998) Biophys. J. 74, 1840-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hille B. (1977) J. Gen. Physiol. 69, 497-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hondeghem L. M. & Katzung, B. G. (1984) Annu. Rev. Pharmacol. Toxicol. 24, 387-423. [DOI] [PubMed] [Google Scholar]