Abstract
The effects of chronic treatment with nitric oxide-containing aspirin (NO-aspirin, NCX-4016) in comparison with regular aspirin or placebo on the development of a chronic disease such as atherosclerosis were investigated in hypercholesterolemic low-density lipoprotein (LDL)-receptor-deficient mice. Male mice were assigned randomly to receive in a volume of 10 ml/kg either placebo (n = 10), 30 mg/kg/day NO-aspirin (n = 10), or 18 mg/kg/day of regular aspirin (n = 10). After 12 weeks of treatment, the computer-assisted imaging analysis revealed that NO-aspirin reduced the aortic cumulative lesion area by 39.8 ± 12.3% compared with that of the placebo (P < 0.001). Regular aspirin did not reduce significantly aortic lesions (−5.1 ± 2.3%) compared with the placebo [P = 0.867, not significant (NS)]. Furthermore, NO-aspirin reduced significantly plasma LDL oxidation compared with aspirin and placebo, as shown by the significant reduction of malondialdehyde content (P < 0.001) as well as by the prolongation of lag-time (P < 0.01). Similarly, systemic oxidative stress, measured by plasma isoprostanes, was significantly reduced by treatment with NCX-4016 (P < 0.05). More importantly, mice treated with NO-aspirin revealed by immunohistochemical analysis of aortic serial sections a significant decrease in the intimal presence of oxidation-specific epitopes of oxLDL (E06 monoclonal antibody, P < 0.01), and macrophages–derived foam cells (F4/80 monoclonal antibody, P < 0.05), compared with placebo or aspirin. These data indicate that enhanced NO release by chronic treatment with the NO-containing aspirin has antiatherosclerotic and antioxidant effects in the arterial wall of hypercholesterolemic mice.
Keywords: atherosclerosis, LDL-receptor-deficient mice
Endothelial dysfunction has been shown in the presence of atherosclerosis (ref. 1 and reviewed in refs. 2–4). Several lines of evidence indicate that restoring nitric oxide (NO)-mediated signaling pathways in atherosclerotic arteries may decrease the disease (2–4). The essential findings are that the biochemical properties of NO allow its exploitation as both a cell signaling molecule through its interaction with redox centers in heme proteins and a rapid reaction with other biologically relevant radical species. The direct reaction of NO with radicals can have, at least in part, antioxidant effects. In arterial cells, the antioxidant properties of NO can be greatly amplified by the activation of signal transduction pathways that lead to the increased synthesis of endogenous antioxidants or down-regulate responses to pro-inflammatory stimuli. Studies in humans and in animal models have shown that low-density lipoprotein (LDL) oxidation may play a pivotal role in the pathogenesis of atherogenesis (reviewed in refs. 5, 6). Recent data indicate that LDL oxidation may promote per se activation of several signaling pathways and transcription factors in human coronary arteries (7–9). Several of these pathways are reduced by concomitant administration of vitamin E (7, 9). Thus, compounds with antioxidant properties may reduce downstream effects induced by LDL oxidation in the arterial wall, and this phenomenon could retard the progression of atherosclerosis.
In preliminary experiments, we evaluated the antioxidant properties in vitro of several nitro-compounds and found that some of these agents had antioxidant properties. In this study, we used male LDL-receptor-deficient mice (10, 11) to address the effects of a NO-containing aspirin derivative (NCX-4016) on the development of a chronic disease such as atherosclerosis and on plasma LDL oxidation and systemic oxidative stress. NO-releasing aspirin (NCX-4016) is a drug well characterized in vitro and in vivo (reviewed in ref. 12). Hypercholesterolemic mice develop hypercholesterolemia on a cholesterol mouse chow diet (10, 13) and extensive atherosclerosis, with lesions progressing from lipid-laden fatty streaks to advanced lesions (10, 11, 13–15). By using this model, we investigated the chronic effects of treatment with NO-aspirin or regular aspirin on aortic lesion development, plasma LDL oxidation, and oxidative stress, as well as oxidation-specific epitopes of LDL in the arterial wall.
Materials and Methods
Drugs and Experimental Protocol.
The experiments conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85–23, revised 1996) and the Guidelines of the American Heart Association. The experiments described here were carried out on male LDL-receptor-deficient mice of 18 weeks on high-cholesterol and cholate-free diet (21% by weight fat, 0.15% by weight cholesterol, and 19.5% by weight casein; no. 8137, Teklad, Madison, WI). LDL-receptor-deficient mice crossed with C57BL/6J mice for 10 generations, develop only “moderately” elevated plasma cholesterol levels (250–300 mg/dl) when fed regular mouse chow (10, 11). However, high cholesterol levels are easily achieved by enriched-cholesterol diets that induce extensive atherosclerosis throughout the arterial tree (10, 11). We selected only male mice to avoid gender-related differences (10). Mice were assigned randomly to be treated for 12 weeks with 30 mg/kg day of NCX-4016 (a generous gift from NicOx; n = 10, 30-mg compound contains 18 mg of aspirin) or 18 mg/kg/day of aspirin (Sigma; n = 10), or placebo (saline vehicle) given by gavage. These drug doses were chosen on the basis of previous studies in vivo (12, 16, 17) and did not affect blood pressure in mice measured by tail cuff (P = NS, not shown). At the end of the study, mice were killed with a lethal dose of sodium pentobarbital and in situ fixation of the aorta at physiologic pressure [100 mmHg (1 mmHg = 33 Pa)] was performed with PBS/paraformaldehyde (4%, 0.1 mol/liter, pH 7.3) for histology and normal saline for immunohistochemistry (see below).
Plasma Determination and LDL Oxidation.
Blood was collected at the time of killing into Eppendorf tubes with 1 mM Na2EDTA. Plasma cholesterol was determined enzymatically (18, 19). LDL particles (d = 1.006 − 1.063 g/ml) were isolated from 2 ml of pooled plasma from two animals of each group by sequential-density ultracentrifugation (18, 19). The protein content of LDL was measured by the method of Lowry (20). Susceptibility of LDL to in vitro oxidation was induced by 1 μM copper sulfate at 37°C for 12 h, as described (18, 19, 21). At the end of the incubation, the formation of thiobarbituric acid reactive substances was determined by the thiobarbituric acid method, as described (18, 19, 21). Lag-time was determined by monitoring the changes measured at 234 nm in the absorbance and observed at room temperature (23°C) every 10 min for a period of 4 h (19, 21). Measurement of the isoprostane 8-epi-PGF2 purified from plasma samples was made by using a commercially available immunoassay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Morphometric Assessment of Lesions and Immunohistochemistry of Lesion Components.
The aorta was dissected, cleaned of adherent fat and fascia, cut open, washed thoroughly with cold sterile PBS containing 2 mM EDTA, placed in ice-cold PBS containing 50 μM butylated hydroxytoluene, 0.001% aprotinin, 50 mM EDTA, and 0.008% chloramphenicol, and equilibrated with nitrogen (10, 11, 22, 23). Each arterial segment then was divided into two parts. One of these was immersed in cysteine prodrug 2-oxothiazolidine-4-carboxylate (5 mM)-containing medium (Dako, Milan) and flash frozen in liquid nitrogen; 7-μm-thick sections were taken and prepared with a cryotome for computer-assisted morphometric determination of lipid-rich lesions (30 cryo-sections from arteries were stained with oil red-O and counterstained with hematoxylin), as described in detail (10, 11, 16, 22, 23). The second part of each arterial segment was fixed in buffered 10% formalin and paraffin embedded; 12–15 serial sections (5-μm thickness) were prepared for immunohistochemistry (10, 11, 16, 22, 23). Duplicate serial sections of the fixed and paraffin-embedded arterial segments were immunostained with E06, murine monoclonal antibody against oxidation-specific-lysine and oxidized phospholipid epitopes of ox-LDL, and F4/80, a monoclonal antibody against mouse monocyte/macrophages-derived foam cells (10, 11, 16, 22, 23). Antibodies were used at a dilution of 1:500. Epitopes recognized by the primary antibody were detected by an avidin-biotin-peroxidase computer-assisted method (10, 11, 16, 22, 23).
Statistical Analysis.
Results are expressed as mean ± SEM. Evaluation of the atherogenesis and the immunohistochemistry were performed in a blinded way regarding the treatment given to mice. A Student's t test was used to compare differences among groups. Statistical significance was defined as P < 0.05.
Results
Lipid Profile.
Plasma cholesterol levels were similar among groups of LDL-receptor-deficient mice (724 ± 68 mg/dl, 746 ± 72 and 738 ± 57 in placebo, NCX-4016 and aspirin-treated groups, respectively; P = NS for all comparisons). Similarly, plasma triglyceride levels were comparable in all three groups of mice (165 ± 22 mg/dl, 172 ± 32, and 170 ± 28 in placebo, NCX-4016, and aspirin-treated groups, respectively; P = NS for all comparisons).
Evaluation of Atherogenesis.
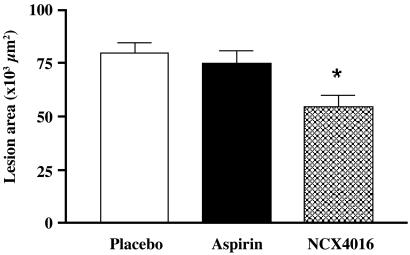
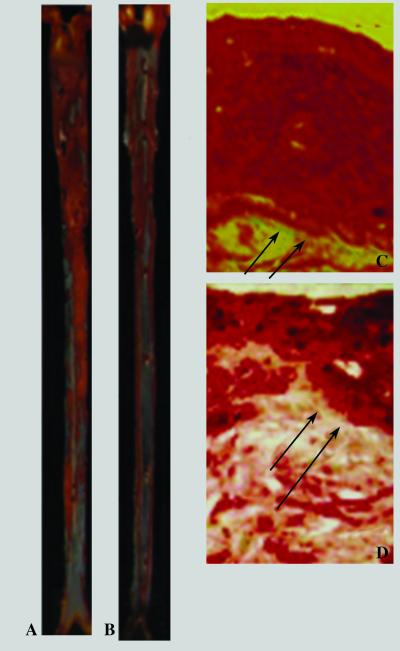
Computer-assisted imaging analysis revealed that 30 mg/kg of NCX-4016 reduced the aortic cumulative lesion area by 39.8 ± 12.3% compared with that of the placebo (P < 0.001). In another set of experiments (n = 5), 30 mg/kg of NCX-4016 reduced the aortic cumulative lesion area by 24.1 ± 10.8% (P = 0.589, NS). The equimolar dose of aspirin (18 mg/kg) did not reduce significantly aortic lesions (−5.1 ± 2.3%) compared with the placebo (P = 0.867, NS; Fig. 1). Fig. 2 shows some examples of high magnifications of oil-red O-stained aorta of a placebo-treated mouse (A) and NCX-4016-treated mouse (B). The reduction of the atherosclerotic lesions was coupled with a marked decrease in the thickness of lesions (oil-red O staining) of a NCX-4016-treated mouse in comparison to the placebo-treated mouse (C).
Fig 1.
Effects of various treatments on atherosclerotic lesion area in male LDL-receptor-deficient mice after 12 weeks of treatment with placebo, regular aspirin (18 mg/kg), or equimolar doses of nitric oxide-releasing aspirin (NCX-4016, 30 mg/kg). Mean lesion area of oil-red O-stained sections was calculated by computer-assisted imaging analysis. Results are expressed as the mean ± SEM of the aortic lesions of 10 animals from each group. *, P < 0.01 vs. placebo-treated mice.
Fig 2.
High-magnifications (×25) of oil-red O-stained thoracic aorta of placebo-treated mouse (A), placebo-treated mouse (B), and nitric oxide-releasing aspirin-treated mouse (30 mg/kg). Sustained reduction of the thickness of lesions of nitric oxide-releasing aspirin-treated mouse (D) in comparison to the placebo-treated mouse (C) (both ×320 magnification). Arrows indicate the degree of staining in relation to the intima.
Immunohistochemistry.
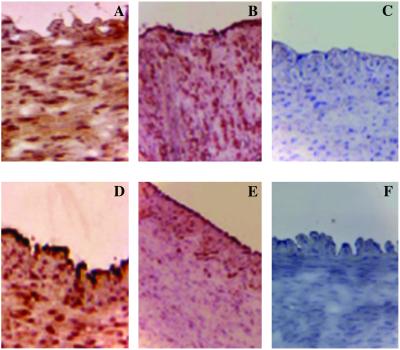
Mice treated with 30 mg/kg/day of NCX-4016 revealed a significant decrease of intimal macrophages–derived foam cells (−28.3 ± 10.2% of F4/80-positive arterial sections, P < 0.05 vs. placebo-treated group) and oxidation-specific epitopes of oxidized LDL by (−35.8 ± 11.9% of E06-positive arterial section, P < 0.01 vs. placebo-treated group; Fig. 3). Thus, NCX-4016 significantly reduced the expression of oxidation-specific epitopes and macrophage accumulation in the arterial wall compared with that of the placebo-treated group as well as aspirin-treated group.
Fig 3.
Treatment with (C) 30 mg/kg nitric oxide-releasing aspirin (NCX-4016) was more effective than (B) 18 mg/kg of regular aspirin or (A) placebo in reducing oxidation-specific epitopes in E06-positive sections (×275). Indeed, placebo-treated animals had a diffuse staining for oxidation-specific epitopes (A). This staining was partially reduced and increased in the subendothelial space in aspirin-treated mice (B). Nitric oxide-releasing aspirin reduced the overall immunostaining throughout the serial section (C). Similarly, macrophage accumulation was reduced in F4/80 positive sections in nitric oxide-releasing aspirin-treated animals (F) when compared with regular aspirin-treated (E) or placebo-treated (D) LDL-receptor-deficient mice (×275). The negative immunostaining (brown) in C and F appears in blue.
Effect of Different Treatments on Plasma LDL Oxidizability and Oxidative Stress.
Treatment with 30 mg/kg/day of NCX-4016 reduced significantly plasma LDL oxidation and systemic oxidative stress compared with both placebo and, to a lesser extent, aspirin (Table 1). This reduction of plasma LDL oxidation was shown by significant reduction of LDL malondialdehyde content of around 40% (P < 0.001 vs. placebo; P < 0.04 vs. aspirin), as well as by the prolongation of lag-time of oxidizability of around 20% (P < 0.01 vs. placebo; P < 0.05 vs. aspirin). In the same group of mice above (n = 5) treated with 10 mg/kg of NCX-4016, the compound reduced LDL malondialdehyde content to 19.3 ± 4.0 nmol/mg of protein (P < 0.05 vs. placebo; P = NS vs. aspirin) and LDL lag-time reached 120 ± 33 min (P = NS vs. placebo and aspirin). Similarly, plasma isoprostanes were reduced significantly by treatment with NCX-4016 (Table 1).
Table 1.
Parameters of susceptibility to ex vivo peroxidation of LDL and systemic oxidative stress in LDL-receptor-deficient mice treated with nitric oxide-releasing aspirin (NCX-4016) or regular aspirin
| LDL lag-time, min | LDL MDA, nmol/mg prot | Plasma isoprostane 8-epi-PGF2, pg/ml | |
|---|---|---|---|
| Placebo-treated LDL-receptor-deficient mice (n = 10) | 112 ± 22 | 24.5 ± 4.2 | 143 ± 37 (n = 6) |
| NCX-4016-treated LDL-receptor-deficient mice (n = 10) | 131 ± 18 | 14.3 ± 2.4 | 119 ± 21 (n = 6) |
| Aspirin-treated LDL-receptor-deficient mice (n = 10) | 115 ± 15 | 22.3 ± 4.7 | 128 ± 38 (n = 6) |
LDL-receptor-deficient mice treated with NCX-4016 (30 mg/kg/day), and aspirin (18 mg/kg/day); MDA, malondialdehyde at 12 h after exposure of LDL to 1 μM copper sulphate (n = 10 for each group). Lag-time represents an index of LDL oxidizability; increased values of lag-time reflect increased resistance of LDL to oxidative modification (n = 10 for each group, see also refs. 19 and 21). In a subset of animals (n = 6), plasma isoprostane levels (8-epi PGF2) were measured.
, P < 0.05 or
, P < 0.01 vs. placebo or aspirin-treated mice;
, P < 0.05 vs. placebo-treated mice by ANOVA followed by t test and Bonferroni's correction. See text for further details.
Discussion
Our article demonstrates that chronic delivery of NO achieved with the NO-releasing aspirin significantly attenuates the development of a chronic disease such as atherosclerosis in hypercholesterolemic LDL receptor-deficient mice without affecting plasma cholesterol levels. The enhancement of the NO pathway may play an important role in antiatherogenic effect of NO-releasing aspirin (reviewed in ref. 4). This study also demonstrates that in parallel to the attenuation of atherosclerosis, NO-aspirin reduced the susceptibility ex vivo of plasma LDL to oxidative modification and systemic oxidative stress measured by plasma isoprostanes. Isoprostane levels are a well recognized indicator of oxidative stress in animal models and in humans (24). Antioxidant protection could be related to the scavenging activity of free radicals by NO-containing aspirin both in plasma and in the arterial wall. Superoxide anion and NO are known to react rapidly to form the stable peroxynitrite anion, and peroxynitrite decomposition generates a strong oxidant with reactivity similar to hydroxyl radical (25). However, the causal role of peroxynitrite in atherogenesis is not established. Nevertheless, the properties of NO-releasing aspirin can also affect multiple radical species generated in the arterial wall. An increasing number of compounds releasing NO or modulating the NO pathway are now available (reviewed in ref. 26). Further studies should evaluate whether these newly developed compounds, clinically used drugs, or other NO-donors could be helpful in retarding atherosclerotic lesion formation and its clinical sequelae.
Oxidative modification of LDL plays a crucial role in human early atherosclerotic lesions (22, 23, 27) leading to atherosclerosis-related diseases (5, 6). Some studies (28) also demonstrated the important role of inhibition of LDL oxidation on the attenuation of atherosclerosis in hypercholesterolemic mice. In the present study, we showed that NCX-4016 reduced formation in the arterial wall of oxidation-specific epitopes of oxidized LDL. Thus, NCX-4016 has a potent antioxidant effect also in the atherosclerotic lesions of mice probably by means of scavenging of the radical-induced oxidation of LDL also in the arterial wall. Oxidized LDL may induce apoptosis in human coronary cells (7, 8). This phenomenon may favor the development of unstable atherosclerotic lesions (7, 8). However, apoptosis in macrophages also may reduce a potential source of mediators which can contribute to destabilizing the plaque (e.g., metalloproteinases and MCP-1). Nevertheless, the reduction of oxidative stress in vivo could also attenuate the degree of unstable atheroma. In another experimental setting, we showed recently that NCX-4016 reduced restenosis after arterial injury and macrophage deposition in hypercholesterolemic mice (16) and in aged rats (17), perhaps, at least in part, through its antioxidant effects. These properties may be particularly useful when applied to hypercholesterolemic or elderly patients. Obviously, the chronic development of atherosclerosis is a completely different pathophysiological condition from restenosis after arterial injury. Indeed, restenotic inflammatory lesions already appear after 14 days from the arterial injury. In the present study, we have also shown that the antiatherogenic effect was coupled to the reduction of macrophage-derived foam cells at the site of lesions. This beneficial effect may contribute to the reduction of lesion progression observed in hypercholesterolemic mice, and it is also consistent with the inhibition of macrophage-dependent LDL oxidation by in vitro NO donors (26, 29).
The findings of the present study are in agreement with an important role of NO in the development of atherogenesis in hypercholesterolemic mice. Accordingly, the role of endogenous NO in the progression of atherosclerosis in apolipoprotein E-knockout mice was recently investigated by using N(omega)-nitro-l-arginine methyl ester (l-NAME), an inhibitor of nitric oxide synthase (NOS) or with the NOS substrate l-arginine for 8 weeks (30). l-NAME treatment resulted in a significant inhibition of NO-mediated vascular responses and a significant increase in the atherosclerotic plaque area in the aorta of these mice. In contrast, l-arginine treatment had no influence on endothelial function and did not alter lesion size. The acceleration in lesion size concomitant to the severely impaired NO-mediated responses indicates that lack of endogenous NO (4, 31) is an important progression factor of atherosclerosis in the apolipoprotein E-knockout mouse.
We conclude that enhanced NO release by chronic treatment with NO-containing aspirin attenuates the development of a chronic disease such as atherosclerosis in hypercholesterolemic mice. Although the natural history of the atherosclerotic disease is different between rodents and humans, these data should be consider a further piece of evidence supporting the key role of NO in atherogenesis. Inhibition of oxidation-sensitive mechanisms by NO-aspirin, and possible other NO-related anti-inflammatory effects (reviewed in ref. 32), both in plasma and in atherosclerotic lesions, together with reduced macrophage accumulation, may have an important role in contributing to this antiatherogenic effect.
Acknowledgments
This work was supported by National Institutes of Health Grants HL57665, HL41371, and HL64793, and by Italian Institutes of Health Grant ISNIH.99.56980.
Abbreviations
LDL, low-density lipoprotein
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ludmer P. L., Selwyn, A. P., Shook, T. L., Wayne, R. R., Mudge, G. H., Alexander, R. W. & Ganz, P. (1986) N. Engl. J. Med. 315, 1046-1051. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro L. J., Cirino, G., Casini, A. & Napoli, C. (1999) J. Cardiovasc. Pharmacol. 34, 876-884. [DOI] [PubMed] [Google Scholar]
- 3.Drexler H. (1999) Cardiovasc. Res. 43, 572-579. [DOI] [PubMed] [Google Scholar]
- 4.Napoli C. & Ignarro, L. J. (2001) Nitric Oxide 5, 88-97. [DOI] [PubMed] [Google Scholar]
- 5.Witztum J. L. & Steinberg, D. (1991) J. Clin. Invest. 88, 1785-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg D. (1997) J. Biol. Chem. 272, 20963-20966. [DOI] [PubMed] [Google Scholar]
- 7.de Nigris F., Maida, I., Palumbo, G., Anania, V. & Napoli, C. (2000) Biochem. Pharmacol. 59, 1477-1487. [DOI] [PubMed] [Google Scholar]
- 8.Napoli C., Quehenberger, O., de Nigris, F., Abete, P., Glass, C. K. & Palinski, W. (2000) FASEB J. 14, 1996-2007. [DOI] [PubMed] [Google Scholar]
- 9.de Nigris F., Youssef, T., Ciafrè, S., Franconi, F., Anania, V., Condorelli, G., Palinski, W. & Napoli, C. (2000) Circulation 102, 2111-2117. [DOI] [PubMed] [Google Scholar]
- 10.Palinski W., Napoli, C. & Reaven, P. D. (2000) in Harvard Series, eds. Simon, D. I. & Rogers, C. (Humana, Totowa, NJ), pp. 149–174.
- 11.Napoli C., de Nigris, F., Welch, J. S., Calara, F. B., Stuart, R., Glass, C. K. & Palinski, W. (2002) Circulation 105, 1360-1367. [DOI] [PubMed] [Google Scholar]
- 12.Del Soldato P., Sorrentino, R. & Pinto, A. (1999) Trends Pharmacol. Sci. 8, 319-323. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S. H., Reddick, R. L., Piedrahita, J. & Maeda, N. (1992) Science 258, 468-471. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima Y., Plump, A. S., Raines, E. W., Breslow, J. L. & Ross, R. (1994) Arterioscler. Thromb. 14, 133-139. [DOI] [PubMed] [Google Scholar]
- 15.Reddick R. L., Zhang, S. H. & Maeda, N. (1994) Arterioscler. Thromb. 14, 141-148. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C., Cirino, G., Del Soldato, P., Sorrentino, R., Sica, V., Condorelli, M., Pinto, A. & Ignarro, L. J. (2001) Proc. Natl. Acad. Sci. USA 98, 2860-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Napoli C., Aldini, G., Wallace, J. L., de Nigris, F., Maffei, R., Abete, P., Bonaduce, D., Condorelli, G., Rengo, F., Sica, V., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napoli C., Chiariello, M., Palumbo, G. & Ambrosio, G. (1996) Cardiovasc. Drugs Ther. 10, 417-424. [DOI] [PubMed] [Google Scholar]
- 19.Napoli C., Mancini, F. P., Corso, G., Malorni, A., Crescenzi, E., Postiglione, A. & Palumbo, G. (1997) J. Biochem. 121, 1096-1101. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O. H., Rosebrough, N. J. & Farr, L. (1951) J. Biol. Chem. 193, 265-275. [PubMed] [Google Scholar]
- 21.Napoli C., Postiglione, A., Triggiani, M., Corso, G., Palumbo, G., Carbone, V., Rocco, A., Ambrosio, G., Montefusco, S., Malorni, A., et al. (1995) Atherosclerosis 11, 263-275. [DOI] [PubMed] [Google Scholar]
- 22.Napoli C., D'Armiento, F. P., Mancini, F. P., Palumbo, G., Witztum, J. L. & Palinski, W. (1997) J. Clin. Invest. 100, 2680-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Napoli C., Witztum, J. L., de Nigris, F., D'Armiento, F. P. & Palinski, W. (1999) Circulation 99, 2003-2010. [DOI] [PubMed] [Google Scholar]
- 24.Witztum J. L. & Berliner, J. A. (1998) Curr. Opin. Lipidol. 9, 441-448. [DOI] [PubMed] [Google Scholar]
- 25.Beckman J. S., Beckman, T. W., Chen, J., Marshall, P. A. & Freeman, B. A. (1990) Proc. Natl. Acad. Sci. USA 87, 1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ignarro L. J., Napoli, C. & Loscalzo, J. (2002) Circ. Res. 90, 21-28. [DOI] [PubMed] [Google Scholar]
- 27.Napoli C., Glass, C. K., Witztum, J. L., D'Armiento, F. P., Deutch, R. & Palinski, W. (1999) Lancet 354, 1234-1241. [DOI] [PubMed] [Google Scholar]
- 28.Aviram M., Maor, I., Keidar, S., Hayek, T., Oiknine, J., Bar-El, Y., Adler, Z., Kertzman, V. & Milo, S. (1995) Biochem. Biophys. Res. Commun. 216, 501-513. [DOI] [PubMed] [Google Scholar]
- 29.Hogg N., Struck, A., Goss, S. P. A., Santanam, N., Joseph, J., Parthasarathy, S. & Kalyanaraman, B. (1995) J. Lipid Res. 36, 1756-1762. [PubMed] [Google Scholar]
- 30.Kauser K., da Cunha, V., Fitch, R., Mallari, C. & Rubanyi, G. M. (2000) Am. J. Physiol. 278, H1679-H1685. [DOI] [PubMed] [Google Scholar]
- 31.Rudic R. D. & Sessa, W. C. (1999) Am. J. Hum. Genet. 64, 673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace J. L., Ignarro, L. J. & Fiorucci, S. (2002) Nat. Rev. Drug Discovery 1, 375-382. [DOI] [PubMed] [Google Scholar]