Abstract
Genetic manipulation of the α2A-adrenergic receptor (α2A-AR) in mice has revealed the role of this subtype in numerous responses, including agonist-induced hypotension and sedation. Unexpectedly, α2-agonist treatment of mice heterozygous for the α2A-AR (α2A-AR+/−) lowers blood pressure without sedation, indicating that more than 50% of α2A-AR must be activated to evoke sedation. We postulated that partial activation of α2A-AR in wild-type α2A-AR+/+ animals could be achieved with partial agonists, agents with variable ability to couple receptor occupancy to effector activation, and might elicit one versus another pharmacological response. In vitro assays reveal that moxonidine is a partial agonist at α2A-AR. Although moxonidine was developed to preferentially interact with imidazoline binding sites, it requires the α2A-AR to lower blood pressure because we observe no hypotensive response to moxonidine in α2A-AR-null (α2A-AR−/−) mice. Moreover, we observe that moxonidine lowers blood pressure without sedation in wild-type mice, consistent with the above hypothesis regarding partial agonists. Our findings suggest that weak partial agonists can evoke response-selective pathways and might be exploited successfully to achieve α2A-AR pharmacotherapy where concomitant sedation is undesirable, i.e., in treatment of depression or attention deficit hyperactivity disorder, in suppression of epileptogenesis, or enhancement of cognition. Furthermore, rigorous physiological and behavioral assessment of mice heterozygous for particular receptors provides a general strategy for elucidation of pathways that might be selectively activated by partial agonists, thus achieving response-specific therapy.
One approach to refining therapeutic specificity is to develop subtype-selective agents to activate or block receptor response. Gene manipulation in mice reveals which receptor subtype elicits a particular physiological or behavioral response in vivo, and has revealed the crucial role of the α2A-adrenergic receptor (α2A-AR) subtype in mediating the ability of α2-agonists to elicit hypotension (1–3), sedation (4), anesthetic sparing and analgesia (4, 5), hypothermia (6, 7), synergism with opiates in the antinociceptive response (5), and enhancement of working memory in mice (10). The latter finding suggests that the beneficial effects of guanfacine in working memory in primates (11) and in the enhancement of attentional focus in patients with attention deficit hyperactivity disorder (12) is attributable to α2A-AR actions. The α2A-AR subtype also is implicated in endogenous catecholamine-mediated suppression of epileptogenesis (8) and protection in models of depression and anxiety (9). The sedative properties of α2-agonists, valuable when used as preanesthetic or anesthetic sparing agents, limit their usefulness in cognitive enhancement (13, 14) or in treating attentional deficits (12, 15, 16). Thus, subtype-selective agonists alone cannot assure optimized therapeutic intervention. However, the present findings suggest that differing α2A-AR responses can be selectively triggered by low-efficacy partial agonists at α2A-AR, and that rigorous characterization of mice heterozygous for a particular receptor may provide a general strategy for inferring which receptor-mediated effects might by amenable to this pharmacological approach.
Materials and Methods
Animals.
Wild-type (WT), α2A-ARD79N/D79N (1), and α2A-AR−/− (2) mice, all on the C57BL/6 background, were maintained at the Vanderbilt University Animal Care Facility in accordance with procedures of the Animal Welfare Act and the 1989 Amendments to this Act.
Cell Culture.
Cells stably expressing HA-tagged WT mouse α2A-AR at two different densities (HEK 293: 7.6 pmol/mg protein, and NIH 3T3: 0.82 pmol/mg protein) were maintained in DMEM supplemented with 10% FCS and 100 units/ml penicillin G sodium with 100 μg/ml streptomycin sulfate.
Measurement of Cardiovascular Responses.
Male mice (8–12 weeks of age, >30 g) were anesthetized with 50 mg/kg sodium pentathol. The left femoral artery and right jugular vein were catheterized with PE-10 polyvinyl tubing. After 48 hours, the arterial catheter was connected by a TXD-300 Pressure transducer to a Gould Amplifier (Orlando, FL); systolic, diastolic, and mean arterial blood pressure and heart rate were recorded continually on a Blood Pressure Analyzer (MicroMed, Portsmouth, NH) and Gould 8 Channel Recorder. Stability of the baseline was established by infusions of saline through the right jugular vein. Vehicle (saline) injections had no effect on the cardiovascular responses. The α2-agonists were evaluated at 10 μg/kg body weight for dexmedetomidine and 300 μg/kg for moxonidine.
Measurement of Sedative Responses.
Male mice (8–12 weeks of age, >30 g) were evaluated in the rotarod and loss of righting reflex tests of sedation, as described (4). Mice were injected i.p. with saline or a single bolus dose of the indicated α2-agonist. Sedation was evaluated at 1,000 μg/kg for moxonidine, brimonidine (UK 14,304), and clonidine, and 433 μg/kg (maximal dose) or 2,165 μg/kg (supramaximal dose) of dexmedetomidine.
Radioligand Binding.
Assessing receptor density in mice of differing genotypes.
Receptor density in mouse brains was evaluated as described (1). Prazosin (1 μM) was added to block radioligand binding to α2B- and α2C-AR subtypes in mouse brain (1). The maximum binding capacity (Bmax; fmol/mg protein) and dissociation constant (Kd; nM) (95% confidence interval) were as follows: α2A-AR+/+, 190 (168–204) and 1.9 (1.6–2.2); α2A-ARD79N/D79N, 38 (35–40) and 1.9 (1.6–2.2); α2A-AR+/−, 92 (78–104) and 0.9 (0.5–1.4). Kd values did not differ between preparations derived from homozygous or heterozygous animals studied in the same experiment.
Competition binding to assess agonist affinity at mouse α2A-AR.
The affinity for agonists at the α2A-AR was assessed by means of competition for binding as described (1), in the presence of Gpp[NH]p (100 μM) to eliminate α2A-AR coupling to G proteins and thus any confounding effects of G protein regulation on receptor affinity for agonists. The Ki for each ligand in competing for radioligand binding to the α2A-AR was calculated from the EC50 for competition (17). Receptor occupancy was calculated as 100% − % [3H] Yohimbine binding remaining in the presence of competing agonist, as described (4).
Measurement of Mitogen-Activated Protein (MAP) Kinase Activation.
Stimulation of MAP kinase activity was assessed as described (18). Parental cell backgrounds expressing no murine HA-α2A-AR were also evaluated and demonstrated no MAP kinase activation in response to epinephrine (administered in the presence of 1 μM propranolol to block endogenous β-adrenergic receptors, should they exist), clonidine, dexmedetomidine, or moxonidine.
γ-[35S]Thio-GTP ([35S]) Binding to Assess Receptor Activation of G Proteins.
Cells expressing the murine HA-α2A-AR at 7.6 pmol/mg protein were harvested and assayed for GTP[γ35S] binding as described (19).
Results
Sedation Requires Greater than 50% α2A-AR Activation.
We initially manipulated the α2A-AR in mice by introducing a point mutation (D79N) into the α2A-AR locus by using hit-and-run homologous recombination strategies (1). Characterization of these mice revealed that instability of the mutant receptor (20, 21) led to an ≈80% loss of steady state functional receptor density (1) in addition to the intended altered coupling of the α2A-AR to G proteins and downstream signaling pathways (22). Mice expressing the D79N α2A-AR (α2A-ARD79N/D79N) lost a variety of α2 agonist-mediated responses, including sedation (Fig. 1; ref. 4). Surprisingly, mice heterozygous for the D79N α2A-AR mutation (α2A-AR+/D79N) also were not sedated in response to maximal (433 μg/kg) or even supramaximal (2,165 μg/kg) doses of the α2-agonist dexmedetomidine. Sedation in heterozygous mice is also lost in response to the α2-agonist UK 14,304, a clonidine analog (data not shown). Given the emerging literature on dimerization of G protein-coupled receptors (reviewed in refs. 23 and 24), one reasonable interpretation of these findings was that the D79N α2A-AR behaves as a dominant negative structure in dimers with the WT receptor, thus eliminating α2A-AR-mediated responses in the α2A-AR+/D79N animals. However, α2A-AR+/− heterozygous mice, created by crossing WT mice with α2A-AR−/− knockout mice, similarly demonstrated no sedative response to supramaximal doses of dexmedetomidine (Fig. 1), indicating that the D79N α2A-AR was not acting as a dominant negative structure in α2A-AR+/D79N mice, but rather that greater than 50% of α2A-AR must be activated to evoke the sedative response.
Fig 1.
Sedative response to the α2A-agonist dexmedetomidine requires more than 50% α2A-AR functional complement. Sedative responses to dexmedetomidine were measured as loss of righting reflex after i.p. injection of dexmedetomidine (Dex). *, No sedative response. The term [α2A-AR] reflects the relative concentration of α2A-AR, quantified by saturation binding, in particulate brain membrane preparations derived from mice of the indicated genotypes. Data are the mean ± SEM of 5–10 mice per group (a given genotype at a given dose of α2-agonist).
Blood Pressure Lowering Requires Less than 50% α2A-AR Activation.
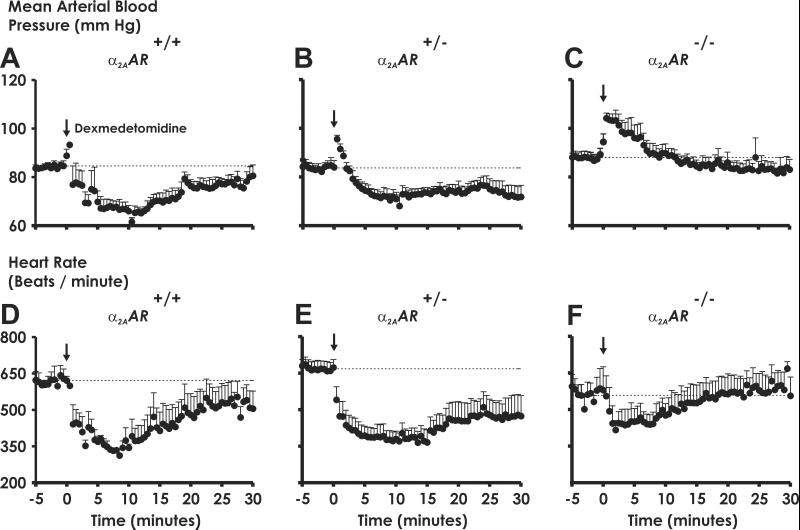
In conscious, freely moving α2A-AR+/+ WT mice, administration of dexmedetomidine evokes a transient hypertensive response followed by a longer-lived hypotensive response (Fig. 2A) paralleled by a decrease in heart rate (Fig. 2D). The α2-agonist-elicited hypotensive response is lost in α2A-AR−/− knockout mice, whereas the transient pressor response, shown to involve the α2B-AR subtype (25), is maintained and even exaggerated in these animals. Agonist treatment of mice heterozygous for the α2A-AR similarly elicits a prolonged hypotensive response (Fig. 2B) and bradycardia (Fig. 2E), in stark contrast to the loss of α2-agonist-elicited sedative responses in α2A-AR+/− mice (see Fig. 1). The finding that heterozygous animals retain cardiovascular responses but lose sedative effects to α2-agonists provides definitive evidence that differing fractional activation of α2A-AR is critical for evoking different receptor-mediated responses and suggests that selective manipulation of α2A-AR-mediated responses in different clinical settings, using drugs rather than differing receptor density to differentially activate the total receptor population, could provide response-specific therapy. Partial agonists, which induce or stabilize a receptor conformation that is less efficient in coupling to downstream effector systems (26), represent potential agents for eliciting a desired therapeutic endpoint (e.g., antihypertensive responses) without unwanted side effects (e.g., sedation).
Fig 2.
Less than 50% of functional α2A-AR is required for α2-agonists to elicit hypotension. Mean arterial blood pressure (A–C) and heart rate (D–F) in conscious, freely moving α2A-AR+/+ (A and D), heterozygous α2A-AR+/− (B and E), and α2A-AR−/− (C and F) mice were measured in response to bolus injections of dexmedetomidine (10 μg/kg) into the right jugular vein, defined as time 0, and were followed by a saline bolus wash of the same volume. ↓, Dexmedetomidine infusion. Data are the mean ± SEM of 5 mice per genotype per dexmedetomidine dose.
Moxonidine, Which Displays Partial Agonism at α2A-ARs, Requires the Expression of the α2A-AR to Lower Blood Pressure.
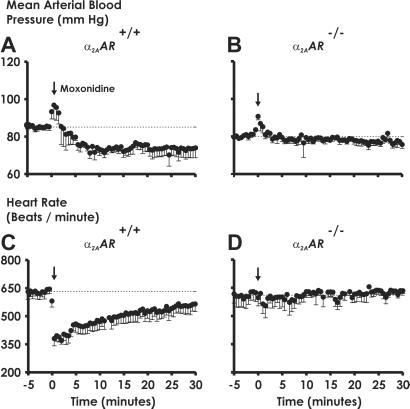
We postulated that moxonidine might behave as a response-selective partial agonist at α2A-ARs. Moxonidine evokes readily detectable cardiovascular responses in α2A-AR+/+ WT mice (Fig. 3 A and C), albeit to 64% of the maximal hypotensive response elicited by dexmedetomidine. The role of the α2A-AR subtype in mediating these effects of moxonidine is conclusively demonstrated by the loss of moxonidine-elicited hypotension (Fig. 3B) and bradycardia (Fig. 3D) in conscious, freely moving α2A-AR−/− knockout mice, consistent with previous findings in D79N α2A-AR mice (29). The ability of moxonidine to lower blood pressure in α2A-AR+/+ mice is not paralleled by a sedative response in these animals (Fig. 4).
Fig 3.
Moxonidine-elicited hypotensive responses require expression of the α2A-AR subtype. Mean arterial blood pressure (A and B) and heart rate (C and D) in conscious, freely moving α2A-AR+/+ (A and C), and α2A-AR−/− (B and D) mice were measured in response to bolus injections of moxonidine (300 μg/kg) as in Fig. 2. ↓, Moxonidine infusion. Data are the mean ± SEM of 5 mice per genotype per moxonidine dose.
Fig 4.
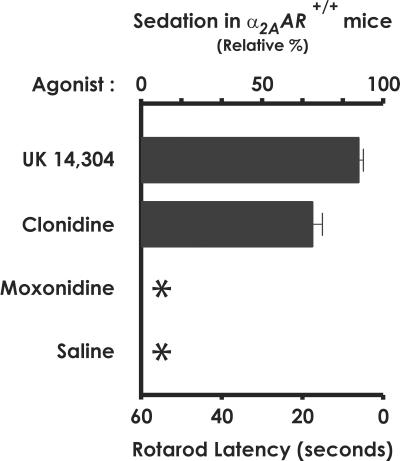
Moxonidine does not cause sedation in α2A-AR+/+ mice. Sedative responses to the α2-agonists UK 14,304, clonidine, moxonidine, or saline were measured as rotarod latency, or the time the mice were able to remain on a rotating bar. (The effects of dexmedetomidine were given in Fig. 1.) UK 14,304 and clonidine (1,000 μg/kg) are sedative in α2A-AR+/+ mice, causing these mice to fall from the rotarod bar after 6 and 17 seconds, respectively. In contrast, moxonidine (1,000 μg/kg) is indistinguishable from saline, e.g., nonsedating. *, No sedative response. Time 0 on the rotarod equals, by definition, 100% sedation. Data are the mean ± SEM of 5 α2A-AR+/+ mice for each α2-agonist evaluated.
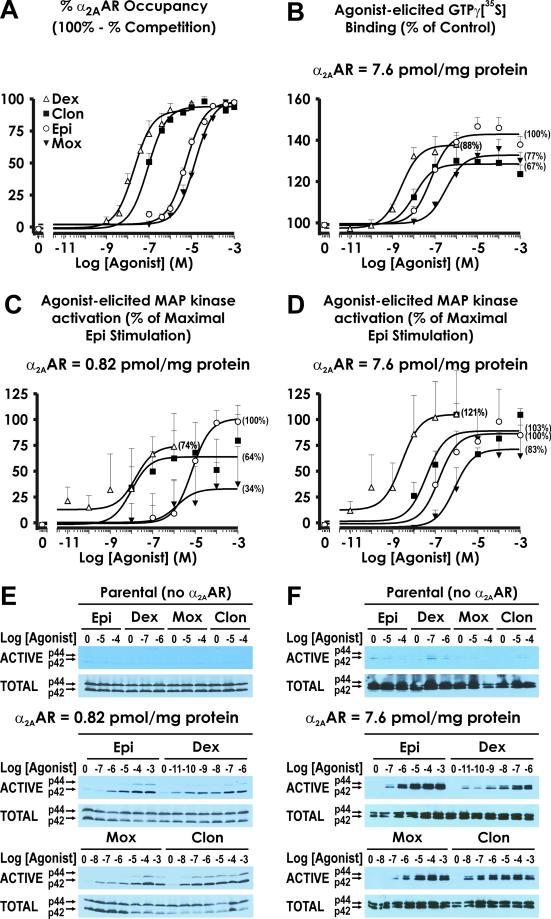
Although moxonidine was developed as an agent preferential for I1-imidazoline binding sites with an intentionally reduced affinity at α2-ARs (27), moxonidine possesses an affinity at the α2A-AR indistinguishable from that of epinephrine (Fig. 5A). We evaluated the ability of moxonidine to activate MAP kinase in cells expressing α2A-AR at 0.82 pmol/mg protein (Fig. 5C), a density comparable to that in mouse brain homogenates (0.2 pmol α2A-AR/mg protein; ref. 1). Dexmedetomidine, clonidine, and moxonidine all behave as partial agonists relative to epinephrine in activating MAP kinase, eliciting 74%, 64%, and 34% of the maximal response of epinephrine, respectively. Because the ability of partial agonists to activate biological systems can be increased in settings of increased receptor density (28), we evaluated the ability of agonists to activate MAP kinase in cells expressing α2A-AR at a nearly 10-fold greater receptor density, i.e., 7.6 pmol/mg protein. As demonstrated in Fig. 5D, the % maximal response for dexmedetomidine, clonidine, and moxonidine increased to 121%, 103%, and 83% of epinephrine, respectively, at this greater receptor density.
Fig 5.
Relationship of receptor occupancy to response. (A) Affinity of epinephrine (Epi, ○), moxonidine (Mox, ▾), dexmedetomidine (Dex, ▵), and clonidine (Clon, ▪) for the α2A-AR was assessed by using competition for [3H] Yohimbine binding under conditions where alterations in receptor affinity caused by receptor coupling to G proteins would not occur (see Materials and Methods). Data are presented as % occupancy (calculated as 100% - % competition for [3H] Yohimbine binding), and are the mean ± SEM for 5–10 independent experiments. (B) Agonist-stimulated GTP[γ35S] binding to G proteins in membrane preparations derived from cells expressing HA-α2A-AR at 7.6 pmol α2A-AR/mg protein is presented as % of control for epinephrine (○), moxonidine (▾), dexmedetomidine (▵), and clonidine (▪), where control represents GTP[γ35S] binding in the absence of agonist. Shown in parentheses in B–D for each curve are % response relative to that of epinephrine (defined as 100%). Data represent the mean ± SEM for 6–12 separate experiments. (C and D) Agonist-stimulated MAP kinase activity in cells expressing HA-α2A-AR at 0.82 pmol α2A-AR/mg protein (C) and at 7.6 pmol α2A-AR/mg protein (D) was calculated as [(p42 and p44 ACTIVE MAP kinase) ÷ (p42 and p44 TOTAL MAP kinase) − baseline (no agonist)] for epinephrine (○), moxonidine (▾), dexmedetomidine (▵), and clonidine (□), and was normalized to % of maximal epinephrine stimulation (maximal epinephrine stimulation: 22.9 arbitrary units = 100% in C, 48.0 arbitrary units = 100% in D). The data shown are mean ± SEM for 3 (C) and 4 (D) independent experiments performed under identical conditions. (E and F) Representative Western blots for ACTIVE (Upper) and TOTAL (Lower) MAP kinase activity. No stimulation of MAP kinase by the agonists evaluated is observed in parental cells serving as the background for the HA-α2A-AR-expressing cells. E and F correspond to a representative experiment performed in cells expressing the α2A-AR at 0.82 pmol/mg protein and 7.6 pmol/mg protein, respectively.
In cells overexpressing the α2A-AR at 7.6 pmol/mg protein, we also evaluated agonist stimulation of GTP[γ35S] binding (Fig. 5B). Moxonidine behaves as a less efficacious agonist than epinephrine in this in vitro system as well, achieving 77% of epinephrine, consistent with previous in vitro findings at human α2A-AR (29). Interestingly, a comparison of MAP kinase activation versus stimulation of GTP[γ35S] binding reveals that at 7.6 pmol/mg protein, dexmedetomidine is a “superagonist” (121% activation) of MAP kinase but a partial agonist (88% stimulation) of GTP[γ35S] binding.
Discussion
The present findings provide several novel insights. First, heterozygous α2A-AR+/− animals retain cardiovascular responses to α2-agonists but, interestingly, are not sedated even at supramaximal doses of dexmedetomidine (Fig. 1) or UK 14,304 (data not shown). These data provide definitive genetic evidence that sedation requires activation of greater than 50% of the α2A-AR population, consistent with the interpretation of previous studies in rats using covalent inactivation of amine-binding receptors (30) or regional antisense strategies (31) to incrementally diminish α2A-AR density. Our data affirm the formal possibility that differing fractional activation of receptors might evoke different receptor-mediated responses in different tissues, or in different neural pathways. In the presence of a full receptor complement (i.e., α2A-AR+/+ WT animals), differing fractional activation can be achieved either by administering incremental doses of a full agonist or by exploiting drugs that have diminished effectiveness in coupling receptor occupancy to effector activation, known as partial agonists.
Our data reveal that moxonidine, an agent developed to selectively interact with I1-imidazoline binding sites, requires the α2A-AR to elicit its effects: no hypotensive response to moxonidine occurs in α2A-AR−/− mice (Fig. 3B), though we cannot rule out the possibility that moxonidine also has independent effects, perhaps via I1-imidazoline binding sites, upstream of the α2A-AR (32). Compared with epinephrine, moxonidine has a decreased maximal activation of MAP kinase stimulation and of GTP[γ35S] binding in vitro. As predicted by our hypothesis that partial agonists might differentially modulate one versus another pharmacological pathway, in vivo treatment of WT α2A-AR+/+ mice with moxonidine recapitulates the phenotype of the α2A-AR+/− heterozygous animals in response to full or strong agonists, i.e., selectively lowering blood pressure without evoking sedation, thus mimicking the clinical profile of moxonidine in human beings (33, 34).
The sedation-free hypotensive effects of moxonidine may result both from its lower efficacy and its moderate affinity at α2A-AR. Thus, moxonidine is 34% as effective as epinephrine in activating MAP kinase in cells expressing 0.82 pmol α2A-AR/mg (Fig. 5 C and E) and is nonsedating. In contrast, clonidine and dexmedetomidine are 64% and 74%, respectively, as effective as epinephrine in activating MAP kinase (i.e., are above the 50% “threshold” suggested in studies of α2A-AR+/− heterozygous mice) and are sedating in vivo. However, it should also be noted that clonidine and dexmedetomidine have a much higher affinity at α2A-AR than either epinephrine or moxonidine (Fig. 5A); in vivo, their partial agonism may be compensated for, at least in part, by their longer lived receptor occupancy. However, we must acknowledge that in vitro assays are, at best, capricious predictors of in vivo response. First, we do not know the precise receptor density of the target cells or cellular pathway(s) in vivo. Furthermore, efficacy can vary for coupling to different effectors. For example, at 7.6 pmol α2A-AR/mg protein, dexmedetomidine is a partial agonist (88%) in evoking GTP[γ35S] binding but a “superagonist” (121% of the maximal response to epinephrine) in eliciting MAP kinase activation (see Fig. 5 B and D).
Our studies unveil a generalizable strategy, i.e., assessing physiological and behavioral responses in mice heterozygous for a particular receptor, to explore whether or not partial agonists might provide highly selective, if not response-specific, therapeutic tools in a particular setting. In cases where one but not another receptor-mediated response is lost in heterozygous animals, subsequent studies could identify partial agonists that are sufficiently efficacious to evoke a desired pharmacological response in WT mice but fortuitously ineffective in eliciting receptor-mediated responses associated with undesired clinical side effects.
Although mice heterozygous for particular G protein-coupled receptors have not been evaluated extensively, findings from mice heterozygous for the M2 muscarinic receptor reveal that greater than 50% receptor density is needed for oxotremorine-elicited tremors (35), suggesting that partial agonists at M2 muscarinic receptors might achieve negative chronotropy without undesired tremorogenic side effects. This same conceptual framework has also proven useful for other receptor families. Thus, findings that mice heterozygous for the nuclear hormone receptor peroxisome proliferator-activated receptor γ had enhanced insulin sensitivity with reduced adipocyte differentiation (36, 37) led to the identification a peroxisome proliferator-activated receptor γ partial agonist that significantly improves insulin sensitivity without significant adipogenic activity (38). These examples suggest that detailed characterization of heterozygous animals may be a fruitful approach for the identification and development of response-specific therapeutic agents for a variety of disease states.
Our data demonstrate that moxonidine behaves as a partial, or less efficacious (39), agonist at α2A-AR, and serves as a prototype for agents capable of selectively modulating cardiovascular responses without sedative side effects. Focusing on the development of α2A-AR partial agonists of differing relative efficacy could lead to refined strategies for treatment of attentional focus in attention deficit hyperactivity disorder (12, 16) or enhancement of cognition in the elderly (12). In addition, because the α2A-AR subtype appears to suppress epileptogenesis (8), it is provocative to speculate that partial α2A-agonists may provide the first antiepileptogenic agents, to complement the existing armamentarium of anticonvulsant drugs (40). The protective role of the α2A-AR against measures of depression and anxiety in mouse models suggest that partial agonists at α2A-AR also might ameliorate these behavioral disorders in human beings. Achieving these therapeutic outcomes without concomitant sedation is an extremely attractive possibility, and the present data provide a definitive proof of concept that the development of partial α2A-agonists with limited efficacy could refine therapeutic specificity, thereby increasing the breadth of treatable physiological and behavioral diseases.
Acknowledgments
We thank Drs. J. H. Exton, J. A. Oates, A. L. George, Jr. (Vanderbilt University), and Dr. A. F. T. Arnsten (Yale University, New Haven, CT) for critical reading of the manuscript, and Dr. T. P. Kenakin for thoughtful discussions. We are grateful to Dr. M. McDonald (Vanderbilt University) and the Murine Behavioral Core Facility for advice concerning the rotarod latency measure of sedation and Mr. E. Price, in the Vanderbilt Murine Cardiovascular Core Facility (Dr. T. Inagami, Director), for mouse cannulations. Supported by National Institutes of Health Grants HL43671 (to L.E.L.), National Institute of Health Medical Science Training Program Grant GM07347 (to M.H.W.), a Vanderbilt University Graduate Fellowship (to L.B.M.), and a Co-Operative Management and Evaluation of Targets (COMET) award from Bristol-Myers Squibb.
Abbreviations
α2-AR, α2-adrenergic receptor
MAP, mitogen-activated protein
GTP[γ35S], γ-[35S]thio-GTP
References
- 1.MacMillan L. B., Hein, L., Smith, M. S., Piascik, M. T. & Limbird, L. E. (1996) Science 273, 801-803. [DOI] [PubMed] [Google Scholar]
- 2.Altman J. D., Trendelenburg, A. U., MacMillan, L., Bernstein, D., Limbird, L., Starke, K., Kobilka, B. K. & Hein, L. (1999) Mol. Pharm. 56, 154-161. [DOI] [PubMed] [Google Scholar]
- 3.Hein L., Altman, J. D. & Kobilka, B. K. (1999) Nature (London) 402, 181-184. [DOI] [PubMed] [Google Scholar]
- 4.Lakhlani P. P., MacMillan, L. B., Guo, T. Z., McCool, B. A., Lovinger, D. M., Maze, M. & Limbird, L. E. (1997) Proc. Natl. Acad. Sci. USA 94, 9950-9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone L. S., MacMillan, L. B., Kitto, K. F., Limbird, L. E. & Wilcox, G. L. (1997) J. Neurosci. 17, 7157-7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter J. C., Fontana, D. J., Hedley, L. R., Jasper, J. R., Lewis, R., Link, R. E., Secchi, R., Sutton, J. & Eglen, R. M. (1997) Br. J. Pharmacol. 122, 1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallinen J., Link, R. E., Haapalinna, A., Viitamaa, T., Kulatunga, M., Sjoholm, B., Macdonald, E., Pelto-Huikko, M., Leino, T., Barsh, G. S., et al. (1997) Mol. Pharm. 51, 36-46. [DOI] [PubMed] [Google Scholar]
- 8.Janumpalli S., Butler, L. S., MacMillan, L. B., Limbird, L. E. & McNamara, J. O. (1998) J. Neurosci. 18, 2004-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schramm N. L., McDonald, M. P. & Limbird, L. E. (2001) J. Neurosci. 21, 4875-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franowicz, J. S., Kessler, L. E., Dailey Borja, C. M., Kobilka, B. K., Limbird, L. E. & Arnsten, A. F. T. (2002) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 11.Avery R. A., Franowicz, J. S., Studholme, C., van Dyck, C. H. & Arnsten, A. F. T. (2000) Neuropsychopharmacology 23, 240-249. [DOI] [PubMed] [Google Scholar]
- 12.Scahill L., Chappell, P. B., Kim, Y. S., Schultz, R. T., Katsovich, L., Shepherd, E., Arnsten, A. F., Cohen, D. J. & Leckman, J. F. (2001) Am. J. Psychiatry 158, 1067-1074. [DOI] [PubMed] [Google Scholar]
- 13.Kita T., Kagawa, K., Mammoto, T., Takada, K., Hayashi, Y., Mashimo, T. & Kishi, Y. (2000) Anesth. Analg. 90, 722-726. [DOI] [PubMed] [Google Scholar]
- 14.Correa-Sales C., Rabin, B. C. & Maze, M. (1992) Anesthesiology 76, 948-952. [DOI] [PubMed] [Google Scholar]
- 15.Huang R. & Hertz, L. (2000) Brain Res. 873, 297-301. [DOI] [PubMed] [Google Scholar]
- 16.Arsten A. F. T., Steere, J. C. & Hunt, R. D. (1996) Arch. Gen. Psychiatry 53, 448-455. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y. & Prusoff, W. H. (1973) Biochem. Pharmacol. 22, 3099-3108. [DOI] [PubMed] [Google Scholar]
- 18.Schramm N. L. & Limbird, L. E. (1999) J. Biol. Chem. 274, 24935-24940. [DOI] [PubMed] [Google Scholar]
- 19.Jasper J. R., Lesnick, J. D., Chang, L. K., Yamanishi, S. S., Chang, T. K., Hsu, S. A., Daunt, D. A., Bonhaus, D. W. & Eglen, R. M. (1998) Biochem. Pharmacol. 55, 1035-1042. [DOI] [PubMed] [Google Scholar]
- 20.Wilson M. H. & Limbird, L. E. (2000) Biochemistry 39, 693-700. [DOI] [PubMed] [Google Scholar]
- 21.Wilson M. H., Highfield, H. A. & Limbird, L. E. (2001) Mol. Pharm. 59, 929-938. [DOI] [PubMed] [Google Scholar]
- 22.Surprenant A., Horstman, D. A., Akbarali, H. & Limbird, L. E. (1992) Science 257, 977-980. [DOI] [PubMed] [Google Scholar]
- 23.Bouvier M. (2001) Nat. Rev. Neurosci. 2, 274-286. [DOI] [PubMed] [Google Scholar]
- 24.Brady A. E. & Limbird, L. E. (2001) Cell. Signal. 14, 297-309. [DOI] [PubMed] [Google Scholar]
- 25.Link R. E., Desai, K., Hein, L., Stevens, M. E., Chruscinski, A., Bernstein, D., Barsh, G. S. & Kobilka, B. K. (1996) Science 273, 803-805. [DOI] [PubMed] [Google Scholar]
- 26.Kenakin T. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 349-379. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler D., Haxhiu, M. A., Kaan, E. C., Papp, J. G. & Ernsberger, P. (1996) J. Cardiovasc. Pharmacol. 27, S26-S37. [DOI] [PubMed] [Google Scholar]
- 28.Furchgott R. F. (1964) Annu. Rev. Pharmacol. 4, 21-50. [Google Scholar]
- 29.Zhu Q. M., Lesnick, J. D., Jasper, J. R., MacLennan, S. J., Dillon, M. P., Eglen, R. M. & Blue, D. R., Jr. (1999) Br. J. Pharmacol. 126, 1522-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabin B. C., Reid, K., Guo, T. Z., Gustafsson, E., Zhang, C. & Maze, M. (1996) Anesthesiology 85, 565-573. [DOI] [PubMed] [Google Scholar]
- 31.Mizobe T., Maghsoudi, K., Sitwala, K., Tianzhi, G., Ou, J. & Maze, M. (1996) J. Clin. Invest. 98, 1076-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Head G. A. (1999) Ann. N.Y. Acad. Sci. 881, 279-286. [DOI] [PubMed] [Google Scholar]
- 33.Mitrovic V., Patyna, W., Hüting, J. & Schlepper, W. (1991) Cardiovasc. Drugs Ther. 5, 967-972. [DOI] [PubMed] [Google Scholar]
- 34.Chrisp P. & Faulds, D. (1992) Drugs 44, 993-1012. [DOI] [PubMed] [Google Scholar]
- 35.Gomeza J., Shannon, H., Kostenis, E., Felder, C., Zhang, L., Brodkin, J., Grinberg, A., Sheng, H. & Wess, J. (1999) Proc. Natl. Acad. Sci. USA 96, 1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miles P. D., Barak, Y., He, W., Evans, R. M. & Olefsky, J. M. (2000) J. Clin. Invest. 105, 287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubota N., Terauchi, Y., Miki, H., Tamemoto, H., Yamauchi, T., Komeda, K., Satoh, S., Nakano, R., Ishii, C., Sugiyama, T., et al. (1999) Mol. Cell 4, 597-609. [DOI] [PubMed] [Google Scholar]
- 38.Rocchi S., Picard, F., Vamecq, J., Gelman, L., Potier, N., Zeyer, D., Dubuquoy, L., Bac, P., Champy, M. F., Plunket, K. D., et al. (2001) Mol. Cell 8, 737-747. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson R. P. (1956) Br. J. Pharmacol. 11, 379-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara J. O. (2001) in Goodman & Gilman's The Pharmacological Basis of Therapeutics, eds. Hardman, J. G. & Limbird, L. E. (McGraw–Hill, New York), pp. 521–547.