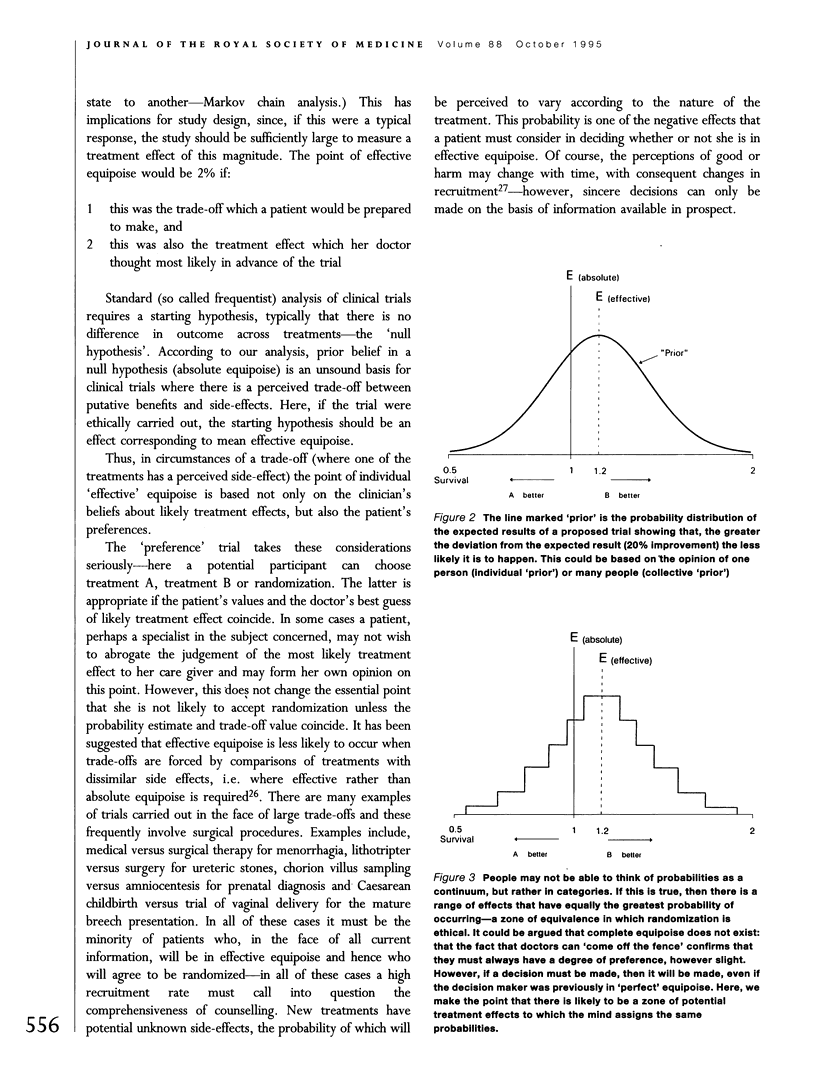
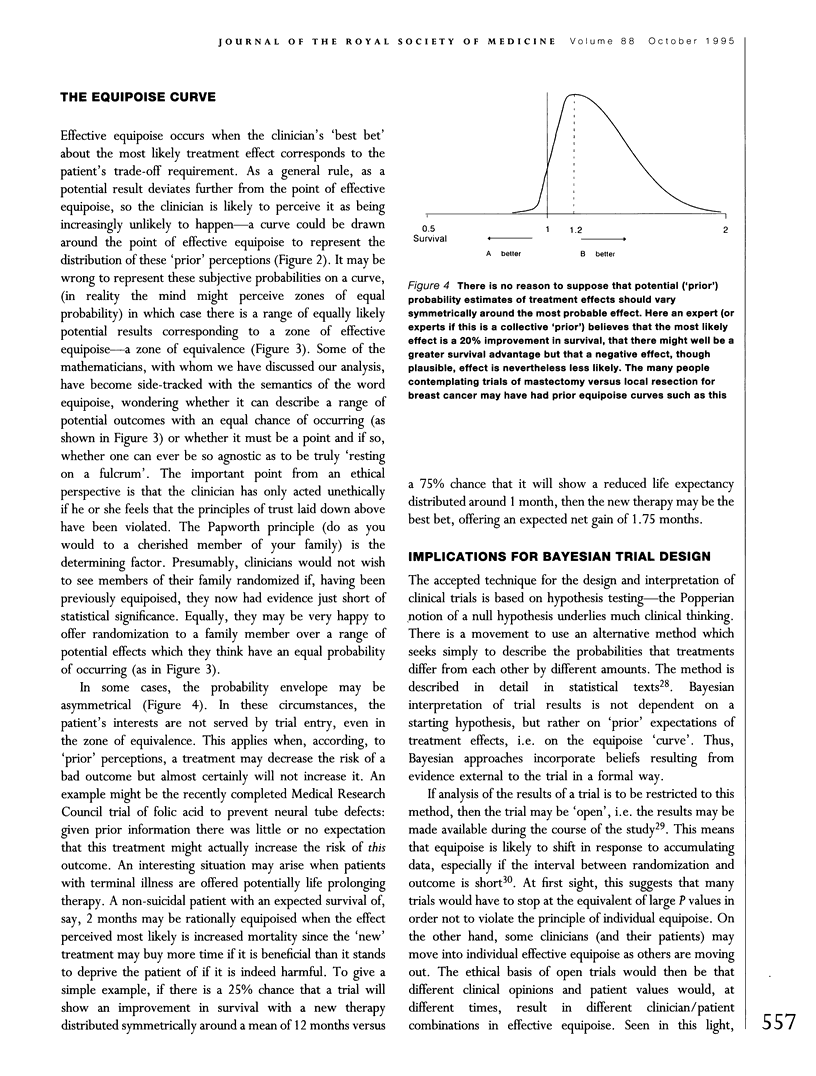
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassileth B. R., Zupkis R. V., Sutton-Smith K., March V. Informed consent -- why are its goals imperfectly realized? N Engl J Med. 1980 Apr 17;302(16):896–900. doi: 10.1056/NEJM198004173021605. [DOI] [PubMed] [Google Scholar]
- Challah S., Mays N. B. The randomised controlled trial in the evaluation of new technology: a case study. Br Med J (Clin Res Ed) 1986 Mar 29;292(6524):877–879. doi: 10.1136/bmj.292.6524.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers I., Chalmers T. C. Randomisation and patient choice. Lancet. 1994 Sep 24;344(8926):892–893. doi: 10.1016/s0140-6736(94)92867-3. [DOI] [PubMed] [Google Scholar]
- Dudley H. A. Stones, lithotripters, trials, and arguments. Br Med J (Clin Res Ed) 1986 Mar 29;292(6524):846–847. doi: 10.1136/bmj.292.6524.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987 Jul 16;317(3):141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- Freedman L. S., Spiegelhalter D. J. Application of Bayesian statistics to decision making during a clinical trial. Stat Med. 1992 Jan 15;11(1):23–35. doi: 10.1002/sim.4780110105. [DOI] [PubMed] [Google Scholar]
- Gilbert J. Evaluation of palliative care. Patients must be told that treatment will be randomised. BMJ. 1995 Jan 14;310(6972):125–125. doi: 10.1136/bmj.310.6972.125c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. The MRC and informed consent. Br Med J (Clin Res Ed) 1986 Jul 19;293(6540):157–158. doi: 10.1136/bmj.293.6540.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore S. M. The consumer principle of randomisation. Lancet. 1994 Jan 1;343(8888):58–58. doi: 10.1016/s0140-6736(94)90913-x. [DOI] [PubMed] [Google Scholar]
- Greenhouse J. B. On some applications of Bayesian methods in cancer clinical trials. Stat Med. 1992 Jan 15;11(1):37–53. doi: 10.1002/sim.4780110106. [DOI] [PubMed] [Google Scholar]
- Hughes M. D. Reporting Bayesian analyses of clinical trials. Stat Med. 1993 Sep 30;12(18):1651–1663. doi: 10.1002/sim.4780121802. [DOI] [PubMed] [Google Scholar]
- Johnson N., Lilford R. J., Brazier W. At what level of collective equipoise does a clinical trial become ethical? J Med Ethics. 1991 Mar;17(1):30–34. doi: 10.1136/jme.17.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadane J. B. Progress toward a more ethical method for clinical trials. J Med Philos. 1986 Nov;11(4):385–404. doi: 10.1093/jmp/11.4.385. [DOI] [PubMed] [Google Scholar]
- Korn E. L., Baumrind S. Randomised clinical trials with clinician-preferred treatment. Lancet. 1991 Jan 19;337(8734):149–152. doi: 10.1016/0140-6736(91)90809-4. [DOI] [PubMed] [Google Scholar]
- Lilford R. J., Johnson N. The alpha and beta errors in randomized trials. N Engl J Med. 1990 Mar 15;322(11):780–781. doi: 10.1056/NEJM199003153221119. [DOI] [PubMed] [Google Scholar]
- Lilford R. J., Thornton J. D. Decision logic in medical practice. The Milroy Lecture 1992. J R Coll Physicians Lond. 1992 Oct;26(4):400–412. [PMC free article] [PubMed] [Google Scholar]
- Sacks H., Chalmers T. C., Smith H., Jr Randomized versus historical controls for clinical trials. Am J Med. 1982 Feb;72(2):233–240. doi: 10.1016/0002-9343(82)90815-4. [DOI] [PubMed] [Google Scholar]
- Simes R. J., Tattersall M. H., Coates A. S., Raghavan D., Solomon H. J., Smartt H. Randomised comparison of procedures for obtaining informed consent in clinical trials of treatment for cancer. Br Med J (Clin Res Ed) 1986 Oct 25;293(6554):1065–1068. doi: 10.1136/bmj.293.6554.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias J. S., Souhami R. L. Fully informed consent can be needlessly cruel. BMJ. 1993 Nov 6;307(6913):1199–1201. doi: 10.1136/bmj.307.6913.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]


