Research on the genetic basis of mental disorders crossed a major watershed this summer. For the first time, specific genes have been discovered that influence susceptibility to schizophrenia, a psychosis that affects nearly 1% of people throughout the world and accounts for about 2.5% of health-care costs (1). In this issue of PNAS, Chumakov and colleagues (2) describe a new human gene, G72, on chromosome 13q34 that interacts with the gene for d-amino acid oxidase (DAAO) on 12q24 to regulate glutaminergic signaling through the N-methyl-d-aspartate (NMDA) receptor pathway. Using traditional positional cloning techniques of linkage and linkage disequilibrium, they show that both of these genes are associated with increased susceptibility to schizophrenia. Therefore, this is the first discovery of a specific gene that also provides a pathogenic molecular mechanism that can account for the major symptoms of a psychiatric disorder. Similarly, two other groups reported this summer that the gene dysbindin on 6p22.3 (3) and the gene neuregulin 1 on 8p (4) also influence susceptibility to schizophrenia and may operate via the same NMDA mechanism. Each of these gene discoveries came from association analysis targeting chromosomal regions first identified by linkage analysis. The success of groups working on three different chromosomal regions of interest confirms the effectiveness of traditional positional cloning techniques in complex mental disorders. Consequently, these results justify optimism for future progress in unraveling complex disorders in which there is interaction among multiple genetic and environmental variables. However, it is important to recognize both the strengths and the limitations of the genetic and functional strategies used by Chumakov and colleagues (2). It is also important to recognize the continuing significance of the prior work that laid the foundation for these particular experiments.
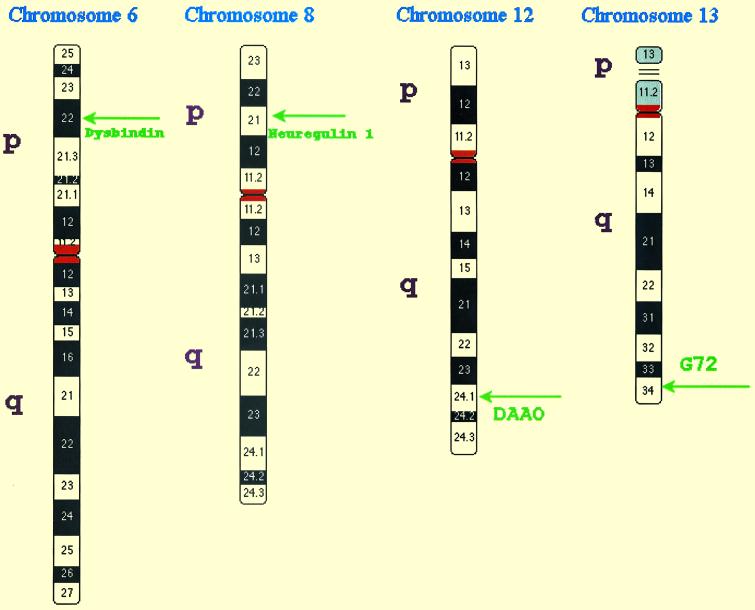
Twin and adoption studies demonstrated that susceptibility to schizophrenia is strongly heritable even if children are reared apart from their biological parents. When one twin has schizophrenia, the risk of schizophrenia in the co-twin is greater in monozygotic twins (45%) than in dizygotic twins (15%). However, 40% of the monozygotic co-twins of a person with schizophrenia are clinically normal (5). Furthermore, the risk of illness decreases with degree of genetic relationship more rapidly than can be explained by a single gene or the sum of effects of several such genes. Thus, the inheritance pattern of schizophrenia suggested that multiple genes, each of small effect, interacted nonlinearly with one another and with environmental factors to influence susceptibility (6, 7). This prediction has now been confirmed by more than 20 genomewide linkage scans in more than 1,200 families of schizophrenics. These studies found evidence for several genes of small effect; that is, genes that modify susceptibility but are neither necessary nor sufficient to cause the disorder. However, no evidence was found for any genes with a large individual effect, such as a Mendelian subtype of schizophrenia. By 1997 there were replications in some, but not all, linkage studies for susceptibility genes in regions of chromosomes 6p, 8p, and 22q (7). Now another 5 years of work by many groups has expanded the list of regions of interest to include target regions on 1q21–q22, 6q21–q22.3, and 13q34, as well as less consistent evidence for broad regions of 2q, 3p, 5q, 10p, and 11q (8). The chromosomal locations of the four recently discovered susceptibility genes for schizophrenia are illustrated schematically in Fig. 1. The linkage of schizophrenia to the 15q14 locus of the α-7 nicotinic receptor has also been replicated, but does not provide a pathogenic mechanism producing the major symptoms of schizophrenia (9).
Fig 1.
Chromosomal locations of four susceptibility genes for schizophrenia.
Until this summer, there was no success in the positional cloning of a susceptibility gene that could explain the major symptoms of schizophrenia or any other nondementing psychiatric disorder. When there are contributions to susceptibility from several genetic and environmental factors, as in schizophrenia, linkage analysis has much less sensitivity for detection of specific genes than does association analysis (10). The most efficient design for detection of specific genes in a complex disorder is the comparison of cases and controls (11). However, association studies in the past often have yielded false positive results because of population stratification and the low prior probability of true association. Consequently, the robustness of findings is improved by working in ethnically homogeneous samples and by targeting chromosomal regions with a high probability of true association based on prior evidence of linkage in the region. This is exactly what was done by Chumakov and colleagues (2). They carried out an association study in the region of 13q34 where they had found prior evidence of linkage. This linkage had been replicated in some other studies (12), but not all (8). Such variability in linkage in different populations is expected for a disorder that depends on the interaction of multiple factors. No particular gene is necessary or sufficient to cause disease, so different genes and environmental factors influence susceptibility in different families. Accordingly, Chumakov and colleagues carried out their initial association study in the sample of the French-Canadian population in which the initial linkage finding had been observed. In addition, they were able to replicate their discovery of a novel human gene they call G72 in an independent Russian sample. The replication of the association findings, following the replication of linkage in the targeted region, provides sound statistical support for G72 being a true susceptibility gene for schizophrenia.
What are even more persuasive, however, are the functional studies that were carried out. The functional studies identify a specific pharmacological mechanism that is already known to induce the symptoms of schizophrenia. G72 was found to interact with the gene for DAAO, which oxidizes d-serine. In turn, the binding of d-serine to the glycine modulatory site on the NMDA receptor is needed for glutamate to activate the receptor. Certain combinations of alleles of G72 and DAAO increased the risk of schizophrenia significantly more than the sum of their individual effects, which is evidence of what is called epistasis, or nonadditive gene–gene interaction (13). Thus the variant forms of genes on 13q and 12q interact to lower the activity of NMDA receptors, which is proposed as the molecular mechanism that increases susceptibility to schizophrenia.
Likewise, available evidence also suggests that the two susceptibility genes on 6p and 8p may also lower glutamate signaling through NMDA receptors. For example, mice with mutant neuregulin 1 have fewer functional NMDA receptors than WT mice and abnormal behavior similar to mouse models of schizophrenia; these abnormal behaviors are partially reversible with clozapine, an atypical antipsychotic drug used to treat schizophrenia (4). Likewise, dysbindin is known to regulate nicotinic receptors and recruit NO synthase, which in turn modulates NMDA receptor activity (3). Thus all four of the recently discovered genes for schizophrenia susceptibility may function by lowering glutamate activity in the brain through different effects on the NMDA receptor pathway. Extensive psychopharmacological work has shown that glutamate antagonists, such as phencyclidine (PCP), nitrous oxide, and ketamine, can induce a schizophrenia-like psychosis in normal individuals and also provoke a prolonged exacerbation of psychosis in stable chronic schizophrenics (14). In contrast, hallucinogens (such as LSD) and dopamine agonists (such as amphetamines) induce the positive symptoms (hallucinations and delusions) but not the negative symptoms (i.e., impoverishment of affect, thought, and initiative) or other cognitive disturbances characteristic of schizophrenia. The convergence of results from genetics and psychopharmacology helps to clarify the nature of this severe and complex mental disorder.
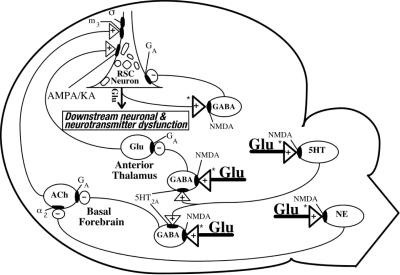
Nevertheless there are serious limitations to reverse genetic strategies in understanding a disorder as complex as schizophrenia. Linkage and linkage disequilibrium try to explain a disorder by identifying one or a few genes that cause the disorder. However, a multifactorial disorder cannot be completely understood in terms of any one-to-one relationships between gene and phenotype. The effects of any gene depend on nonlinear interactions with other genes and environmental factors that are expressed in varying situations during development across the lifespan. This complexity can be illustrated by considering the complex neural networks by which low glutamate activity or high dopamine activity can induce the symptoms of schizophrenia (14, 15). Glutamate is the principal excitatory neurotransmitter in the brain and also a major regulator of inhibitory tone. In certain brain circuits, glutamate activates NMDA receptors on γ-aminobutyric acid (GABA)ergic, serotonergic, and noradrenergic neurons, which in turn inhibit the activity of excitatory glutaminergic and cholinergic pathways. The circuitry is illustrated schematically in Fig. 2. Consequently, reducing glutamate activity leads to disinhibition of primary neurons in neocortex and limbic brain regions, which produce the symptoms of schizophrenia. The release of glutamate at NMDA receptors appears to be regulated by dopamine DRD2 receptors (14), which may explain how DRD2 antagonists are highly effective in reducing positive psychotic symptoms like hallucinations.
Fig 2.
The NMDA receptor hypofunction model of schizophrenia is based on the disinhibition of certain limbic brain regions and neocortical regions, such as the retrosplenial cortical (RSC) neurons. The disinhibition circuitry of this model is depicted here. Glutamate acts through NMDA receptors on GABAergic, serotonergic, and noradrenergic neurons to maintain tonic inhibitory control over two major excitatory pathways that convergently innervate RSC neurons. Systemic administration of an NMDA antagonist blocks NMDA receptors in multiple brain regions, thereby abolishing inhibitory control over both of the excitatory inputs to the RSC neuron. The disinhibited excitatory pathways then simultaneously hyperactivate the RSC neuron. Abnormal functioning of the hyperactivated RSC neuron would produce derangements in neuronal and neurotransmitter function downstrean from the hyperactivated RSC neuron. This circuit diagram focuses exclusively on RSC neurons. A similar disinhibition mechanism and similar, but not necessarily identical, neural circuits and receptor mechanisms probably exist in other corticolimbic brain regions. +, Excitatory input; −, inhibitory input; ACh, acetylcholine; NE, norepinephrine; 5HT, serotonin; α2, α2 subtype of adrenergic receptor; GA, GABA type A subtype of GABA receptor; m3, m3 subtype of muscarinic cholinergic receptor; AMPA/KA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainic acid subtype of Glu receptor; NMDA, NMDA subtype of Glu receptor; σ, sigma site; 5HT2A, 5HT2A subtype of serotonin receptor. Asterisks indicate the postulated sites where dopamine inputs may presynaptically regulate Glu release. This diagram was provided by John W. Olney, John W. Newcomer, and Nuri B. Farber, Washington University School of Medicine.
Given the complex interaction of the brain networks that underlie schizophrenia, it is uncertain whether it will be possible to subdivide schizophrenics in a useful way by identifying cases with low d-serine or modified expression of G72 or DAAO, as proposed by Chumakov and colleagues (2). It is possible that such a subset of schizophrenics will differ physiologically or clinically from other schizophrenics because of differences in such susceptibility genes. However, the impact of any one of the genes that have been discovered on susceptibility to schizophrenia is quite modest. Furthermore, whenever multifactorial disorders are studied, there will always be influences from interactions with genes and environmental factors that are not measured or detected, leading to inconsistent results. We can try to reconstruct the whole as the sum of its parts, but this simply does not work when the parts are interactive rather than additive. Nevertheless, it is possible that changes in one gene with a small contribution to risk of disorder may result in a dramatic shift in the nonlinear dynamics of a complex system, but such “butterfly” effects are unpredictable.
Consequently, additional functional analyses, such as those already carried out by Chumakov and colleagues (2) to discover the interaction of G72 with DAAO, will be increasingly important to clarify the role of specific genes in the operation and interaction of complex brain networks in disorders like schizophrenia. For example, they suggest that protein differential expression and d-serine local concentration measurements will elucidate the relationship of genotype to variability in the expression profile. Much is known already about the neural networks related to low glutamate signaling through NMDA receptors, as well as about information processing in individuals with schizophrenia (14–17), but little is known about the genetics of these networks. Functional genomics can help to distinguish primary causal factors from secondary consequences that are confounded in physiological data about isolated individuals. Insights about gene function at the levels of cells, brain networks, and interactions between networks will help us to understand and predict the phenotypic effects of genes at the clinical level.
Better understanding of the pathway from genotype to phenotype offers hope for improved treatment of schizophrenia. The company Genset (www.genset.fr) in Paris has already applied for patents on the genes reported here by Chumakov and colleagues (2). Likewise, deCODE Genetics (www.decode.com/news) in Reykjavik, Iceland, has also applied for patents on their discoveries on 8p (3) and has formed a contractual alliance with Roche Diagnostics for drug discovery (18). Atypical neuroleptics are already known to ameliorate underactivity of the NMDA receptor pathway (4, 13). Critical enzymes, like DAAO, may also be useful targets for drug discovery (19). Thus the discovery of susceptibility genes this summer opens the door to improved understanding of the pathogenesis of schizophrenia, which can lead to better prevention and treatment of disability from the disease. The discovery of some of the pathogenic molecular mechanisms associated with schizophrenia is truly a landmark event in the history of psychiatry.
See companion article on page 13675.
References
- 1.Meltzer D. (1999) J. Clin. Psychiatry 60, Suppl. 3, 32-35. [PubMed] [Google Scholar]
- 2.Chumakov I., Blumenfeld, M., Guerassimenko, O., Cavarec, L., Palicio, M., Abderrahim, H., Bougueleret, L., Barry, C., Tanaka, H., La Rosa, P., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13675-13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub R. E., Jing, Y., MacLean, C. J., Ma, Y., Webb, B. T., Myakishev, M. V., Harris-Kerr, C., Wormley, B., Sadek, H., Kadambi, B., et al. (2002) Am. J. Hum. Genet. 71, 337-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefansson, H., Sigurdsson, E., Steinthorsdottir, V., Bjornsdottir, S., Sigmundsson, T., Ghosh, S., Brynjolfsson, J., Gunnarsdottir, S., Ivarsson, O., Chou, T. T., et al. (2002) Am. J. Hum. Genet., in press. [DOI] [PMC free article] [PubMed]
- 5.Gottesman I. I. & Shields, J., (1982) Schizophrenia: The Epigenetic Puzzle (Cambridge Univ. Press, Cambridge, U.K.).
- 6.Risch N. (1990) Am. J. Hum. Genet. 46, 222-228. [PMC free article] [PubMed] [Google Scholar]
- 7.Cloninger C. R. (1997) Curr. Opin. Psychiatry 10, 5-10. [DOI] [PubMed] [Google Scholar]
- 8.Levinson D. F., Holmans, P. A., Straub, R. E., Owen, M. J., Wildenauer, D. B., Gejman, P. V., Pulver, A. E., Laurent, C., Kendler, K. S., Walsh, D., et al. (2000) Am. J. Hum. Genet. 67, 652-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman R. & Leonard, S. (2001) Am. J. Med. Gen. 105, 655-657. [DOI] [PubMed] [Google Scholar]
- 10.Risch N. J. (2000) Nature 405, 847-856. [DOI] [PubMed] [Google Scholar]
- 11.Morton N. E. & Collins, A. (1998) Proc. Natl. Acad. Sci. USA 95, 11389-11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzustowicz L. M., Hodgkinson, K. A., Chow, E. W., Honer, W. G. & Bassett, A. S. (2000) Science 288, 678-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culverhouse R., Suarez, B. K., Lin, J. & Reich, T. (2002) Am. J. Hum. Genet. 70, 461-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olney J. W., Newcomer, J. W. & Farber, N. B. (1999) J. Psychiatr. Res. 33, 523-533. [DOI] [PubMed] [Google Scholar]
- 15.Lisman J. E. & Otmakhova, N. A. (2001) Hippocampus 11, 551-568. [DOI] [PubMed] [Google Scholar]
- 16.Newcomer J. W. & Krystal, J. H. (2001) Hippocampus 11, 529-542. [DOI] [PubMed] [Google Scholar]
- 17.Csernansky J. G., Joshi, S., Wang, L., Haller, J., Gado, M., Miller, J. P., Grenander, U. & Miller, M. I. (1998) Proc. Natl. Acad. Sci. USA 95, 11406-11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wade, N. (June 18, 2002) N.Y. Times, Section F, pp. 1, 4.
- 19.Roses A. D. (2000) Nature 405, 857-865. [DOI] [PubMed] [Google Scholar]