Climatologists tell us that Earth's climate is changing (1): It currently seems clear that a warmer climate is developing in the northern hemisphere, and that the weather will become more variable (2, 3). As part of this global change, seasonal patterns are being altered to make spring conditions occur earlier in the year in the north (4), without necessarily corresponding changes in more southern latitudes (5).
We know much less about the ecological effects of such climate changes. Studying recent changes (6) and fluctuations (7) over the last few decades may help us improve our understanding of ecological responses to climate changes. This is the approach taken by Winkler et al. (8), in this issue of PNAS, in their study of the consequences of climate-induced shifts in breeding date on one of the most important life history traits in birds: clutch size (8). Their contribution adds to a rapidly growing literature on ecology–climate interactions (6, 7).
Phenology, a key feature in the article by Winkler et al. (8), is the study of seasonal plant and animal activity driven by environmental factors (4). The phenology of a species is typically evolved through natural selection to match the environmental conditions to maximize its fitness (9). In the temperate region, reproduction in birds starts in late spring or early summer, although timing often varies depending on climate. The effect of climate on the phenology, and in particular the timing of reproduction, is well known for plants, amphibians, and birds (10–12). These studies have demonstrated that onset of reproduction in spring may have advanced by a week or two due to recent changes in climate over much of North America and Europe (6, 7). We know much less about the implications of such phenological changes on the population dynamics of the species. Winkler et al. (8) are among the first to ask: (i) what are the consequences of earlier egg-laying on demographic rates (such as clutch size and survival)? (ii) To what extent do phenological changes in one species match changes in the rest of the environment? (iii) Finally, are the relationships between climate, phenology, and other life history traits simply linear, or are these relationships nonlinear? Here we highlight these three issues and suggest a more general framework, within which such ecological responses to climate change might be studied.
Seasonality: Onset of Breeding and Its Relation to Clutch Sizes
In birds, there is a strong effect of laying date on clutch size, and one may reckon that earlier breeding may lead to larger clutch sizes (8). However, the relationship between laying date and clutch size may be relative to the mean for the entire population within a year rather than absolute date of egg laying. Earlier breeding may therefore not necessarily affect clutch size, and if it does, such relationships need not be linear (13). Egg-laying dates of tree swallows (Tachycineta bicolor) across North America, for instance, have shifted up to 9 days over the period 1959–1991 (14). Tree swallows lay only a single clutch within a given year, and Winkler et al. (8) showed that these swallows respond to the absolute date of laying in their clutch sizes. Their analyses revealed that both laying dates and, to a lesser extent, the relationship between laying date and clutch size have changed. Surprisingly, there was nevertheless no trend toward larger clutches with earlier spring conditions and advancing temperatures (8). This result may suggest that birds shift their clutch sizes in response to higher temperatures only to some critical level, beyond which no major changes occur. If such a threshold does exist, the consequences of global warming on phenology may not be as pronounced as previously established (linear) relationships between laying date and clutch size may suggest. If relationships are truly nonlinear (13), linear relationships between climate, clutch size, and laying date will not be sufficient for extrapolations on future global change.
Match, and Mismatch, Between Phenological Changes Within a Species and Its Environment
Winkler et al. (8) found the lowest variances in laying dates during the most recent and warmest years, suggesting some genetic constraint preventing birds from laying eggs earlier. Such constraints may be due to a change from a match to a mismatch to the environmental conditions. Because no plant or animal lives in isolation from other species, they cannot be seen in isolation from each other. Many animals (e.g., migrating species) also depend on the environmental conditions in more than one area. Any differential impact of climatic variability on two species, such as predator and prey (or two areas, such as winter or summer ground), may affect the dynamics through a switch from matching to mismatching the environmental conditions. This idea of match–mismatch derives from the marine literature describing the relationship between the growth and survival of cod larvae depending on temporally matching production of early stages of zooplankton, their main food items (15, 16). Terrestrial examples of match–mismatch include the relationships between winter moth (Operophtera brumata) egg hatching and oak (Quercus robur) bud burst to temperature (17). Recent warmer spring conditions have resulted in more asynchronous phenology of the two species: an increase in spring temperatures (affecting the oak) occurs without a corresponding decrease in the incidence of freezing spells in winter (affecting the hatching moth eggs; see also figure 3 in ref. 7).
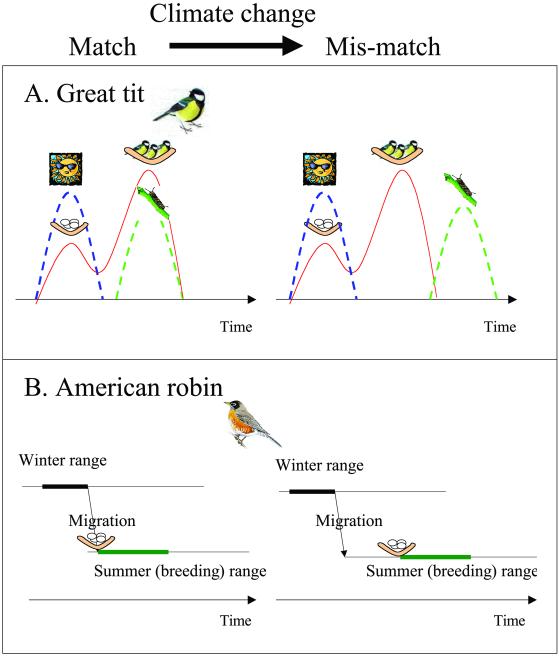
Visser et al. (18) reported a similar case for birds. In seasonal environments, the main selection pressure on the timing of reproduction is so that synchrony between offspring requirements and food availability is achieved. Egg laying must take place long before the energetically most expensive period of feeding the young. Birds therefore use environmental cues other than peak food availability to initiate egg laying at the right time. Climate change may alter the sequence of such spring events used as cues by the birds. As a result, the environmental cues previously being used to initiate reproduction no longer correspond to favorable conditions when offspring demands are the largest (18). The result is a mismatch between peak food abundance and peak food requirements (see Fig. 1A), generating a new selection pressure.
Figure 1.
A central concept when studying the relationship between climate, phenology, and other life history traits is the match vs. mismatch of the climate–phenology response within different components of the ecological system. (A) The environmental cues triggering onset of egg laying change in asynchrony to the environmental conditions prevailing when chicks are reared and when birds' energetic demands are the highest, as shown for Great tit (18). (B) The differential climate change between summer and winter ranges may lead to problems in the transition for migratory birds, such as the American robin (21).
Climate change may not be homogenous in space (1), possibly resulting in a mismatch condition for species depending on more than one area to fulfill their life cycle. This principle may refer to vastly different spatial scales. In black-throated blue warblers (Dendroica caerulescens), demographic rates in both tropical winter quarters and north temperate breeding grounds varied with fluctuations in El Niño Southern Oscillation (19). Adult survival and fecundity were found to be lower during El Niño years and higher during La Niña years. Fecundity, in turn, was found to be positively correlated to subsequent recruitment of new individuals (19). However, similar phenomena may also occur at a much smaller spatial scale. Climate warming typically leads to both higher temperature and more precipitation during the winter. At lower altitudes, this warming leads to less snow (because the temperatures are higher). However, at higher altitudes, with colder conditions, more precipitation results in more snow. This pattern has been reported for both Europe (20) and North America (21). Contrasting effects of climate change at high and low altitude are subsequently reported on phenology. The disjunction between phenology at low and high altitudes may create difficulties for species, such as many birds, migrating over altitudinal gradients. For instance, for the American robin (Turdus migratorius) in the Rocky Mountains, currently arriving about 14 days earlier than they did in 1981, the interval between arrival date and first date of bare ground has grown by 18 days (21), an example of a mismatch (see Fig. 1B).
Differential Linear and Nonlinear Responses to Climate in the Various Components of the Ecosystem
The above examples of match–mismatch to the environment suggest that we may expect to find nonlinearities between demographic rates and climate. Such nonlinearities may arise for different reasons and take different forms. It is interesting that the fixed effects of lay date and geography on clutch size reported by Winkler et al. (8) are often nonlinear. Freezing represents another example of a nonlinear effect of climate variability with a biological basis (22). Formation of ice crystals in plant tissue often leads to the death of the plant, or at least damage to sensitive parts including flower buds, ovaries, and leaves (22). In the Rocky Mountains, the major effect of warming was less frequent freezing of the plant Helianthella quinquenervis (23). Surprisingly, milder winters may sometimes lead to more frost damage, as not all parts of the plants are equally prone to freezing, and reduced snow cover during mild winters may result in more exposure. For example, mild periods in winter may generate premature spring growth and cause severe dieback of nonhardy shoots (24). During years with lower snow accumulation, an early-blooming herbaceous perennial (Delphinium nelsonii) experienced colder temperatures between the period of snowmelt and flowering (25). Flowering becomes delayed, floral production lowered, and flowering curves more negatively skewed in years of low snow accumulation (25). Also, climate indices such as El Niño Southern Oscillation or the North Atlantic Oscillations may be nonlinearly related to weather in a specific area, leading to a nonlinear relationship between a life history trait and climate (26).
Such nonlinear effects of climate have earlier been demonstrated for the European dipper (Cinclus cinclus) feeding on insects under water and thus highly sensitive to long-term freezing of the water surface (27). At northern latitudes, the amount of ice strongly affects which areas are feasible winter-feeding habitats. Fewer individuals are therefore recruited after cold winters. Mean winter temperature becomes also closely correlated with the annual variation in the number of days with ice cover. A 2.5°C increase in winter temperature in this region may therefore be expected to increase the carrying capacity by as much as 58% (27).
The autumnal moth (Epirrita autumnata) (28–30), an irruptive species with dramatic defoliation effects on birch trees (Betula spp.) in Northern Scandinavia, represents yet another example of a nonlinear response to climate. Eggs of the autumnal moth are known to die at ≈−36°C (28–30). The result of low winter temperatures will be seen along the mountain slopes during the subsequent summer if there is an outbreak. In the bottom of the valleys, there will be almost no defoliated birches if the winter temperature passes the critical freezing tolerance of the eggs, whereas heavily defoliated trees will occur at higher warmer (in winter) altitudes in these northern areas. Warming and a changing frequency of extreme cold events may thus lead to expansion of the outbreak areas, whereas the extent of refuge areas with no outbreaks may decrease (30, 31). However, warming may also lead to an increase in parasitoid abundances, which are likely to play an important role in the regulation of moth populations (32). This example then emphasizes the importance of considering different pathways when analyzing the consequences of climatic change and that both direct and indirect effects of climate change need to be considered.
Ecological Responses to Climate: A Suggested Framework
Recently, we have seen much progress in predicting the patterns of population dynamics by using knowledge of life-history characteristics and/or temporal variation in certain demographic traits (e.g., ref. 33). Variations in life history and foraging ecology can serve as a basis for grouping species' responses to climate change, leading to more rapid progress in our ability to predict changes. Given the diversity of invertebrate responses to climate change, Winkler et al. (8) reason that the avian insectivores that will be the least affected by climate change will be those with the greatest diversity of suitable prey during the egg-laying and chick-rearing periods. These will be the species whose requirements will best match the new conditions. The enterprise of trying to improve the relevant knowledge base represents a challenge to a great variety of scientists, including ecologists, climatologists, and statisticians (7). Our ultimate goal is to be able to provide assessments, and related advice, regarding the possible effects of climate change. This is not an easy undertaking. We have to rely on past climatic variability to study effects on ecological systems, whereas we may in the near future see temperature ranges not recorded in the past, thus requiring extrapolation. In such efforts, there is no shortcut to in-depth studies of specific systems. However, it is important to remind ourselves of the incredible progress we have seen in this topic during the last few years (6, 7). Hence, we have good reason to be quite optimistic regarding ecologists' ability to provide politicians with increasingly better advice regarding the effects of climate change. Hopefully, such insight will not come too late. With appropriate funding, ecologists certainly have the ability to provide improved insight into the ecological effects of climate change, an example of which is the study by Winkler et al. (8).
Footnotes
See companion article on page 13595.
References
- 1.Intergovernmental Panel on Climate Change. Climate Change 2001: Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, U.K.: Cambridge Univ. Press; 2001. [Google Scholar]
- 2.Easterling D R, Meehl G A, Parmesan C, Changnon S A, Karl T R, Mearns L O. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 3.Meehl G A, Karl T, Easterling D R, Changnon S, Pielke R, Changnon D, Evans J, Groisman P Y, Knutson T R, Kunkel K E, et al. Bull Am Met Soc. 2000;81:413–416. [Google Scholar]
- 4.Menzel A, Fabian P. Nature. 1999;397:659. [Google Scholar]
- 5.Doran P T, Priscu J C, Lyons W B, Walsh J E, Fountain A G, McKnight D M, Moorhead D L, Virginia R A, Wall D H, Clow G D, et al. Nature. 2002;415:517–520. doi: 10.1038/nature710. [DOI] [PubMed] [Google Scholar]
- 6.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T J C, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 7.Stenseth N C, Mysterud A, Ottersen G, Hurrell J W, Chan K-S, Lima M. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 8.Winkler D W, Dunn P O, McCulloch C E. Proc Natl Acad Sci USA. 2002;99:13595–13599. doi: 10.1073/pnas.212251999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Futuyma D J. Evolutionary Biology. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 10.Beebee T J C. Nature. 1995;374:219–220. [Google Scholar]
- 11.Crick H Q P, Dudley C, Glue D E, Thomson D L. Nature. 1997;388:526. [Google Scholar]
- 12.Forchhammer M C, Post E, Stenseth N C. Nature. 1998;391:29–30. [Google Scholar]
- 13.Crick H Q P, Gibbons D W, Magrath R D. J Anim Ecol. 1993;62:263–273. [Google Scholar]
- 14.Dunn P O, Winkler D W. Proc R Soc Lond Ser B. 1999;266:2487–2490. doi: 10.1098/rspb.1999.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjort J. Rapp P-v Reun Cons Int Explor Mer. 1914;20:1–228. [Google Scholar]
- 16.Cushing D H. In: Sea Fisheries Research. Jones H, editor. London: Elek; 1974. pp. 399–412. [Google Scholar]
- 17.Visser M E, Holleman L J M. Proc R Soc Lond Ser B. 2001;268:289–294. doi: 10.1098/rspb.2000.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser M E, Van Noordwijk A J, Tinbergen J M, Lessells C M. Proc R Soc Lond Ser B. 1998;265:1867–1870. [Google Scholar]
- 19.Sillett T S, Holmes R T, Sherry T W. Science. 2000;288:2040–2042. doi: 10.1126/science.288.5473.2040. [DOI] [PubMed] [Google Scholar]
- 20.Mysterud A, Yoccoz N G, Stenseth N C, Langvatn R. J Anim Ecol. 2000;69:959–974. [Google Scholar]
- 21.Inouye D W, Barr B, Armitage K B, Inouye B D. Proc Natl Acad Sci USA. 2000;97:1630–1633. doi: 10.1073/pnas.97.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inouye D W. Ecol Lett. 2000;3:457–463. [Google Scholar]
- 23.De Valpine P, Harte J. Ecology. 2001;82:637–648. [Google Scholar]
- 24.Crawford R M M. New Phytol. 2000;147:257–281. [Google Scholar]
- 25.Inouye D W, McGuire A D. Am J Bot. 1991;78:997–1001. [Google Scholar]
- 26.Mysterud A, Stenseth N C, Yoccoz N G, Langvatn R, Steinheim G. Nature. 2001;410:1096–1099. doi: 10.1038/35074099. [DOI] [PubMed] [Google Scholar]
- 27.Sæther B-E, Tufto J, Engen S, Jerstad K, Røstad O W, Skåtan J E. Science. 2000;287:854–856. doi: 10.1126/science.287.5454.854. [DOI] [PubMed] [Google Scholar]
- 28.Niemelä P. Rep Kevo Subarctic Res Station. 1979;15:33–36. [Google Scholar]
- 29.Tenow O, Nilssen A. J Appl Ecol. 1990;27:723–734. [Google Scholar]
- 30.Danell K, Hofgaard A, Callaghan T V, Ball J P. Ecol Bull. 1999;47:8–15. [Google Scholar]
- 31.Niemelä P, Chapin F S I, Danell K, Bryant J P. Clim Change. 2001;48:427–440. [Google Scholar]
- 32.Ruohomäki K, Tanhuanpää M, Ayres M P, Kaitaniemi P, Tammaru T, Haukioja E. Pop Ecol. 2000;42:211–223. [Google Scholar]
- 33.Sæther B-E, Engen S, Matthysen E. Science. 2002;295:2070–2073. doi: 10.1126/science.1068766. [DOI] [PubMed] [Google Scholar]