Abstract
Thermal stress in living cells produces multiple changes that ultimately affect membrane structure and function. We report that two members of the family of small heat-shock proteins (sHsp) (α-crystallin and Synechocystis HSP17) have stabilizing effects on model membranes formed of synthetic and cyanobacterial lipids. In anionic membranes of dimyristoylphosphatidylglycerol and dimyristoylphosphatidylserine, both HSP17 and α-crystallin strongly stabilize the liquid-crystalline state. Evidence from infrared spectroscopy indicates that lipid/sHsp interactions are mediated by the polar headgroup region and that the proteins strongly affect the hydrophobic core. In membranes composed of the nonbilayer lipid dielaidoylphosphatidylethanolamine, both HSP17 and α-crystallin inhibit the formation of inverted hexagonal structure and stabilize the bilayer liquid-crystalline state, suggesting that sHsps can modulate membrane lipid polymorphism. In membranes composed of monogalactosyldiacylglycerol and phosphatidylglycerol (both enriched with unsaturated fatty acids) isolated from Synechocystis thylakoids, HSP17 and α-crystallin increase the molecular order in the fluid-like state. The data show that the nature of sHsp/membrane interactions depends on the lipid composition and extent of lipid unsaturation, and that sHsps can regulate membrane fluidity. We infer from these results that the association between sHsps and membranes may constitute a general mechanism that preserves membrane integrity during thermal fluctuations.
The physical state of the membrane lipids is a central feature of current models of membrane organization (1). Although biological membranes maintain an overall bilayer structure under physiological conditions, they contain substantial amounts of lipids like phosphatidylethanolamine (PE) and monogalactosyldiacylglycerol (MGDG) with strong preference to organize into nonbilayer structures, such as inverted hexagonal phase (HII). Eukaryotic membranes are usually highly enriched in PE, whereas MGDG is the major lipid component of thylakoid membranes in plant cells. A dominant structural feature of these lipids is a small polar headgroup compared with large acyl chains, resulting in a conical shape (2). This shape can induce local high-membrane curvature, resulting in the formation of inverted micelles. It is known that membrane physical properties are maintained well within the lamellar liquid-crystalline state but with a constant potential for forming nonbilayer structures. Numerous data suggest that the formation of such structures is precisely regulated, and that they are of fundamental importance for fusion, endocytosis, signaling, and for the activity of certain membrane-bound enzymes (2–4). However, nonbilayer structures can be induced by environmental stress, such as elevated temperatures, thereby producing transient membrane defects and affecting permeability (5). Therefore, during thermal stress, it is essential to prevent their formation and to preserve membrane integrity.
Virtually all organisms respond to high temperature by synthesizing heat-shock proteins, including the small heat-shock proteins (sHsps). The sHsps are a diverse group of stress proteins that are sequence related to the vertebrate lens α-crystallins. They are defined by a conserved C-terminal domain of ≈100 aa referred to as the α-crystallin domain and have monomeric masses of 16–40 kDa (6). Many sHsps have been shown to be efficient at binding denatured proteins, and current models propose that sHsps function as molecular chaperones preventing irreversible protein aggregation (7, 8). Increased expression of sHsps can increase tolerance to a variety of stresses, such as heat, salt, drugs, and oxidants (9).
α-Crystallin, a major component of the lens in vertebrate eyes, is a member of the sHsp family and is composed of two highly homologous subunits, αA and αB (10). Initially thought to be lens-specific proteins, both αA- and αB-crystallin have been identified also in heart, liver, and brain (11). Despite numerous reports, the biological role of α-crystallin is not yet firmly established. It is thought to inhibit stress-induced aggregation in vitro, suggesting a potential role in maintenance of lens transparency (8, 12). α-Crystallin has been shown to bind to ocular plasma membranes and synthetic phospholipid vesicles (13–18), but the mechanism of interaction is not yet characterized, and the role these interactions may play in lens biology is not clear. A recent report (19) suggests that increased membrane association of α-crystallin is a critical event in the pathogenesis of cataracts.
A correlation between the level of sHsps and thermotolerance in a cyanobacterium Synechocystis PCC 6803 was published recently (20). Inactivation of the single Synechocystis sHsp gene, hsp17, reduced the photosynthetic oxygen evolution in heat-stressed cells. It was shown that part of the protein is associated with thylakoid membranes and, by increasing the membrane physical order, antagonizes the heat-induced increase in fluidity, thus protecting the membranes from thermal damage (21).
The objective of this work is to reveal the mode of interactions between sHsps and membranes composed of synthetic and cyanobacterial lipids. We used two members of the sHsp family, α-crystallin and HSP17, which share low sequence homology [22% in the C-terminal extension, 24% in the α-crystallin domain, and no homology in the N-terminal region (22)] but affect lipid membranes in a similar way. The study documents a novel feature of α-crystallin and HSP17; namely, they stabilize the bilayer liquid-crystalline phase relative to the inverted hexagonal phase in lipids with high propensity for nonbilayer structures (PE, MGDG). We found that sHsps strongly modulate the membrane properties of anionic phospholipids, suggesting a protection by sHsps at both thermal extremes. This novel property of sHsps implies local (domain-restricted) membrane functions, provided by the discrete localization of membrane lipids. The data also suggest that the association between sHsps and membranes may constitute a general mechanism, which protects membranes against thermal fluctuations.
Materials and Methods
Materials.
Synthetic dimyristoylphosphatidylcholine, dimyristoylphosphatidylglycerol (sodium salt) (DMPG), dimyristoylphosphatidylserine (sodium salt) (DMPS), and dielaidoylphosphatidylethanolamine (DEPE) were purchased from Avanti Polar Lipids and used without further purification. Synechocystis PCC 6803 was grown as in ref. 23, and Synechocystis thylakoids were isolated as in ref. 23. MGDG and phosphatidylglycerol (PG) were isolated from Synechocystis thylakoids by using chloroform/methanol extraction (2:1) and purified by two-dimensional thin-layer chromatography as in ref. 24. α-Crystallin was purchased from StressGen Biotechnologies (Victoria, Canada). HSP17 was expressed in Escherichia coli (strain GI724, Invitrogen) carrying the plasmid pAL-hsp17, and purified as in ref. 25.
Sample Preparation.
Liposomes were prepared by drying aliquots of the lipid under an N2 stream, followed by at least 2 h on a lyophilizer. Lipids were hydrated with 10 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (Tes), pH 7.1, and heated to a temperature above the gel to liquid-crystalline phase transition, followed by shaking. Multilamellar liposomes with a lipid concentration of 100 mg/ml and 60 mg/ml were used for the differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR) studies, respectively. α-Crystallin or HSP17 in 10 mM Tes, pH 7.1, was incubated with the liposomes above the temperature of phase transition for 30 min before the studies. The incubation temperatures were 30°C for DMPG, 42°C for DMPS, and DEPE, 30°C for Synechocystis MGDG and 33°C for Synechocystis PG. The protein concentrations were 50 mg/ml and 30 mg/ml for the DSC and FTIR studies, respectively.
DSC.
DSC studies were carried out on a Perkin–Elmer DSC7 and on a high-sensitivity DSC from Calorimetry Sciences (Provo, UT). The scanning rate was 2°C/min and 12°C/hr for the Perkin–Elmer DSC and the high-sensitivity DSC, respectively. Sample volumes were 20 μl for the Perkin–Elmer DSC and 100 μl for the high-sensitivity DSC.
FTIR.
Spectra were obtained with a Perkin–Elmer 2000 infrared spectrometer. About 4 μl of lipid/protein suspension was loaded on CaF2 widows. Sample temperature was controlled with a Peltier device (Page, Davis, CA) and monitored separately by a thermocouple. For each spectrum, eight interferograms were collected at 4 cm−1 resolution and 3,600–900 cm−1 wave-number range. Spectral analysis was carried out by using spectrum 2000 (Perkin–Elmer).
sHsp-Binding Experiments.
sHsp/lipid-binding studies were carried out as in ref. 26. α-Crystallin and HSP17 (0.25 mg/ml) in Tes buffer, pH 7.1, were incubated with extruded unilamellar liposomes (100 nm) (1 mg/ml) for 30 min at a temperature above the phase transition (see sample preparation). Liposomes were sedimented by centrifugation at 259,000 × g for 1 h in a TL100 ultracentrifuge (Beckman, Palo Alto, CA). Pellet and supernatant fractions were analyzed by SDS/PAGE (using 5% stacking and 17% separating gels) and were visualized by Coomassie blue staining. Lipid concentration of the pellet and supernatant was determined by analytical thin-layer chromatography by using an Iatroscan TH-10 (Iatron Laboratories, Tokyo). The developing medium contained chloroform/methanol/water (65:35:0.5) with 1% acetic acid (27).
Fatty Acid Analysis.
The extracted MGDG and PG were methylated by using HCl/methanol (5:95, wt/wt) at 80°C for 2 h. The esterified fatty acids were analyzed by gas chromatography (HP3396A Hewlett–Packard) on a SP2330 column (Supelco, Bellefonte, PA) as in ref. 23.
Results
Molecular interactions between proteins and lipid membranes can be studied by monitoring discrete frequency ranges in the FTIR spectrum. In this work, we observed changes in the vibrational frequency of the methylene symmetric stretching mode (νCH2) at 2,850 cm−1, which reflects the membrane fluidity, and the methylene scissoring band (δCH2) at 1,468 cm−1, which is diagnostic for the lateral packing in the depth of the hydrophobic core (28, 29). We also followed changes in the lipid phosphate asymmetric stretching band (νPO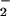 ) at 1,220 cm−1 and the serine carboxylate stretching band (νCO
) at 1,220 cm−1 and the serine carboxylate stretching band (νCO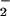 ) at 1,420 cm−1, which report on the immediate environment of the lipid headgroup region (30).
) at 1,420 cm−1, which report on the immediate environment of the lipid headgroup region (30).
Influence of α-Crystallin and HSP17 on Bilayer-Forming Lipids.
In a previous study, we showed that HSP17 did not affect the gel to liquid-crystalline phase transition temperature (Tm) in dimyristoylphosphatidylcholine membranes but reduced the fluidity in the liquid-crystalline state at elevated temperatures (25). In the present study, in DMPG membranes, both HSP17 and α-crystallin decrease the transition temperature (about 4°C), as shown by the DSC thermograms (Fig. 1A Inset, Table 1). Thermal profiles of the methylene stretching band show that the two proteins decrease the Tm and stabilize the liquid-crystalline state (Fig. 1A). In addition, HSP17 has a fluidizing effect on the membrane in the gel state, as evidenced by the higher vibrational frequency of the CH2 band (Fig. 1A). The two proteins do not influence the fluidity of DMPG in the liquid-crystalline state. Possible lipid/protein binding was studied by examining the phosphate stretching band (Fig. 1 B and C). The fairly broad contour of this band in the gel state is changed, and its position is shifted from 1,205 cm−1 in the control (31) to 1,210 cm−1 in the presence of the proteins (Fig. 1B). In the liquid-crystalline state, the vibrational frequency of this band is also increased in the presence of the proteins (from 1,210 cm−1 in the control to 1,215 cm−1 in DMPG/sHsps) (Fig. 1C). Such an upshift is consistent with disruption of hydrogen bonding around the phosphates and suggests a binding between the protein and the lipid headgroups. Further evidence for lipid/protein binding arises from the pattern of the CH2 scissoring band (Fig. 1 D and E), which reflects the interactions between lipid acyl chains (28, 29). The appearance of a doublet in the δCH2 is characteristic of a significant rearrangement of the acyl chain packing (31), which is induced by lipid headgroup/protein interactions.
Figure 1.
(A) Thermal profiles of the CH2 stretching band in DMPG liposomes in10 mM Tes, pH 7.1, in the presence of HSP17 and α-crystallin (DMPG/sHsp molar ratio 55:1). (Inset) DSC heating thermograms. FTIR profiles of the phosphate stretching band in gel (5°C) (B), and in liquid-crystalline state (34°C) (C). FTIR profiles of the CH2 scissoring band in gel (5°C) (D), and in liquid-crystalline state (34°C) (E). Frequency variations between three independent FTIR experiments were 0.08 cm−1 at 5°C and 0.14 cm−1 at 40°C.
Table 1.
Phase transitions in lipid membranes in the presence of α-crystallin and HSP17 (molar ratio lipid/protein in parentheses)
| Lipid | Pretransition, °C | Main transition, °C | Nonlamellar transition, °C |
|---|---|---|---|
| DMPG in buffer | 18.0 ± 0.2 | 28.0 ± 0.3 | |
| DMPG/αC (55:1) | 17.0 ± 0.3 | 24.3 ± 0.4 | |
| DMPG/HSP17 (55:1) | 16.6 ± 0.3 | 24.2 ± 0.3 | |
| DMPS in buffer | 39.6 ± 0.2 | ||
| DMPS/αC (55:1) | 38.0 ± 0.4 | ||
| DMPS/HSP17 (55:1) | 37.4 ± 0.3 | ||
| DEPE in buffer | 38.1 ± 0.2 | 61.3 ± 0.4 | |
| DEPE/HSP17 (110:1) | 38.0 ± 0.3 | 64.4 ± 0.5 | |
| DEPE/HSP17 (55:1) | 37.6 ± 0.2 | 67.8 ± 0.6 | |
| DEPE/αC (110:1) | 38.0 ± 0.3 | 63.8 ± 0.6 | |
| DEPE/αC (55:1) | 37.6 ± 0.4 | 67.1 ± 0.6 | |
| MGDG in buffer | 29.7 ± 0.3 | ||
| MGDG/αC (55:1) | 30.8 ± 0.3 | ||
| MGDG/HSP17 (55:1) | 32.0 ± 0.2 |
Data are derived from DSC studies and are means of at least three measurements in three independent preparations.
In DMPS membranes, both HSP17 and α-crystallin induce a downward shift of the Tm, as evidenced by the DSC thermograms and thermal profiles of the CH2 band (Fig. 2A Inset, Table 1). HSP17 produces a marked increase in the fluidity of the gel state, similarly to its effect on DMPG membranes. The serine headgroup of DMPS that contributes a second negative charge through the amino acid carboxyl group shows an up-scale shift in the position and change in the shape in the liquid-crystalline state (from 1,414 cm−1 in the control to 1,419 cm−1 in the samples) (Fig. 2C), consistent with disruption of the lipid–lipid and lipid–water hydrogen bonding network in the polar region. This shift in the CO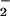 peak of DMPS results from the DMPS/sHsp interactions and is not due to a contribution arising from the proteins, as shown on the spectra of α-crystallin and HSP17 without lipid (see Fig. 2 B and C). Further, changes in the hydrophobic core were estimated by the pattern of the CH2 scissoring band (Fig. 2 D and E). The shape change of this band in the presence of the proteins suggests that the acyl chain packing is influenced by the proteins in the liquid-crystalline state. These data demonstrate that the two proteins stabilize the liquid-crystalline state in anionic saturated lipids, and that the interactions occur at the headgroup moiety.
peak of DMPS results from the DMPS/sHsp interactions and is not due to a contribution arising from the proteins, as shown on the spectra of α-crystallin and HSP17 without lipid (see Fig. 2 B and C). Further, changes in the hydrophobic core were estimated by the pattern of the CH2 scissoring band (Fig. 2 D and E). The shape change of this band in the presence of the proteins suggests that the acyl chain packing is influenced by the proteins in the liquid-crystalline state. These data demonstrate that the two proteins stabilize the liquid-crystalline state in anionic saturated lipids, and that the interactions occur at the headgroup moiety.
Figure 2.
(A) Thermal profiles of the CH2 stretching band in DMPS liposomes in 0.1 M NaCl/10 mM Tes, pH 7.1, in the presence of HSP17 and α-crystallin (DMPS/sHsp molar ratio 55:1). (Inset) DSC heating thermograms. Frequency variations between three independent FTIR experiments were 0.12 cm−1 at 20°C and 0.2 cm−1 at 48°C. FTIR profiles of the DMPS serine carboxyl stretching band in gel (18°C) (B), and in liquid-crystalline state (43°C) (C). FTIR profiles of the methylene scissoring band in gel (18°C) (D), and in liquid-crystalline state (43°C) (E). B and C show spectra of HSP17 and α-crystallin in 10 mM Tes, pH 7.1, without lipid in the region of the serine carboxylate band.
Influence of α-Crystallin and HSP17 on Nonbilayer-Forming Lipids.
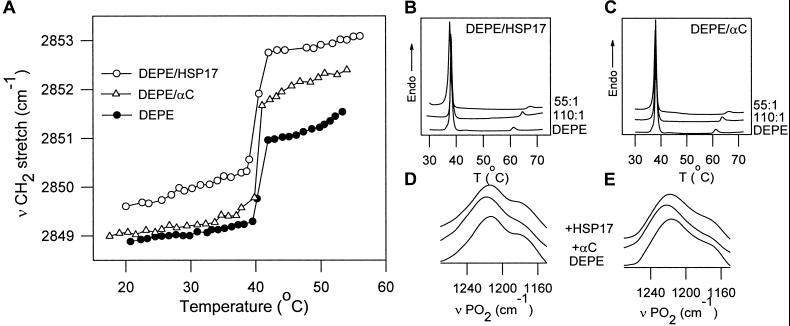
DEPE membranes are known to display a phase sequence of gel→liquid-crystalline→inverted hexagonal phase (Lβ→Lα →HII) during heating. A large endotherm at 38°C, corresponding to the main-phase transition, is followed by a much smaller endotherm (Lα→HII) at 61°C (32). Thermograms of DEPE membranes containing increasing amounts of α-crystallin and HSP17 clearly show that these proteins shift the transition of the nonlamellar phase to higher temperatures (from 61°C in the control to about 68°C with sHsps) and slightly decrease the Tm (Fig. 3 B and C, Table 1). Thermal profiles of the CH2 band in DEPE/α-crystallin and DEPE/HSP17 liposomes show a major increase in the vibrational frequency throughout the main transition (see Fig. 3A), consistent with considerable conformational disorder of the lipid acyl chains in the presence of the proteins. On the other hand, the phosphate stretching band, which was at 1,217 cm−1 in the liquid-crystalline state for DEPE without protein, increased in frequency to 1,222 cm−1 and to 1,219 cm−1 in the presence of α-crystallin and HSP17, respectively (Fig. 3E). Such an upshift is indicative of a change in the polar environment in the vicinity of the phosphate groups and a disruption of the hydrogen bonding, which is consistent with interactions between the proteins and the phosphates. However, the patterns of the CH2 scissoring band in the control and the samples were very similar (data not shown), suggesting no significant change in the acyl chain packing, which implies that the DEPE/sHsp interactions are mediated by the headgroup region.
Figure 3.
(A) Thermal profiles of the CH2 stretching band in DEPE liposomes in 10 mM Tes, pH 7.1, in the presence of HSP17 and α-crystallin (DEPE/sHsp molar ratio 55:1). Frequency variations between three independent FTIR experiments were 0.1 cm−1 at 20°C and 0.2 cm−1 at 55°C. DSC heating thermograms of DEPE in the presence of HSP17 (B) or α-crystallin (C) (DEPE/sHsp molar ratios shown on the scans). FTIR profiles of the phosphate stretching band in gel state (20°C) (D), and in liquid-crystalline state (43°C) (E).
It should be noted that neither α-crystallin nor HSP17 in buffer showed any thermotropic transition in the range between 20 and 70°C. HSP17 showed a major change in secondary structure at 75°C, measured by FTIR (data not shown).
Influence of α-Crystallin and HSP17 on Synechocystis Lipids.
Synechocystis MGDG, another lipid with high propensity for forming HII structure, consists predominantly of 16:0, 18:2, and 18:3 fatty acids (Table 2). MGDG membranes showed a phase transition at 29°C, which corresponds to a direct transformation from lamellar gel into inverted hexagonal phase (33) (Fig. 4A Inset, Table 1). α-Crystallin and HSP17 induced an upward shift in this transition to about 31 and 32°C, respectively, suggesting that the proteins stabilize the membrane bilayer structure. The thermal profile of the MGDG CH2 band reveals an ordering effect of the proteins on the membrane, which is illustrated by the decrease in the vibrational frequency of this band (Fig. 4A) due to sHsp/lipid association. The CH2 thermal profile of Synechocystis PG (Fig. 4B) shows a broad thermal event between 17 and 38°C with a gradual increase in the CH2 vibrational frequency, typical for most naturally derived lipids. Both sHsps induce a decrease in the fluidity of PG membranes (Fig. 4B), although the rigidifying effect caused by the proteins is less pronounced than in MGDG. Contrary to MGDG that contains ≈29% trienoic fatty acids, PG contains only 2% trienoic fatty acids (Table 2). These results suggest that HSP17 and α-crystallin modulate the membrane properties of cyanobacterial MGDG and PG by increasing the molecular order in unsaturated membranes.
Table 2.
Fatty acid composition of MGDG and PG isolated from thylakoid membranes of Synechocystis PCC 6803
| Lipid | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | γ18:3 | α18:3 | DBI |
|---|---|---|---|---|---|---|---|---|
| MGDG | 47.2 | 2.3 | 0.6 | 4.7 | 16.2 | 28.8 | 0.0 | 126.2 |
| PG | 53.2 | 1.0 | 1.5 | 8.8 | 33.4 | 1.0 | 1.2 | 82.8 |
Values are presented as mol % and are averages from three independent experiments with SDs within 2%. DBI refers to double bond index for each lipid.
Figure 4.
Thermal profiles of the CH2 stretching band of MGDG (A) and PG (B) liposomes in 10 mM Tes, pH 7.1, in the presence of HSP17 and α-crystallin (lipid/sHsp molar ratio 55:1). (A Inset) DSC heating scans of MGDG. Lipids were isolated from Synechocystis thylakoid membranes (see text). Frequency variations between three independent FTIR experiments were 0.08 cm−1 at 4°C and 0.14 cm−1 at 38°C.
Binding Studies.
Unilamellar liposomes from different lipids were incubated with either α-crystallin or HSP17 for 30 min and then centrifuged. Binding of the sHsps was estimated by SDS/PAGE of the pellets and supernatants (Fig. 5). HSP17 was highly enriched in the pellet of cyanobacterial PG and MGDG, whereas in DEPE and DMPS, there was a more similar distribution of HSP17 between pellet and supernatant, suggesting somewhat lower affinity of the protein for these lipids. α-Crystallin was most abundant in the pellets of DMPS and DEPE and to a lesser extent in Synechocystis MGDG and PG. No membrane-bound sHsps were observed for dimyristoylphosphatidylcholine liposomes. Lipid analysis of the pellet and supernatant showed that between 80 and 90% of the lipids were sedimented (data not shown), supporting the SDS/PAGE data that the sHsp partitioning is driven by their affinity for the lipids.
Figure 5.
Binding of α-crystallin (Upper) and HSP17 (Lower) to unilamellar liposomes (see text for details). Liposomes (1 mg/ml) made of each lipid were incubated with either α-crystallin or HSP17 (0.25 mg/ml) for 30 min above the Tm and were centrifuged at 259,000 × g. Supernatants and pellets were analyzed by SDS/PAGE. Lane 6 shows protein that was centrifuged without liposomes.
Discussion
At physiological temperatures, the membranes of poikilothermic organisms are in a fluid-like state, with a high degree of molecular motion. During cold stress, the membranes may pass into an ordered gel state, whereas when temperatures are elevated, transient nonbilayer lipid structures may form. Both effects may lead to cell injury due to leakage and loss of membrane integrity (34, 35). These organisms, therefore, have to operate an adaptive mechanism that is able to attenuate rapidly and effectively the effects of thermal fluctuations. On the basis of the present study, we propose that besides the well established adaptive remodeling of membranes involving changes in the extent of lipid unsaturation and ratio between lipid classes, a lipid-associated pool of sHsps may fulfill such membrane protective functions. By fluidizing the high-temperature melting lipids, sHsp/lipid interactions could prevent the damaging effect of chilling on the membrane. In contrast, by rigidifying the highly unsaturated molecular species, together with an upshift of the temperature of formation of nonbilayer structures (in PE and MGDG), sHsp–membrane associations may confer elevated thermotolerance to the organism. The data suggest a protective role of sHsps against heat and cold stress but also imply that in some organisms or under certain environmental conditions, their function may be redundant to other cellular components.
sHsps are found in virtually all prokaryotic and eukaryotic cells, and their amounts increase during heat stress, in support of the hypothesis that they play a role in cell survival. However, the protective function(s) of sHsps has yet to be demonstrated in vivo. In mammalian cells, sHsp expression confers increased thermotolerance, as does expression of plant class I sHsp or αB-crystallin in E. coli (36, 37). More interestingly, expression of sHsp from chestnut (Castanea sativa) in E. coli enhanced cell viability not only to heat stress but also to low temperature (4°C) (38). Clearly, these findings fundamentally question the view that the ability of sHsps to attenuate both heat- and cold-induced cell injury is solely due to their chaperone function. Instead, such universal protective effects of sHsps at both thermal extremes support the notion that it could be also related to their membrane stabilizing effect (21, 25).
This study reveals the mode of interaction between sHsps and lipid membranes. The data show that the nature of the interactions is highly specific and depends on the membrane lipid headgroup and the extent of fatty acid unsaturation. In DMPG and DMPS membranes, both sHsps produced a downward shift in the transition temperature (Figs. 1 and 2), consistent with stabilization of the liquid-crystalline state. In addition, HSP17 increased the fluidity in the gel state, suggesting a membrane-protective role at low temperatures. In contrast, in cyanobacterial PG liposomes, consisting of both saturated and unsaturated fatty acids (Table 2), both proteins reduced the fluidity in the liquid-crystalline state (Fig. 4B), supporting the finding that sHsps modulate the membrane properties by increasing the fluidity in the gel state and by rigidifying the liquid-crystalline state. FTIR data showed changes in the polar environment at the membrane interface consistent with lipid/sHsp association but no evidence for electrostatic interactions between the sHsps and the lipid headgroups (Figs. 1 and 2C). Alterations in the spectral pattern of the acyl chains point to a significant rearrangement in this region induced by the sHsps/lipid association (Figs. 1 and 2E), suggesting that this association affects the hydrophobic interior.
There are important differences in the thermotropic properties of the sHsp/lipid membranes, with phase transitions being more cooperative in the presence of α-crystallin than in the presence of HSP17 (Figs. 1 and 2). HSP17 was more effective in influencing DMPS and DEPE than α-crystallin at equal concentrations (Figs. 2 and 3), but it showed higher affinity for binding to Synechocystis MGDG and PG (Fig. 5). This apparent discrepancy further illustrates the extreme efficiency of HSP17 as “lipid chaperone.” The differences between the effects of HSP17 and α-crystallin most likely reflect differences in the morphology of the lipid/sHsp assemblies and may be related to the strong tendency of sHsps to dissociate depending on the temperature (39, 40). Like most sHsps, α-crystallin and HSP17 have variable and dynamic quaternary structure of oligomers, shaped by a continuous exchange of monomeric subunits. For instance, wheat HSP16.9 has a dodecameric structure and undergoes a reversible dissociation into smaller subunits (most likely dimers) between 35 and 45°C (40). In HSP17, this dissociation occurs with exposure of hydrophobic domains that act as binding sites for the substrates (25). Because our results show sHsp/membrane interactions well below this temperature, we propose that the membranes interact with dissociated species and thereby shift the equilibrium toward dissociation.
The most intriguing result is that in DEPE membranes, both HSP17 and α-crystallin strongly inhibit the formation of the inverted hexagonal phase and stabilize the bilayer structure (Fig. 3 B and C). It is known that a potential for lipid polymorphic structural changes is a requirement of biological membranes, and that the formation of HII structures is regulated (2, 5). However, high temperatures induce local nonbilayer structures, thus affecting membrane permeability and integrity (2). sHsps counteract this effect by stabilizing the bilayer structure, suggesting that they may regulate the membrane lipid polymorphism. In Synechocystis MGDG, both α-crystallin and HSP17 induced an upward shift in Thex (Fig. 4A), although this shift is less pronounced than for the other lipids. More importantly, the two proteins caused an increase in the molecular order after the MGDG chain-melting transition (Fig. 4A). Furthermore, the apparent lipid selectivity of both proteins implies that the membrane binding of sHsps may confine the location of sHsps to membrane microdomains (1).
Numerous data show that sHsps are found in diverse types of tissues (11). For instance, overexpression of αB-crystallin in myocardial tissue has a protective effect against ischemia and markedly reduces oxidative stress (41). Similarly, Hsp70 in H9c2 heart myoblasts attenuates membrane lipid peroxidation during oxidative stress and preserves membrane functions (42). These findings suggest that while reactive oxygen species are generated, Hsps may reduce cardiomyocyte apoptosis by virtue of their ability to decrease membrane lipid peroxidation.
Various soluble proteins have different effects on membrane structure and properties. For instance, polylysine associates with the surface of anionic lipids and increases the Tm, whereas insertion proteins typically have no effect on Tm (43). Protein penetration into membranes can occur independently of lipid headgroup charge (44), and integral membrane proteins induce marked effects on the local membrane organization with only minor effect on the phase behavior (45). The pattern observed with sHsps, however, does not appear to fit entirely to these models, as sHsps associate with the polar headgroups and affect the hydrophobic core. Although sHsps are soluble, they also associate with the membranes in support of the proposed role of these proteins as amphitropic (25, 46). Their amphitropism makes them ideal for protecting membranes during stress without altering the membrane function under nonstress conditions.
In conclusion, this study shows that sHsps can regulate membrane physical properties in vitro, thereby preventing the formation of “hyperfluid” or ordered gel states. Thus, we propose that HSP17 and α-crystallin associate reversibly with membrane lipids in vivo and, by regulating membrane fluidity, preserve membrane structure and integrity during the initial stages of stress conditions. Further, we suggest that this stabilization precedes the thermal adaptation that occurs by adjustment of the lipid composition. On the basis of these and earlier data (47, 48), we propose that membrane domains may act as sensors where stress-induced membrane perturbations are transduced as a signal leading to activation of heat-shock genes (21). The association of sHsps with membranes causing increased molecular order may lead to down-regulation of the gene expression. Such “crosstalk” between the primary stress sensor in the membranes and sHsps suggests a feedback mechanism in the regulation of heat-shock genes (48) and provides a model for sHsp function.
Acknowledgments
We are grateful to Drs. Ann Oliver, Gordon Tollin, and Michael Brown for critically reading the manuscript. N.M.T., W.F.W., L.M.C., F.T., and J.H.C. acknowledge Grants HL61204 and NHLBI 57810-01 from the National Institutes of Health, and N66001-00-C-8048 and 981711 from Defense Advanced Research Planning Agency. E.V. acknowledges Grant GM42762 from the National Institutes of Health. I.H. and L.V. acknowledge grants from the Hungarian National Scientific Research Foundation (OTKA) (T029883 and T038334) and from the Hungarian National Research and Development Program (1/040/2001).
Abbreviations
- sHsp
small heat-shock protein
- DMPG
dimyristoylphosphatidylglycerol
- DMPS
dimyristoylphosphatidylserine
- DEPE
dielaidoylphosphatidylethanolamine
- MGDG
monogalactosyldiacylglycerol
- PG
phosphatidylglycerol
- HII
inverted hexagonal structure
- Tm
phase transition from gel to liquid-crystalline state
- Thex
phase transition from lamellar to hexagonal phase
- FTIR
Fourier transform infrared spectroscopy
- DSC
differential scanning calorimetry
- Tes
N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid
References
- 1.Simons K, Ikonen E. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.de Kruijff B. Nature. 1987;329:587–588. doi: 10.1038/329587a0. [DOI] [PubMed] [Google Scholar]
- 3.Escriba P V, Ozaita A, Ribas C, Miralles A, Fodor E, Farkas T, Garcia-Sevilla J A. Proc Natl Acad Sci USA. 1997;94:11375–11380. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brink-van der Laan E, Dalbey R E, Demel R A, Killian A, Kruijff B. Biochemistry. 2001;40:9677–9684. doi: 10.1021/bi002903a. [DOI] [PubMed] [Google Scholar]
- 5.Dowhan W. Annu Rev Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- 6.de Jong W W, Caspers G-J, Leunissen J A M. Int J Biol Macromolecules. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 7.Derham B K, Harding J J. Biochem J. 1997;328:763–768. doi: 10.1042/bj3280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz J. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrigo A-P, Landry J. In: The Biology of Heat Shock Proteins and Molecular Chaperones. Morimoto R, Tissieres A, Georgopoulos C, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 335–373. [Google Scholar]
- 10.de Jong W W, Leunissen J A M, Voorter C E M. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan A N, Nagineni C N, Bhat S P. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- 12.Andley U P, Mathur S, Griest T A, Petrash J M. J Biol Chem. 1996;271:31973–31980. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Zeng J, Yin H, Tang D, Borchman D, Patterson C A. Curr Eye Res. 1999;18:56–61. doi: 10.1076/ceyr.18.1.56.5387. [DOI] [PubMed] [Google Scholar]
- 14.Liang J J N, Li X-Y. Exp Eye Res. 1992;54:719–724. doi: 10.1016/0014-4835(92)90026-o. [DOI] [PubMed] [Google Scholar]
- 15.Tang D, Borchman D, Yappert M C, Cenedella R J. Exp Eye Res. 1998;66:559–567. doi: 10.1006/exer.1997.0467. [DOI] [PubMed] [Google Scholar]
- 16.Sato H, Borchman D, Ozaki Y, Lamba O P, Byrdwell W C, Yappert M C, Patterson C A. Exp Eye Res. 1996;62:47–53. doi: 10.1006/exer.1996.0006. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekher G, Cenedella R. Exp Eye Res. 1995;60:707–717. doi: 10.1016/s0014-4835(05)80012-0. [DOI] [PubMed] [Google Scholar]
- 18.Cobb B A, Petrash J M. J Biol Chem. 2000;275:6664–6672. doi: 10.1074/jbc.275.9.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobb B A, Petrash J M. Biochemistry. 2002;41:483–490. doi: 10.1021/bi0112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Owen H A, Prochaska D J, Barnum S R. Curr Microbiol. 2000;40:283–287. doi: 10.1007/s002849910056. [DOI] [PubMed] [Google Scholar]
- 21.Horvath I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Miller W. Adv Appl Math. 1991;12:337–357. [Google Scholar]
- 23.Vigh L, Los D, Horvath I, Murata N. Proc Natl Acad Sci USA. 1993;90:9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigh L, Horvath I, Thompson G A. Biochim Biophys Acta. 1988;937:42–50. doi: 10.1016/0005-2736(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 25.Török Z, Goloubinoff P, Horvath I, Tsvetkova N M, Glatz A, Balogh G, Varvasovszki V, Los D, Vierling E, Crowe J H, Vigh L. Proc Natl Acad Sci USA. 2001;98:3098–3103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Török Z, Horvath I, Goloubinoff P, Kovacs E, Glarz A, Balogh G, Vigh L. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver A E, Fisk E, Crowe L M, de Araujo P S, Crowe J H. Biochim Biophys Acta. 1995;1267:92–100. doi: 10.1016/0167-4889(95)90001-w. [DOI] [PubMed] [Google Scholar]
- 28.Snyder R G. J Mol Spectrosc. 1961;7:116–144. [Google Scholar]
- 29.Snyder R G. J Chem Phys. 1967;47:1316–1360. [Google Scholar]
- 30.Casal H L, Mantsch H H. Biochim Biophys Acta. 1984;779:381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y-P, Lewis R N A, McElhaney R N. Biophys J. 1996;72:779–793. doi: 10.1016/s0006-3495(97)78712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koynova R, Caffrey M. Chem Phys Lipids. 1994;69:1–34. doi: 10.1016/0009-3084(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 33.Mannock D A, McElhaney R N. Biochem Cell Biol. 1991;69:863–867. doi: 10.1139/o91-128. [DOI] [PubMed] [Google Scholar]
- 34.Quinn P J. Cryobiology. 1985;22:128–146. doi: 10.1016/0011-2240(85)90167-1. [DOI] [PubMed] [Google Scholar]
- 35.Watson P F, Morris G J. J Exp Biol. 1987;41:311–340. [PubMed] [Google Scholar]
- 36.Plater M, Goode D, Crabbe M J C. J Biol Chem. 1996;271:28558–28566. doi: 10.1074/jbc.271.45.28558. [DOI] [PubMed] [Google Scholar]
- 37.Yeh C-H, Chang P-F L, Yeh K-W, Lin W-C, Chen Y-M, Lin C-Y. Proc Natl Acad Sci USA. 1997;94:10967–10972. doi: 10.1073/pnas.94.20.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soto A, Allona I, Collada C, Guevara M-A, Casado R, Rodiguez-Cerezo E, Aragoncillo C, Gomez L. Plant Physiol. 1999;120:521–528. doi: 10.1104/pp.120.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bova M P, Mchaourab H S, Han Y, Fung B K-K. J Biol Chem. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- 40.van Montfort R L M, Basha E, Friedrich K L, Slingsby C, Vierling E. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- 41.Ray P S, Martin J L, Swanson E A, Otani H, Dillmann W H, Das D K. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 42.Su C-Y, Chong K-Y, Edelstein K, Lille S, Khardori R, Lai C-C. Biochem Biophys Res Commun. 1999;265:279–284. doi: 10.1006/bbrc.1999.1649. [DOI] [PubMed] [Google Scholar]
- 43.McElhaney R N. Biochim Biophys Acta. 1986;864:361–421. doi: 10.1016/0304-4157(86)90004-3. [DOI] [PubMed] [Google Scholar]
- 44.Davletov B, Perisic O, Williams R L. J Biol Chem. 1998;273:19093–19096. doi: 10.1074/jbc.273.30.19093. [DOI] [PubMed] [Google Scholar]
- 45.Gil T, Ipsen J H, Mouristen O G, Sabra M C, Sperotto M M, Zuckerman M J. Biochim Biophys Acta. 1998;1376:245–266. doi: 10.1016/s0304-4157(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 46.Johnson J E, Cornell R B. Mol Membr Biol. 1999;16:217–235. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- 47.Vigh L, Maresca B, Harwood J. Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 48.Vigh L, Maresca B. In: Cell and Molecular Responses to Stress. Storey K B, Storey J M, editors. Amsterdam: Elsevier; 2002. pp. 173–188. [Google Scholar]