Abstract
The enzyme dimethylarginine dimethylaminohydrolase (DDAH) hydrolyses asymmetrically methylated arginine residues that are endogenously produced inhibitors of nitric oxide synthases (NOS). We and others have proposed that DDAH activity is a key determinant of intracellular methylarginine concentrations and that factors that regulate the activity of DDAH may modulate nitric oxide (NO) production in vivo. We recently solved the crystal structure of a bacterial DDAH and identified a Cys-His-Glu catalytic triad [Murray-Rust, J., Leiper, J. M., McAlister, M., Phelan, J., Tilley, S., Santa Maria, J., Vallance, P. & McDonald, N. (2001) Nat. Struct. Biol. 8, 679–683]. The presence of a reactive cysteine residue (Cys-249) in the active site of DDAH raised the possibility that DDAH activity might be directly regulated by S-nitrosylation of this residue by NO. In the present study, we demonstrate that recombinant DDAH is reversibly inhibited after incubation with NO donors in vitro. Similarly mammalian DDAH in cytosolic extracts is also reversibly inhibited by NO donors. In cultured endothelial cells, heterologously expressed human DDAH II was S-nitrosylated after cytokine induced expression of the inducible NOS isoforms. The implication of these findings is that under certain conditions when NO generation increases, S-nitrosylation diminishes DDAH activity and this would be expected to lead to accumulation of asymmetric dimethylarginine and inhibition of NOS. This observation may help explain why expression of iNOS often leads to inhibition of activity of constitutively expressed NOS isozymes. We also identify Cys-His-Glu as a nitrosylation motif that is conserved in a family of arginine handling enzymes.
Dimethylarginine dimethylaminohydrolase (DDAH) catalyses the hydrolysis of NG-monomethyl-l-arginine (l-NMMA) and asymmetric dimethylarginine (ADMA) to citrulline and either mono- or di-methylamine, respectively (1). ADMA and l-NMMA are competitive inhibitors of all three isoforms of NO synthase, and we and others (2–4) have suggested that the accumulation of endogenous methylarginines (particularly ADMA) is an important mechanism to regulate nitric oxide (NO) production. Consistent with this suggestion, ADMA levels are elevated in various disease states in which a reduction of NO production contributes to pathology, including renal failure, hypertension and hypercholesterolaemia (3). Indeed in renal failure, plasma ADMA levels are strong predictors of mortality and cardiovascular morbidity (5).
DDAH activity is a key determinant of asymmetric methylarginine concentrations (6), but factors that regulate its activity have not clearly been identified. Recently, we solved the crystal structure of a microbial DDAH and identified a Cys-His-Glu catalytic triad (Cys-249, His-162, and Glu-114) (7). Equivalent cysteine and histidine residues and a glutamic or aspartic acid residue are conserved in all known DDAHs, including both of the isoforms present in humans (8). The presence of a reactive cysteine residue in the active site of DDAH raised the possibility that DDAH activity might be altered in situations of oxidative or nitrosative stress. In the present study, we demonstrate that DDAH activity is regulated by NO and show that the mechanism is by reversible nitrosylation of Cys-249. This observation extends the interactions between NOS and DDAH and identifies a potential homeostatic mechanism to control NO generation. It also defines a novel catalytic triad that is susceptible to regulation by NO.
Materials and Methods
Materials.
Restriction enzymes and TaqDNA polymerase were purchased from Roche. Arginine, ADMA, symmetric dimethylarginine, and l-NMMA were from Calbiochem. The 2-(N,N-dimethylamino)-diazenolate-2-oxide⋅Na (DEANONOate) was purchased from Alexis Biochemicals, Lausen, Switzerland. Oxidized DEANONOate was produced by incubation of a solution of DEANONOate (10−2 M) in PBS at room temperature overnight. Anti-S-nitrosocysteine Ab was from Calbiochem. Horseradish peroxidase-conjugated anti-rabbit secondary Ab was from Amersham Pharmacia. Trimethylrhodamine isothiocyanate-conjugated anti-mouse secondary Ab and calcium ionophore A23187 were from Sigma. BIOTRAK cGMP enzymeimmunoassay kit was from Amersham Pharmacia.
Construction of DDAH Expression Plasmids.
The WT Pseudomonas aeruginosa DDAH (PaDDAH) gene was amplified by PCR and cloned into the bacterial expression vector pProEX.Hta as previously described (9). Site-specific mutagenesis was achieved by a PCR-based approach by using the WT PaDDAH expression plasmid as a template. For His-162 and Glu-114, pairs of complementary oligonucleotides spanning the target codon were designed to introduce nucleotide changes to encode alanine at residue 162 or isoleucine at residue 114. In separate PCR reactions, one of the mutagenic primers was used with the appropriate downstream vector primer. The PCR products were gel purified and mixed and the DNA mixture used as a template for a second PCR reaction in which only the vector primers were included. In this way, full-length PaDDAH sequences incorporating the desired mutations were produced. Mutated sequences were digested with EcoRI and HindIII and cloned into EcoRI and HindIII digested pProEX.Hta. For Cys-249 a single mutagenic oligonucleotide was designed. This oligonucleotide encodes the cysteine to serine (Cys249Ser) mutation followed by the natural stop codon and a downstream HindIII site. PCR was performed by using WT PaDDAH as a template and included Cys249Ser and a 5′-vector primer. After PCR, the appropriate-sized product was purified, restriction digested, and cloned into pProEX.Hta as described above.
To produce an epitope-tagged human DDAH II expression plasmid, the DDAH II ORF was amplified by using a 5′-oligonucleotide that encodes a HindIII site, a sequence encoding the 9E10 epitope from N-myc followed by a sequence complementary to nucleotides 3–27 of human DDAH II and a 3′-oligonucleotide complementary to residues 836–858 of human DDAH II and a downstream XbaI site. After amplification, the appropriately sized product was purified, digested with HindIII and XbaI, and cloned into similarly digested pcDNA3.1Hygro. All sequences were verified by automated DNA sequencing.
Recombinant Expression of DDAH.
WT and mutant PaDDAH proteins were expressed in Escherichia coli BL21 essentially as described (9). After induction of expression, cells were collected by centrifugation (3,000 × g, 5 min at 4°C) and resuspended (1 g cells ml−1) in phosphate buffer (100 mM sodium phosphate/300 mM NaCl, pH 6.8) containing 10 mM imidazole. Cells were disrupted by sonication (5 × 10-sec bursts) and soluble proteins separated from insoluble material by centrifugation (10,000 × g, 20 min at 4°C). Cleared lysates (5 ml) were mixed with nickel-nitrilotriacetic acid agarose resin (1 ml of 50% suspension preequilibrated in phosphate buffer) for 60 min at 4°C to bind polyhistidine tagged recombinant PaDDAH proteins. The lysate/resin suspension was transferred to a minicolumn and unbound proteins allowed to flow through. The resin was washed with phosphate buffer containing 50 mM imidazole (2 × 5 ml) and bound proteins eluted in phosphate buffer containing 250 mM imidazole (2 × 1 ml). Eluted proteins were dialyzed against phosphate buffer (2 × 1 liter for 1 h at 4°C). Protein purity was determined by SDS/PAGE analysis.
Endothelial Cell Expression of Human DDAH II.
Plasmid pcDNA3-NmycDDAH II was expressed in the murine endothelial cell line sEnd.1 by stable transfection. sEnd1 cells were cultured in DMEM supplemented with 1 mM glutamine, 10% FBS, and penicillin and streptomycin in a humidified incubator at 37°C with 5% CO2. Cells (60–70% confluence) were transfected by using Transfast transfection reagent according to the manufacturer's instructions (Promega). Transfected cells were selected with hygromycin (500 μg/ml) 48 h after transfection. Hygromycin-resistant clones were isolated and analyzed for recombinant DDAH II expression by Western blotting by using the 9E10 anti-myc epitope mAb. Where indicated, culture medium was supplemented with lipopolysaccharide (5 μg/ml), IFN-γ (100 units/ml), and tumor necrosis factor-α (10 ng/ml) for 24 h to induce iNOS expression. DDAH assays and immunofluorescence were performed as described (10). NO production was assessed by measuring nitrite in the culture medium by using the Griess reaction. In some experiments, sEnd.1 cells were stimulated with calcium ionophore A23187 (100 μM) for 15 min before measurement of intracellular cGMP by using a cGMP enzymeimmunoassay system according to the manufacturer's instructions.
In Vitro Nitrosylation Assays.
Aliquots of partially purified recombinant PaDDAH proteins (≈1 mg) were incubated with the NO donor DEANONOate (10−5–10−2 M) in a final volume of 50 μl of phosphate buffer at 37°C for 10 min. After incubation, reactions were dialyzed against phosphate buffer (1 liter for 1 h at 4°C) before incubation in the presence or absence of DTT (20 mM final concentration) for 10 min on ice. Reactions were again dialyzed against phosphate buffer before assaying DDAH activity as previously described. Aliquots of reactions also were analyzed for the presence of S-nitrosocysteine by SDS/PAGE under nonreducing conditions followed by Western blotting using a rabbit polyclonal anti-S-nitrosocysteine Ab. In other experiments, the effect of NO donors on DDAH activity in rat kidney extracts was determined. Rat kidneys were homogenized in ice-cold phosphate buffer (30% w/vol) by using a UltraTurrax tissue homogenizer (Scientific Laboratory Supplies, Nottingham, U.K.). Homogenates were centrifuged at low speed (14,000 × g for 10 min at 4°C) to remove intact tissue and supernatants then centrifuged at high speed (100,000 × g for 30 min at 4°C) to produce a cytosolic extract. Aliquots of cytosolic extracts (50 μl) were incubated with DEANONOate and DTT before assaying for DDAH activity (6).
Results
NO Donors Inhibit the Activity of Purified DDAH in Vitro.
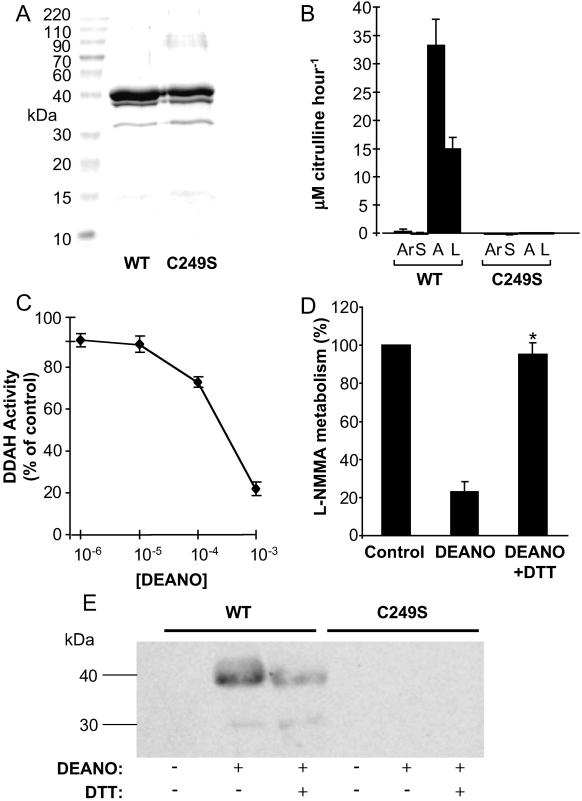
WT and mutant PaDDAH were expressed in E. coli and partially purified as described. SDS/PAGE analysis followed by Coomassie blue staining revealed the presence of a predominant band of ≈40 kDa (the expected size on N-terminally polyhistidine-tagged DDAH) and a minor proteolytic product that migrates at ≈30 kDa. WT and mutant proteins were expressed to similar levels and were >90% pure after purification on nickel agarose (Fig. 1A). DDAH activity assays indicated that only the WT protein possessed measurable DDAH activity as judged by the conversion of ADMA and l-NMMA to citrulline (Fig. 1B). Aliquots of WT protein (≈1 μg) were incubated with increasing concentrations of the NO donor DEANONOate, dialyzed against PBS, and assayed for DDAH activity. Incubation with DEANONOate resulted in a concentration-dependent inhibition of DDAH activity (Fig. 1C). In contrast, oxidized DEANONOate had no effect on DDAH activity (data not shown).
Figure 1.
Recombinant expression and S-nitrosylation of PaDDAH. (A) WT and cysteine to serine mutated (C249S) PaDDAH were expressed in E. coli and purified as described in Materials and Methods. Aliquots of proteins (≈200 μg) were resolved by SDS/PAGE and proteins visualized by Coomassie blue staining. (B) Aliquots of purified recombinant proteins were assayed for the ability to metabolize either arginine (Ar), SDMA (S), ADMA (A), or l-NMMA (L). Data are expressed as micromol (μM) of citrulline produced per milligram (mg) of protein per hour (n = 4). (C) Purified WT PaDDAH (≈10 μg) was incubated with the indicated concentrations of the NO donor DEANONOate. After incubation, DDAH activity was determined. Data are presented as the percentage of control DDAH activity remaining after incubation (n = 4). (D) WT PaDDAH was incubated with 10−3 M DEANONOate before dialysis and treatment with DTT. Samples were again dialyzed and then assayed for DDAH activity. Data are presented as the percentage of control activity remaining after each treatment (*, P < 0.01, n = 4). (E) Aliquots of WT and Cys-249→Ser (C249S) mutant DDAH proteins treated with either DEANONOate and/or DTT as indicated were processed for Western blotting by using an anti-S-nitrosocysteine Ab.
Inhibition of DDAH Activity Is Reversed by DTT.
To test the hypothesis that inhibition of DDAH activity was caused by S-nitrosylation of cysteine residues within the protein, we determined whether the effects of DEANONOate could be reversed by the reducing agent DTT. WT DDAH protein was incubated with DEANONOate (10−3 M), dialyzed, incubated in the presence or absence of DTT, redialyzed, and assayed for DDAH activity. Incubation in the presence of DTT resulted in a 95 ± 2.8% (P < 0.01) reversal of the DEANONOate induced inhibition of DDAH activity (Fig. 1D). Incubation with DTT alone had no effect on DDAH activity (data not shown).
Cys-249 Is the Major Site of S-Nitrosylation in DDAH.
PaDDAH contains five cysteine residues including Cys-249, which forms part of the catalytic triad of residues in the active site. This active site cysteine residue is essential for DDAH activity, and we therefore performed Western blotting by using a commercial Ab raised against S-nitrosocysteine to determine whether this residue could be S-nitrosylated by NO donors. WT PaDDAH did not react with the anti-S-nitrosocysteine Ab; however, after treatment with DEANONOate, the protein reacted with the Ab, indicating the presence of S-nitrosocysteine (Fig. 1D). Treatment of the nitrosylated protein with DTT reduced the intensity of the immunoreactive band indicating that S-nitrosylation of DDAH is partially reversed under reducing conditions. In contrast to WT PaDDAH, the Cys-249→Ser mutant, a single atom change, was not nitrosylated in the presence of DEANONOate (Fig. 1E), suggesting that Cys-249 is the predominant if not exclusive target for S-nitrosylation in PaDDAH. Incubation of His-162 or Glu-114 mutant proteins with DEANONOate did not abolish S-nitrosylation, but given the nonquantitative nature of Western blotting, we were unable to determine whether these proteins are less efficiently S-nitrosylated than WT DDAH (data not shown).
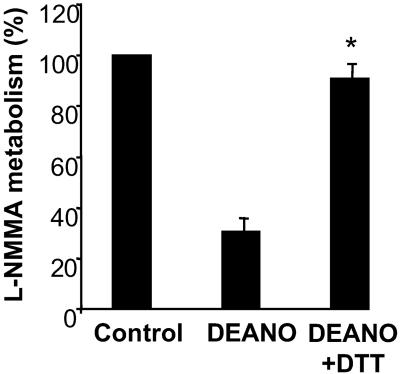
Mammalian DDAH Is also Reversibly Inhibited by NO Donors.
Similar to the effects on PaDDAH, DEANONOate inhibited kidney cytosolic DDAH activity, and this effect was reversed by 92.4 ± 3.2% (P < 0.01) after incubation with DTT (Fig. 2).
Figure 2.
Reversible inhibition of DDAH in kidney lysates by NO donors. Aliquots of rat cytosolic extracts (≈200 μg) were incubated with 10−3 M DEANONOate before dialysis and treatment with DTT. Samples were again dialyzed and then assayed for DDAH activity. Data are presented as the percentage of control activity remaining after each treatment (*, P < 0.01, n = 4).
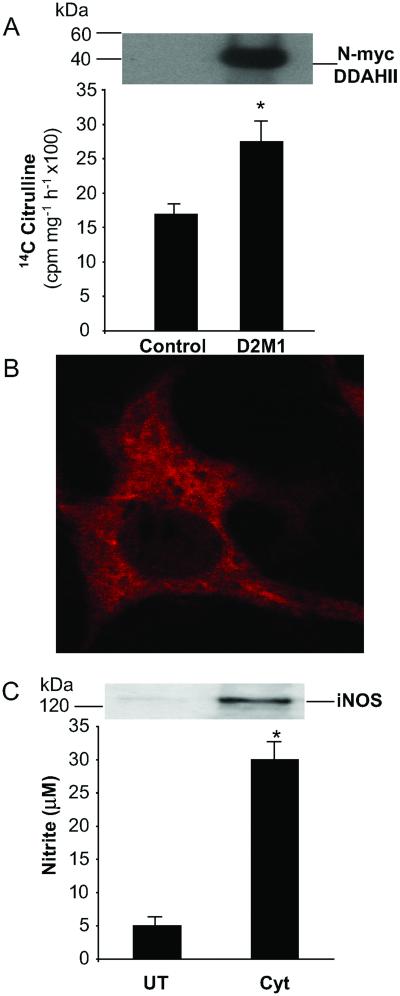
Mammalian DDAH Is S-Nitrosylated After iNOS Induction in Endothelial Cells in Culture.
We produced several sEnd.1 cell lines that stably overexpress N-terminally Myc-tagged human DDAH II. Western blot analysis by using the anti-Myc mAb 9E10 revealed the presence of an immunoreactive band of ≈40 kDa in clone D2M1, which was absent in mock-transfected control cells (Fig. 3A). Consistent with overexpression of functionally active DDAH I, D2M1 cells had higher DDAH activity than mock-transfected controls (26.2 ± 4.2 vs. 17.4 ± 1.8 cpm/mg/h × 100, P < 0.05) (Fig. 3A). Immunofluorescent staining of D2M1 cells indicated that N-myc DDAH II is distributed throughout the cytoplasm (Fig. 3B) whereas no staining was apparent in mock-transfected cells (data not shown). Under normal conditions in culture, sEnd.1 cell express the endothelial isoform of NOS and make low but detectable levels of NO (Fig. 3C). After cytokine treatment, sEnd.1 cells expressed the inducible NOS isoform and produced higher levels of NO (4.8 ± 1.8 μM/mg/24 h vs. 28 ± 4.3 μM/mg/24 h, P < 0.01) (Fig. 3C).
Figure 3.
Overexpression of human DDAH II in endothelial cells. (A) Murine endothelial cells sEnd.1 (control) and sEnd.1 cells stably overexpressing N-terminally myc-tagged human DDAH II (D2M1) were assayed for DDAH expression by either Western blotting (Upper) or by DDAH activity assay (Lower). DDAH activity is presented as cpm in 14C citrulline produced per milligram of cellular protein per hour (*, P < 0.05, n = 3). Western blot data are from a single representative experiment. Similar results were obtained in three independent experiments. (B) D2M1 cells were fixed permeabilized and stained by using the anti-myc 9E10 Ab followed by a trimethyl rhodomine isothiocyanate-conjugated secondary Ab. Fluorescence staining was visualized by confocal microscopy. (C) D2M1 cells were either cultured under standard conditions (UT) or cultured in the presence of cytokines (Cyt) for 24 h to induce iNOS expression. iNOS expression was confirmed by Western blotting (Upper) and determination of NO production by using the Griess assay for nitrite accumulation in the culture medium (Lower) (*, P < 0.01, n = 3).
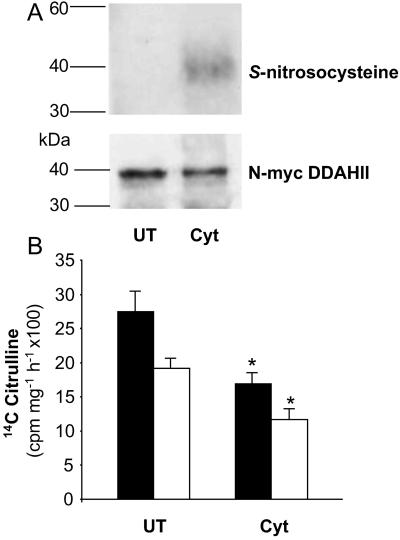
We examined the S-nitrosylation state of N-myc DDAH II from D2M1 cells under normal growth conditions and after cytokine treatment. In untreated cells, immunoprecipitated N-myc DDAH II was not detected on Western blots by using an S-nitrosocysteine Ab, although the protein was readily detected with the 9E10 Ab (Fig. 4A). In contrast, after cytokine treatment, N-myc DDAH II was readily detected by the anti-S-nitrosocysteine Ab. These data indicate that, in situations of high level endogenous NO production, DDAH II is S-nitrosylated in intact cells. Consistent with a functional effect after cytokine treatment DDAH activity decreased in D2M1 and control cells 27.3 ± 4.4 vs. 17 ± 2.8 (P < 0.05) and 19.1 ± 3.4 vs. 12.2 ± 2.9 (P < 0.05), respectively (Fig. 4B). In a separate series of studies, we stimulated endothelial NOS activity in sEnd.1 cells with calcium ionophore (A23187; 100 μM). Although cGMP levels increased, there was no change in DDAH activity (n = 12, from three independent experiments; data not shown).
Figure 4.
S-nitrosylation and inhibition of DDAH II in endothelial cells. (A) D2M1 cells were either cultured under standard conditions (UT) or in the presence of cytokines (Cyt) for 24 h. Lysates of cells were processed for immunoprecipitation by using an anti-myc Ab followed by Western blotting using either an Ab against S-nitrosocysteine (Upper) or an anti-myc Ab (Lower). Data are from a single representative experiment. Similar results were obtained in three independent experiments. (B) D2M1 (solid bars) and sEnd.1 cells (open bars) treated as above were assayed for DDAH activity. Data are presented as cpm in 14C citrulline produced per milligram of cellular protein per hour (*, P < 0.05, n = 3).
Discussion
Dimethylarginine dimethylaminohydrolase (DDAH) metabolizes and inactivates the endogenous NOS inhibitors l-NMMA and ADMA (1). The results of this study show that NO donors reversibly inhibit DDAH activity in vitro and that the mechanism of the effect is S-nitrosylation of Cys-249. S-nitrosylation of DDAH also occurs after induction of iNOS in endothelial cells providing a potential feedback mechanism to regulate NO production.
DDAH is highly conserved throughout evolution and previously we reported the existence of bacterial DDAHs (9, 11). Recently we solved the crystal structure of DDAH from P. aeruginosa and proposed a reaction mechanism for DDAH (7). This involved nucleophilic attack of the sulfur atom of Cys-249 on the C atom of the guanidino group of ADMA. In the present study, we expressed mutant enzyme in which this cysteine residue was replaced by serine, a single atom change. The protein is soluble and folds normally (7) but is unable to metabolize ADMA to citrulline. Cys-249 forms part of a catalytic triad that is conserved in the active sites of all DDAHs identified and mutation of the other two residues, His-162 and Glu-114, also resulted in loss of enzyme activity. These findings confirm that Cys-249 is essential for enzyme activity and indicate that the other two residues are important to maintain the cysteine in an activated state, possibly as a thiolate-imidazolinium ion pair with His-162. These findings suggested that DDAH might be susceptible to regulation by cysteine nitrosylation.
Purified recombinant bacterial DDAH was inhibited by addition of the NO donor DEANONOate. The effect was concentration-dependent and was reversed by addition of the reducing agent DTT. The effect of the NO donor was substantial, with the highest concentration inhibiting DDAH activity by >90%. It is difficult to be certain of the concentration of NO that would have reached the DDAH but the concentration range of DEANONOate over which inhibition of DDAH activity occurred is similar to that reported for other enzymes that are regulated by nitrosylation (12) and also was seen with endogenous NO generation. To test whether the effects were because of nitrosylation, we used a specific Ab that recognizes S-nitrosocysteine residues. This Ab did not detect native DDAH but recognized DDAH treated with DEANONOate. The effect was reversed by DTT and these results indicate that treatment with DEANONOate S-nitrosylated cysteine on DDAH.
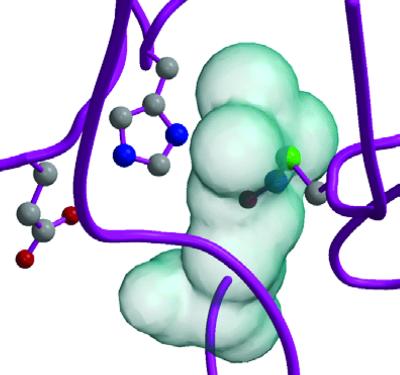
There are five cysteine residues in the DDAH protein (9), and any or all of these might be targets for S-nitrosylation. To test the hypothesis that S-nitrosylation occurs preferentially on the active site cysteine that is held in an NO-responsive state, we expressed the Cys-249→Ser mutant. Unlike the WT protein, when incubated with DEANONOate, the Cys-249→Ser mutant was not recognized by the anti-S-nitrosocysteine Ab. These observations confirm that the active site cysteine is the major, or possibly the only, cysteine residue that is nitrosylated and confirm that the action of the NO donor to inhibit DDAH activity is because of S-nitrosylation of this residue. Consistent with these observations, molecular modeling of the effects on S-nitrosylation of Cys-249 predict that this modification would both deactivate the sulfur atom of this residue and cause interference of protein/substrate binding (Fig. 5). Nitrosylation of cysteine residues or transition metal centers is increasingly being recognized as a posttranslational modification that is used as a regulatory mechanism in a wide spectrum of functionally diverse proteins (13). As found in the present study, specific nitrosylation of a single cysteine residue is usually observed, and some success has been achieved in the prediction of nitrosylation sites from sequence data. The primary sequence adjacent to Cys-249 of DDAH does not contain the acid/base nitrosylation motif identified by Stamler et. al (14); however, spatial juxtaposition of the cysteine with acidic and basic residues within the protein fold, recognized by these authors as a more general criterion (15, 16), is found in DDAH. Interestingly, the disposition of catalytic residues of DDAH around the substrate-binding site resembles that of the cysteine proteases, a family of proteins which are well known to be inhibited by NO donors (17). Hess et al. (15) also observe that “hydrophobic tunnels” may serve to channel NO toward its target thiols, and the buried nature of the active site in DDAH suggests that this could be a feature of its interaction with NO. We have previously identified the Cys-His-Glu/Asp triad in a superfamily of arginine-handling enzymes, and it seems likely that the other members (arginine glycine amidino transferase and arginine deiminase) will also be susceptible to regulation by S-nitrosylation.
Figure 5.
Structural modeling of S-nitrosylation of Cys-249 of DDAH. The active site of PaDDAH has His-162 and Glu-114 on one side of the substrate and Cys-249 on the other [Protein Data Bank (PDB) ID code 1H70]. The protein backbone is in magenta, with the three catalytic sidechains in a ball-and-stick representation. The substrate position is indicated by the transparent molecular surface of ADMA. The nitrosylated sidechain of Cys-249 was modeled by analogy with that of Cys-93 of S-nitroso-nitrosyl human hemoglobin A (PDB ID code 1BUW). Manual adjustment of the sidechain torsion angles was unable to produce a model in which there was no steric clash with either other protein sidechains (not shown) or the substrate.
Studies of the isolated enzyme were undertaken by using bacterial DDAH because this expresses to high levels and folds normally in the bacterial expression system. To determine whether the findings are also relevant to mammalian DDAH, we examined the effect of DEANONOate on DDAH activity in rat tissue. Like PaDDAH, incubation of rat kidney cytosolic extracts with DEANONOate resulted in inhibition of DDAH activity, which was reversed by DTT. These data indicate that inhibition by NO has been conserved in DDAH enzymes from species as widely divergent as microbes and mammals. As rat kidney cytosol contains both DDAH I and II it seems likely that both isoforms were inhibited after incubation with DEANONOate.
To test the hypothesis that endogenously produced NO would also S-nitrosylate and inhibit DDAH, we generated endothelial cell lines that stably overexpressed human DDAH II. This system has the advantage that the cell line used expresses iNOS when stimulated with cytokines, and therefore it was possible to test whether basal NO synthesized by eNOS or induced NO synthesized by iNOS has the capacity to S-nitrosylate DDAH. Immunoprecipitation of the tagged DDAH showed that under basal conditions DDAH was not S-nitrosylated to any great extent. However, after cytokine stimulation higher levels of NO were generated and under these conditions DDAH was S-nitrosylated. This demonstrates that the amounts of NO generated within cells, at least after cytokine activation, is sufficient to S-nitrosylate DDAH. Furthermore, cytokine treatment was associated with a significant decrease in DDAH activity. We also have found that DDAH activity is reduced in rats treated with endotoxin to induce iNOS, suggesting that the phenomenon also occurs in vivo (unpublished observations). However we cannot exclude the possibility that there is a low degree of nitrosylation (below the detection limit of our assay) that regulates DDAH activity in the basal state. Although in the present study, stimulation of eNOS activity with calcium ionophore did not alter DDAH activity, it remains possible that an increase in intracellular NO per se, whatever the source, would nitrosylate DDAH under certain conditions.
Recently, Stamler and coworkers (18) have reported that NO produced by each of the NOS isoforms S-nitrosylates a distinct subset of cellular proteins. This isoform-specific nitrosylation was proposed to result in part from the different intracellular localizations of the NOS isoforms within the cell. Interestingly, DDAH was S-nitrosylated after the induction of the iNOS enzyme, which localizes to the cytosol where DDAH II is found.
DDAH I and II have emerged as key enzymes regulating the cellular and tissue levels of the endogenous NOS inhibitor ADMA. The studies described here clearly show that DDAH activity is regulated by exogenous NO and endogenous NO derived from iNOS and that the mechanism is S-nitrosylation of Cys-249. The implication of the finding is that under certain conditions when NO generation increases, S-nitrosylation diminishes DDAH activity and this would be expected to lead to accumulation of ADMA and inhibition of NOS. Because ADMA inhibits all three isoforms of NOS, this may help explain why induction of high output NO generation can subsequently suppress endothelium-dependent vasodilator responses (19).
Acknowledgments
We thank Dr. Guy Whitley (St. Georges Hospital Medical School, London) for discussion and critical reading of this manuscript and Dr. E Wagner (Institute of Molecular Pathology, Vienna) for the kind gift of sEnd-1 cells. This work was supported by British Heart Foundation Program Grants RG94008 and RG200007 (to P.V.).
Abbreviations
- DDAH
dimethylarginine dimethylaminohydrolase
- NOS
nitric oxide synthase
- iNOS
inducible NOS
- ADMA
asymmetric dimethylarginine
- DEANONOate
2-(N,N-dimethylamino)diazenolate-2-oxide⋅Na
- DTT
dithiothreitol
- l-NMMA
NG-monomethyl-l-arginine
- PaDDAH
P. aeruginosa DDAH
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ogawa T, Kimoto M, Sasaoka K. J Biol Chem. 1989;264:10205–10209. [PubMed] [Google Scholar]
- 2.Vallance P, Leone A, Calver A, Collier J, Moncada S. Lancet. 1992;339:572–573. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 3.Leiper J M, Vallance P. Cardiovasc Res. 1999;43:542–548. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Tsao P S, Adimoolam S, Kimoto M, Ogawa T, Cooke J P. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 5.Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frolich J, et al. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 6.MacAllister R J, Parry H, Kimoto M, Ogawa T, Russell R J, Hodson H, Whitley G S, Vallance P. Br J Pharmacol. 1996;119:1533–1540. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray-Rust J, Leiper J M, McAlister M, Phelan J, Tilley S, Santa Maria J, Vallance P, McDonald N. Nat Struct Biol. 2001;8:679–683. doi: 10.1038/90387. [DOI] [PubMed] [Google Scholar]
- 8.Leiper J M, Santa Maria J, Chubb A, MacAllister R J, Charles I G, Whitley G S, Vallance P. Biochem J. 1999;343:209–214. [PMC free article] [PubMed] [Google Scholar]
- 9.Santa Maria J, Vallance P, Charles I G, Leiper J M. Mol Microbiol. 1999;33:1278–1279. doi: 10.1046/j.1365-2958.1999.01580.x. [DOI] [PubMed] [Google Scholar]
- 10.Birdsey G M, Leiper J M, Vallance P. Acta Physiol Scand. 2000;168:73–79. doi: 10.1046/j.1365-201x.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- 11.Tran C T, Fox M F, Vallance P, Leiper J M. Genomics. 2000;68:101–105. doi: 10.1006/geno.2000.6262. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y M, Talanian R V, Billiar T R. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 13.Jaffery S R, Erdjument-Bromage H, Ferris C D, Temps P, Snyder S H. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 14.Stamler J S, Toone E J, Lipton S A, Sucher N J. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 15.Hess D T, Matsumoto A, Nudelman R, Stamler J S. Nat Cell Biol. 2001;3:E46–E49. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- 16.Stamler J S, Lamas S, Fang F C. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 17.Mannick J B, Hausladen A, Liu L, Hess D T, Zeng M, Miao Q Z, Kane L S, Gow A J, Stamler J S. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 18.Gow A J, Chen Q, Hess D T, Day B J, Ischiropoulos H, Stamler J S. J Biol Chem. 2002;277:9637–9640. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 19.Kessler P, Bauersachs J, Busse R, Schini-Kerth V B. Arterioscler Thromb Vasc Biol. 1997;17:1746–1755. doi: 10.1161/01.atv.17.9.1746. [DOI] [PubMed] [Google Scholar]