Abstract
Controlling cell division is fundamental. One environmental cue that exerts profound control over both the orientation and frequency of cell division in vivo is a naturally occurring, wound-induced electric field (EF). Wounds in rat corneas generate endogenous EFs in the plane of the epithelial sheet because the transcorneal potential difference (TCPD; +40 mV internally positive) collapses at the wound edge, but is maintained at normal levels at 0.5 mm back from the wound. We manipulated the endogenous EF this creates by using drugs with differing actions. The wound-induced EF controlled the orientation of cell division; most epithelial cells divided with a cleavage plane parallel to the wound edge and perpendicular to the EF vector. Increasing or decreasing the EF pharmacologically, respectively increased or decreased the extent of oriented cell division. In addition, cells closest to the wound edge, where the EF was highest, were oriented most strongly by the EF. Remarkably, an endogenous EF also enhanced the frequency of cell division. This also was regulated by enhancing or suppressing the EF pharmacologically. Because the endogenous EF also regulated the wound healing rate, it may act as one control of the interplay between cell migration and cell division during healing.
Keywords: cleavage plane‖electric fields‖cell migration‖corneal epithelium
Cell division and migration are fundamental in development, wound healing, and pathology. Many cell types undergo oriented cell division and directed migration. Understanding the controls of these behaviors is crucial. In the Drosophila CNS, neuroepithelial cells delaminate basally from the neuroectoderm and divide asymmetrically along the apical–basal axis, producing a neuroblast and a ganglion mother cell. The neuroblast remains adjacent to the neuroectoderm and retains stem cell-like properties, whereas the ganglion mother cell divides once more to produce differentiated neurons or glia (1, 2). Cortical neurons in the mouse CNS also are generated from asymmetric divisions (3). Cells in the germinal layers lining the ventricles can divide with a vertical cleavage plane and remain in the germinal layer, or divide horizontally, generating one germinal cell and a daughter cell, which will migrate away and differentiate into a neuron. A variety of proteins become distributed asymmetrically during these cell divisions and play crucial roles in determining the extent of symmetry and the orientation of cell division (see Discussion and refs. 4 and 5). Oriented division and directional migration are essential for correctly locating postmitotic neurons in the developing nervous system.
Several environmental cues contribute to oriented cell division and directional migration. Appropriate cell–cell contact directs the orientation of mitotic spindles (6, 7), whereas chemotaxis modulates the direction of cell migration (8–13). Naturally occurring EFs also orient division and direct cell migration (14–16). For example, corneal epithelial cells in culture migrate toward a cathode in a physiological EF and divide with a cleavage plane perpendicular to the EF vector (14, 17–19). Endogenous EFs exist in many situations where cells divide and migrate (20, 21). Disrupting the endogenous EFs associated with the neural plate and neural tube in amphibia causes specific abnormalities of CNS development (22–24). Similarly, the cellular events underpinning wound healing depend on the wound-induced EF. If this is inhibited, healing is compromised (25, 26). Perhaps disruptions arise because EFs control cell division, cell proliferation, and cell migration in vivo, in a manner similar to that demonstrated directly in vitro.
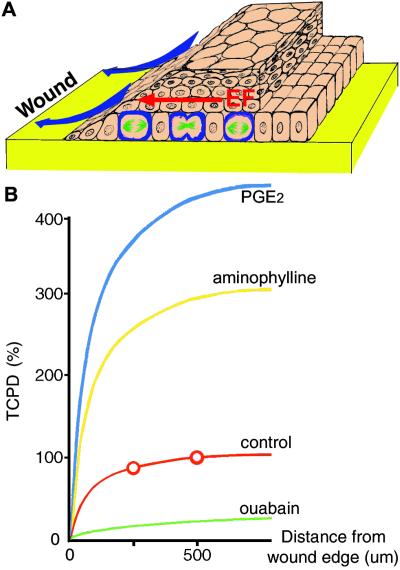
The mammalian cornea establishes an internally positive transcorneal potential difference (TCPD; +40 mV) by pumping Na+ and K+ in and Cl− out (27–29). A dc EF arises instantaneously on wounding, because a current sink is created at the lesion when the epithelium is breached. The TCPD here falls to zero, but it is maintained at normal levels 0.5–1 mm back from the lesion, and this creates a laterally oriented voltage drop along the plane of the epithelium (Fig. 1A) (25, 30). The wounded cornea is therefore an excellent model in which to test the effects of a naturally occurring EF. In addition, because there are many pharmacological ways to alter the TCPD (and the resultant EF), the effects of manipulating the naturally occurring EF also can be assessed.
Figure 1.
Summary diagram indicating the origin and effects of the wound-induced EF in rat cornea. (A) The EF (red) directed cell division (green/blue) and controlled wound healing by directing cell migration (blue arrows). (B) Profiles of the lateral voltage gradients near a corneal wound are shown and are based on previous direct measurements (30). The transcorneal potential difference (TCPD) has collapsed to 0 mV per mm at the wound, but retains its normal value in the unwounded epithelium about 500 μm from the wound edge (30). This creates a steep lateral EF near the wound (red), where the points were determined by direct measurement (30). Pharmacologically enhancing (blue and yellow) or reducing (green) the TCPD (which is sustained 500 μm back from the wound edge), respectively, will increase or decrease the endogenous EF gradients as shown. We make the assumption that the lateral resistance does not change with the drug treatments.
We demonstrate that the natural EF at a wound controlled the orientation of cell division with the cleavage plane lying perpendicular to the EF vector, regulated the rate of wound healing, and modulated the frequency of cell division. This is evidence at a single-cell level of physiological effects of naturally occurring EFs in vivo.
Materials and Methods
TCPD Measurement.
Corneas were clamped in Ussing chambers with a 3-mm-diameter hole immediately after dissection, perfused continuously at 10 ml/min with Krebs–Ringer solution (pH 7.4), and equilibrated with 95% O2/5% CO2. TCPD was recorded by using a DVC-1000 amplifier (World Precision Instruments, Sarasota, FL). Aminophylline, prostaglandin E2 (PGE2), neomycin, and ouabain were added to both sides of the corneas, at final concentrations of 10 mM, 0.1 mM, 10 mM, and 10 mM, respectively. Aminophylline and PGE2 were used to enhance the TCPD and hence the wound-induced lateral EF, whereas ouabain was used to reduce TCPD. Neomycin had no effect on TCPD.
Circular Epithelial Wounds.
Sprague–Dawley rats (27–30 days old, male and female) were anesthetized with intramuscular Hypnom (0.3 ml/kg) and i.p. diazepam (0.5 ml/kg). Central circular (3.5 mm diameter) corneal epithelial wounds were made through the whole epithelium with a trephine. The epithelium within the lesion, with the basement membrane intact, was removed under an ophthalmic microscope. Sterile conditions were maintained for all experiments. Postsurgical recovery was uneventful and corneal wound healing proceeded without infection.
Application of Drugs.
Aminophylline (10 mM), 0.1 mM PGE2, 10 mM ouabain, and 10 mM neomycin were applied topically to lesioned corneas every 2 h after wounding, for a 30-h period. Balanced salt solution was used for control corneas. All agents were diluted in balanced salt solution: NaCl 140 mM/KCl 5 mM/CaCl2 1.8 mM/MgCl2 0.5 mM/glucose 5 mM/Hepes 10 mM, pH 7.4.
Scanning Confocal Microscopy.
Control and drug-treated animals were killed by using CO2 at 12 h after wounding. Although the peak of proliferation occurs at about 24 h after wounding (31, 32), we observed in our preliminary experiments that drugs that enhanced the EF could cause complete wound healing within 24 h. Therefore, we chose to study the early responses of corneal epithelial cells to endogenous EF within 12 h.
Wounded corneas were removed, fixed, permeabilized, and incubated with 1:200 FITC-conjugated monoclonal anti-α-tubulin (Sigma) to label spindle microtubules during mitosis, and with rhodamine-labeled phalloidin (1:50, Molecular Probes) to label F-actin. All dividing cells within the first 0–600 μm of the wound edge were counted under confocal microscopy (×100 objective lens). Cells dividing at 0–200, 200–400, and 400–600 μm from the wound edge and in the limbus (1530–1700 μm) also were assessed. Cell proliferation and the orientation of division were assessed within a sector with a surface area of 200 × 100 μm. The angle between the cleavage plane and the EF vector was measured to quantify orientation of division. The orientation of division was analyzed by Rayleigh's distribution to give a mean polarization index (PI) of (∑ncos[2(θ−90)]/n), where θ is the angle between the cleavage plane and the EF vector (33). A population of cells dividing with all cleavage planes either perpendicular or parallel to the EF vector would give a PI of 1, or −1, respectively. Cells dividing at random angles would have a mean PI of 0. The number of cells dividing in each area (200 × 100 μm) was counted.
Statistical Analysis.
Two-sided Pearson χ2 test and Student's t test were used.
Results
A dc EF arises instantaneously at a corneal wound (see Introduction) and is regulated by many drugs with disparate actions. PGE2, which enhances Cl− efflux, and aminophylline, which inhibits the phosphodiesterase breakdown of cAMP and enhances Cl− efflux, were chosen to increase the TCPD and the wound-generated lateral EF this creates. The TCPD increased 3- to 4-fold (425 ± 25% and 288 ± 13%, respectively; n = 3, P < 0.01). Ouabain, which inhibits Na+/K+-ATPase, reduced the TCPD 5-fold (to 18.8 ± 3.2%, n = 6, P < 0.01: Fig. 1B). Neomycin, which inhibits phospholipase C, was used because it (i) enhanced the wound healing rate by a mechanism that did not involve a change in TCPD (see below) and (ii) inhibited some EF-induced cell behaviors (34, 35). We chose these drugs for their effects on TCPD (to increase, reduce, or leave it unchanged), but in particular because they induce their effects by different mechanisms.
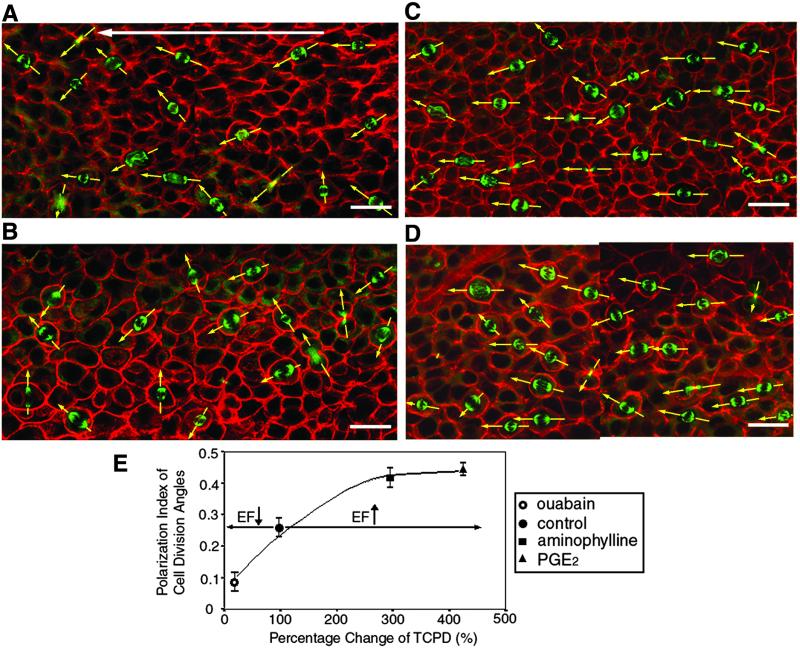
The naturally occurring EF regulated the axis of cell division within 500 μm of the wound edge. At untreated wounds, the cleavage plane was not random, and spindles lay roughly parallel to the EF vector with cleavage occurring perpendicularly (Fig. 2). The mean PI was 0.26 ± 0.03 (n = 529 cells, five corneas, P < 0.001, Fig. 2 A and E). Enhancing the wound-generated EF pharmacologically with PGE2 or aminophylline markedly enhanced this effect, nearly doubling the PI to 0.44 ± 0.03 and 0.41 ± 0.03, respectively (n = 578 and 462 cells, six corneas each, Fig. 2 C–E). Cleavage planes, therefore, lay more perpendicular to the EF vector when its magnitude was increased (P < 0.01 compared with controls, Student's t test).
Figure 2.
Wound-generated endogenous EFs controlled the proportion and axis of cell division. Mitotic spindles stained with FITC-conjugated anti-α-tubulin (green) are shown in whole mount corneas (12 h post lesion), and double-stained with rhodamine-phalloidin (red). In wounded but untreated corneas (A), the cleavage plane was nonrandom and lay roughly perpendicular to the wound edge (at left), and hence, to the EF vector (arrow). PGE2 (C) and aminophylline (D) significantly enhanced perpendicular orientation of cleavage relative to the EF vector. By contrast, ouabain (B) significantly reduced the mean polarization index (PI) almost to zero, indicating a random orientation of cell division relative to the wound edge. All images are projections from three separate experiments. (Bars = 20 μm.) (E) PI of cell division as a function of the wound-generated EF. Enhancing or reducing the wound-induced EF pharmacologically (x axis) respectively enhanced and reduced the PI of cell division angles (mean ± SEM; y axis). The higher the PI, the more perpendicular the cleavage planes are with respect to the EF vector. Regression formula for the correlation between TCPD and division angle PI is y = 0.05 + 0.002x − (3⋅E−6)χ2, Pearson correlation = 0.93, correlation is significant at 0.05 level.
By contrast, reducing the wound-generated EF to less than 20% of normal with ouabain reduced the mean PI to 0.09 ± 0.03 (Fig. 2E, n = 480 cells, six corneas), indicating random orientation of cleavage (Fig. 2 B and E, P < 0.05 compared with control cells). The value of the TCPD and of the PI were highly correlated (Fig. 2E), (Pearson correlation = 0.93; P < 0.05), indicating that the naturally occurring EF controlled the orientation of cell division in vivo. Interestingly, neomycin completely inhibited wound-induced orientation of cleavage, but did not influence the TCPD (Fig. 3).
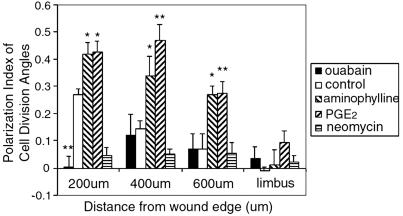
Figure 3.
Endogenous EFs controlled the orientation of cell division. In controls, the closer cells were to the wound edge, the higher the PI. Cells in the limbus had a PI of −0.01, indicating a randomly oriented cleavage plane. Pharmacologically increasing the EF with PGE2 and aminophylline significantly increased the PI within the first 600 μm from wound edge. Reducing the endogenous EF with ouabain significantly decreased the PI within the first 200 μm (P < 0.01). Neomycin inhibited oriented division in all areas. *, P < 0.05; **, P < 0.01.
The EF profile at a bovine corneal wound has been measured directly (30). It is steepest at the wound and declines sharply over the first 500 μm, becoming undetectable 0.5–1 mm back from the edge (Fig. 1B). If the endogenous EF in the rat is causal in directing the axis of cell division, then its effects should be highest at the wound edge and decline away from this point, as the EF declines. To test this idea, we assessed the average PI of cells dividing 0–200, 200–400, and 400–600 μm back from the wound and also in the distant limbus, around 1700 μm away. At untreated corneal wounds, as predicted, the PI dropped in proportion to the distance from the wound edge. In the first 200 μm from the edge, the PI was 0.27 ± 0.02 (n = 124 cells from 16 corneas; P < 0.001), but had declined to 0.14 ± 0.03 at 200–400 μm (P = 0.003). At 400–600 μm, the PI was 0.07 ± 0.05, P > 0.05, indicating randomly directed cleavage. In the distant limbus, where the endogenous EF also would be zero, the PI was −0.01 ± 0.02 (P > 0.05; Fig. 3). PI and distance from the wound were correlated inversely (Pearson correlation = −0.89, P < 0.05), indicating that the axis of cell division was regulated as a function of distance from the wound, as predicted by the decline in the EF, and that the effect of the naturally occurring EF was lost between 400 and 600 μm from the wound (Fig. 1B).
Next, we tested the predictions that enhancing the TCPD would enhance oriented division at given distances from the edge because it would create a larger EF gradient, and that inhibiting the TCPD would suppress EF-oriented division at a wound, because this would impose a smaller EF gradient (Fig. 1B). PGE2 and aminophylline (which increase the EF) increased the PI significantly, at 0–200, 200–400, and 400–600 μm from the wound edge (Fig. 3, PGE2 0–200 μm, P < 0.05 compared with control; 200–400 and 400–600 μm, P < 0.01; aminophylline P < 0.05 throughout; minimum of five experiments). Ouabain decreased the PI within 200 μm of the wound edge to 0.003 ± 0.12 (P < 0.01, five experiments), indicating that randomly oriented cleavage had replaced perpendicular cleavage (Fig. 3). Neomycin, which had no effect on the EF, completely inhibited oriented division, with a PI at all distances from the wound of roughly zero (Fig. 3). None of the drugs used altered the PI of cells in the limbus (Fig. 3). Both PGE2 and aminophylline amplified the effect of the endogenous EF with a significant inverse correlation between PI and the distance from the wound edge (Fig. 3, Pearson correlation value = −0.93 and −0.99, respectively).
We found that (i) the PI of oriented division dropped to zero as a function of distance from the wound edge, (ii) there was no oriented division in the distant limbus where the EF will be zero, (iii) increasing the EF increased the PI at all distances back from the wound, and (iv) decreasing the EF abolished oriented division particularly at the wound edge, providing strong evidence that the naturally occurring EF at a wound regulates the direction of cell division in vivo.
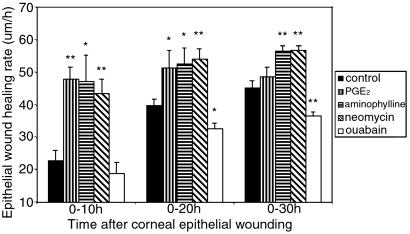
DC EFs may control wound healing (17, 26), and wounds that close faster may induce more radial tension, which could orient cell division. We therefore assessed the rate of wound healing in normal and drug-treated corneas. In untreated wounds (with endogenous EFs), healing rates almost doubled after 10 h (Fig. 4). Wounds treated with PGE2 or aminophylline, to enhance the wound-induced EF, healed much quicker than controls (2.5 times faster in the first 10 h), those treated with neomycin (which did not affect the wound-induced EF) also healed much faster, whereas those treated with ouabain, to inhibit the natural EF, were slower to heal, especially after 20–30 h (Fig. 4).
Figure 4.
Endogenous EFs controlled the corneal epithelium wound healing rate in vivo. Compared with controls, PGE2, aminophylline, and neomycin all enhanced the epithelium wound healing rate significantly throughout most time periods. Ouabain significantly reduced epithelial wound healing at 20 h and 30 h. *, P < 0.05; **, P < 0.01.
Because wounds heal by both directed cell migration and enhanced cell proliferation, we assessed the effects of the wound-induced EF on cell proliferation.
Dividing cells were very rare in unwounded corneas. In untreated corneas within the first 600 μm of the wound edge, there were 242 ± 26 dividing cells per mm2. Enhancing the wound-generated EF with PGE2 and aminophylline increased the frequency of cell division by up to 40% (335 ± 27 and 327 ± 30 cells per mm2, respectively; P < 0.01, compare Fig. 2 A, C, and D). Neomycin did not affect the mean number of dividing cells (250 + 20 per mm2), whereas in corneas treated with ouabain, cell divisions decreased to 177 ± 21 cells per mm2 (P < 0.05). This finding indicates that modulating the endogenous EF affects the cell cycle, alters the frequency of cell division, and could contribute to wound healing rate.
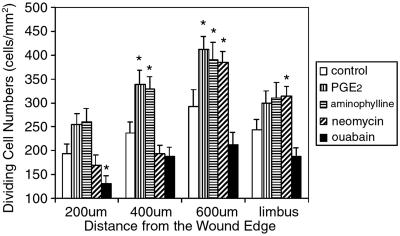
Because the endogenous EF declines with distance from the cut edge (Fig. 1B), we determined whether proliferation also was a function of distance from the wound. In untreated wounds, cells divided more 400–600 μm from the edge (294 ± 35 cells per mm2, mean ± SEM, n = 8 corneas) than within 200 and 400 μm (194 ± 20, and 238 ± 22 cells per mm2, respectively). The difference between 200 and 600 μm was significant (P < 0.05). Cell division was less common, therefore, at the wound edge (Fig. 5).
Figure 5.
Endogenous EFs controlled the frequency of cell division. In all groups, cells tended to divide more at 400 and 600 μm away from the leading edge, rather than in the first 200 μm from the wound. PGE2 and aminophylline significantly increased cell divisions at 400 and 600 μm, but had no effect at 200 μm, or in the limbus. Ouabain significantly decreased cell divisions in the first 200 μm, but had no effect in other areas. Neomycin significantly increased cell divisions at 600 μm and in the limbus, but had no effect at 200 and 400 μm. *, P < 0.05.
Enhancing the endogenous EF with PGE2 or aminophylline increased proliferation in the first 200 μm, and did this more so at 400 μm and 600 μm (Fig. 5, P < 0.05 compared with control, 400 μm, or 600 μm). Neither PGE2 nor aminophylline affected the number of cell divisions in the distant limbus area (Fig. 5). Enhancing the endogenous EF therefore increased proliferation at 200, 400, and 600 μm, but the extent of the effect again was less evident at the leading edge (Fig. 5). Reducing the endogenous EF with ouabain significantly reduced cell division especially in the first 200 μm (131 ± 16 cells per mm2; Fig. 5, P < 0.05, compared with control).
Discussion
We report five findings: (i) the axis of cell division was oriented at a wound edge; (ii) orientation declined with distance from the edge; (iii) increasing the wound-induced EF increased orientation and decreasing the EF decreased it; (iv) healing was faster when the wound EF was increased and slower when it was decreased; and (v) the proliferation of epithelial cells was regulated by the wound-induced EF. Applying a physiological EF to cultured epithelial cells in a chamber designed to isolate the EF influence stimulated faster and more direct cathodic migration and oriented cell division perpendicular to the EF (14, 15). Because these were direct responses to the EF, it is likely that these in vivo findings also were a direct response to the wound-induced EF. Are there alternative explanations?
Cells moving into a wound pull those behind them, and this tension might align the mitotic spindle and orient division. Faster wound healing would enhance this process. Two observations indicate that this cannot explain our findings: (i) Untreated wounds healed at normal rates and showed strong orientation of division. Neomycin doubled the healing rate, had no effect on the EF, but completely inhibited oriented division. (ii) PGE2, aminophylline, and neomycin-treated corneas all healed rapidly. Both PGE2 and aminophylline enhanced the EF and enhanced the PI of oriented division; neomycin did not affect the EF, but it totally abolished oriented cell division. Thus there was no correlation between the rate of wound healing and the extent of oriented division.
Many growth factors and cytokines regulate proliferation and migration at a wound. A standing gradient of such factors, although not demonstrated, might orient cell division at untreated wounds (where the EF gradient has been measured directly, ref. 30). However, we chose four different drugs with different mechanisms of action (see Results) for their ability to enhance, reduce, or leave unaffected the wound-induced EF. There is no evidence that any of these drugs have any effect on growth factor production or release, so it is extremely unlikely that they could have had the same pattern of effects, to increase, reduce, and leave unaffected growth factor release as they did on EFs. Nevertheless, the proportion of oriented divisions was regulated by these pharmacological treatments and this was entirely consistent with EF-driven mechanisms. Neomycin had no effect on EF magnitude, but inhibited oriented division. Neuronal growth cones and myoblasts show striking orientation in a physiological EF and both are inhibited by neomycin (34, 35). Presumably, although the EF magnitude remains unchanged, the neomycin-induced inhibition of phospholipase C prevents signaling of the EF. Inhibition of oriented division by neomycin, therefore, is consistent with this being induced by an EF and implicates phospholipase C mechanistically. Because alternative explanations are unlikely, this result indicates that the axis of cell division, the rate of wound healing, and the extent of proliferation were all regulated by a wound-induced EF in vivo.
How Could the Endogenous EF Control Orientation of Division?
The mitotic spindle and spindle microtubules assemble and correctly position the cleavage furrow (36). An endogenous EF could orient spindle microtubules along the EF vector. In corneal epithelial cells an EF enriches lipids and associated epidermal growth factor receptors at the cathodal side (4). If the cytoplasmic tail of such molecules captured the + ends of aster microtubules, this could orient the mitotic spindle and the cleavage plane. In Drosophila, intact adherens junctions are essential for symmetrical division within the plane of the neuroectoderm (2). Two adherens junction-related proteins may be involved in a spindle capture and orientation process (5). These proteins are EB1, the end budding protein in yeast, which binds the + ends of astral microtubules, extending to subcortical regions, and the adenomatous polyposis coli protein, which may link EB1 and microtubule + ends (5). Whether either molecule is involved in EF-induced orientation of epithelial cell division through capture and alignment of aster microtubules is untested.
Several proteins control asymmetric cell division in Drosophila, including Numb, Miranda, Prospero, Inscuteable, Bazooka, Lgl, Dlg, and SpoIIE (37–42). For example, Bazooka binds with Inscuteable in neuroblasts, and it directs both the asymmetrical localization of Numb/Miranda and the apical–basal orientation of the spindle. Lacking Bazooka function, Inscuteable does not localize asymmetrically, and the spindles of neuroblasts are oriented randomly. Perhaps these proteins, Bazooka in particular, are involved in the EF-induced orientation of the mitotic spindle.
How Does an Endogenous EF Modulate Directional Cell Migration?
Physiological EFs control the axis of division and the direction of cell migration. Corneal epithelial cells migrate cathodally faster in an applied EF and in vivo the wound is a cathode (Fig. 1) (17, 18). Because pharmacological manipulation of the EF regulated the rate of healing, this finding indicates that an EF could be a useful adjunct therapy in nonhealing wounds.
EF-directed migration of cultured corneal epithelial cells is serum dependent (18), restored in serum-free medium by the addition of selected growth factors, and involves the cathodally-directed enrichment of lipids and of the epidermal growth factor receptor, which is up-regulated by the EF (15). There is also asymmetric intracellular signaling with the cathodal accumulation of ERK 1 and ERK 2 and of F-actin (4). Similar mechanisms may operate in vivo.
Because a steady dc EF and charged molecules such as growth factors and cytokines coexist at a wound, interactions must occur. EFs do establish chemical gradients. Injecting fluorescently charged protein into the pre-limb-bud region of amphibian embryos, where an endogenous EF has been measured, resulted in a comet-tail-like distribution, driven by extracellular electrophoresis, rather than symmetrical diffusion of the fluorescent probe (43). Similar events could contribute to directed cell division and cell migration in vivo, but the initiating stimulus would be the EF.
An Endogenous EF Influences Cell Proliferation.
The interactions between proliferation, oriented division, and directed migration are important. EF-oriented division was influenced maximally at the wound edge, where cells migrate the fastest. Back from the edge, where the EF declines (25, 30), cell migration rates also dropped (44). EF effects on proliferation, however, were suppressed at the edge and more evident 400–600 μm back. In the limbus, where the endogenous EF would be zero, there was no effect on proliferation or migration. Perhaps the EF strikes a balance between migration and division, with leading-edge cells migrating but dividing infrequently, and trailing cells dividing more readily. Mitosis is rare during migration (31, 44). For example, there were few G2/M phase cells in regenerated epithelium at a corneal wound (no more than in unwounded controls), but cell division increased significantly by 12 h within 4 mm back from the wound (45). Re-epithelialization was not dependent on cell division, but depended more on the centripetal migration of cells into the regenerating area (31, 32). Thus, where cells migrate they divide less.
The EF effect to promote proliferation may be actively suppressed at the leading edge. This effect is in keeping with recent findings in Drosophila where proliferation is suppressed as migration occurs during gastrulation. Local expression of the gene tribbles, which induces proteolytic inactivation of the cell cycle regulator cdc25, controls this action (46, 47).
In conclusion, a naturally occurring EF controls multiple aspects of cell behavior in vivo. Because EFs are present during development and in pathology in many places where cells divide and migrate, their effects may be far-reaching and profound.
Acknowledgments
We thank Dr. Gordon McEwan for helping with TCPD measurements, and the Wellcome Trust for generous support.
Abbreviations
- EF
electric field
- TCPD
transcorneal potential difference
- PGE2, prostaglandin E2
PI, polarization index
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Giansanti M G, Gatti M, Bonaccorsi S. Development (Cambridge, UK) 2001;128:1137–1145. doi: 10.1242/dev.128.7.1137. [DOI] [PubMed] [Google Scholar]
- 2.Lu B, Roegiers F, Jan L Y, Jan Y N. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 3.Chenn A, McConnell S K. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhao M, Pu J, Forrester J V, McCaig C D. FASEB J. 2002;16:857–859. doi: 10.1096/fj.01-0811fje. [DOI] [PubMed] [Google Scholar]
- 5.Bienz M. Nat Cell Biol. 2001;3:E67–E68. doi: 10.1038/35060140. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein B. J Cell Biol. 1995;129:1071–1080. doi: 10.1083/jcb.129.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S W, Griffin F J, Clark W H., Jr Development (Cambridge, UK) 1997;124:773–780. doi: 10.1242/dev.124.4.773. [DOI] [PubMed] [Google Scholar]
- 8.Jin T, Zhang N, Long Y, Parent C A, Devreotes P N. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- 9.Servant G, Weiner O D, Herzmark P, Balla T, Sedat J W, Bourne H R. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker L V, Segal A W. Science. 2000;287:982–985. doi: 10.1126/science.287.5455.982. [DOI] [PubMed] [Google Scholar]
- 11.Weiner O D, Servant G, Welch M D, Mitchison T J, Sedat J W, Bourne H R. Nat Cell Biol. 1999;1:75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent C A, Blacklock B J, Froehlich W M, Murphy D B, Devreotes P N. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 13.Parent C A, Devreotes P N. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 14.Zhao M, Forrester J V, McCaig C D. Proc Natl Acad Sci USA. 1999;96:4942–4946. doi: 10.1073/pnas.96.9.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M, Dick A, Forrester J V, McCaig C D. Mol Biol Cell. 1999;10:1259–1276. doi: 10.1091/mbc.10.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe L F. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Agius-Fernandez A, Forrester J V, McCaig C D. Invest Ophthalmol Visual Sci. 1996;37:2548–2558. [PubMed] [Google Scholar]
- 18.Zhao M, Agius-Fernandez A, Forrester J V, McCaig C D. J Cell Sci. 1996;109:1405–1414. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, McCaig C D, Agius-Fernandez A, Forrester J V, Araki-Sasaki K. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- 20.Robinson K R. J Cell Biol. 1985;101:2023–2027. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCaig C D, Rajnicek A M, Song B, Zhao M. Trends Neurosci. 2002;25:354–359. doi: 10.1016/s0166-2236(02)02174-4. [DOI] [PubMed] [Google Scholar]
- 22.Shi R, Borgens R B. Dev Dyn. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- 23.Borgens R B, Shi R. Dev Dyn. 1995;203:456–467. doi: 10.1002/aja.1002030408. [DOI] [PubMed] [Google Scholar]
- 24.Hotary K B, Robinson K R. Dev Biol. 1994;166:789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 25.Sta Iglesia D D, Vanable J W., Jr Wound Repair Regen. 1998;6:531–542. doi: 10.1046/j.1524-475x.1998.60606.x. [DOI] [PubMed] [Google Scholar]
- 26.Sta Iglesia D D, Cragoe E J, Jr, Vanable J W., Jr J Exp Zool. 1996;274:56–62. doi: 10.1002/(SICI)1097-010X(19960101)274:1<56::AID-JEZ6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Candia O A. Biochim Biophys Acta. 1973;298:1011–1014. doi: 10.1016/0005-2736(73)90407-0. [DOI] [PubMed] [Google Scholar]
- 28.Klyce S D. Am J Physiol. 1975;228:1446–1452. doi: 10.1152/ajplegacy.1975.228.5.1446. [DOI] [PubMed] [Google Scholar]
- 29.Candia O A, Cook P. Am J Physiol. 1986;250:F850–F859. doi: 10.1152/ajprenal.1986.250.5.F850. [DOI] [PubMed] [Google Scholar]
- 30.Chiang M, Robinson K R, Vanable J W., Jr Exp Eye Res. 1992;54:999–1003. doi: 10.1016/0014-4835(92)90164-n. [DOI] [PubMed] [Google Scholar]
- 31.Zagon I S, Sassani J W, Ruth T B, McLaughlin P J. Brain Res. 2000;882:169–179. doi: 10.1016/s0006-8993(00)02864-x. [DOI] [PubMed] [Google Scholar]
- 32.Zagon I S, Sassani J W, McLaughlin P J. Brain Res. 1999;839:243–252. doi: 10.1016/s0006-8993(99)01722-9. [DOI] [PubMed] [Google Scholar]
- 33.Bixby J L, Harris W A. Annu Rev Cell Biol. 1991;7:117–159. doi: 10.1146/annurev.cb.07.110191.001001. [DOI] [PubMed] [Google Scholar]
- 34.McCaig C D, Dover P J. J Cell Sci. 1991;98:497–506. doi: 10.1242/jcs.98.4.497. [DOI] [PubMed] [Google Scholar]
- 35.Erskine L, Stewart R, McCaig C D. J Neurobiol. 1995;26:523–536. doi: 10.1002/neu.480260406. [DOI] [PubMed] [Google Scholar]
- 36.Strome S. Cell. 1993;72:3–6. doi: 10.1016/0092-8674(93)90041-n. [DOI] [PubMed] [Google Scholar]
- 37.Bellaiche Y, Radovic A, Woods D F, Hough C D, Parmentier M L, O'Kane C J, Bryant P J, Schweisguth F. Cell. 2001;106:355–366. doi: 10.1016/s0092-8674(01)00444-5. [DOI] [PubMed] [Google Scholar]
- 38.Tio M, Udolph G, Yang X, Chia W. Nature. 2001;409:1063–1067. doi: 10.1038/35059124. [DOI] [PubMed] [Google Scholar]
- 39.Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Nature. 2000;408:593–596. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- 40.Schober M, Schaefer M, Knoblich J A. Nature. 1999;402:548–551. doi: 10.1038/990135. [DOI] [PubMed] [Google Scholar]
- 41.Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- 42.Kraut R, Chia W, Jan L Y, Jan Y N, Knoblich J A. Nature. 1996;383:50–55. doi: 10.1038/383050a0. [DOI] [PubMed] [Google Scholar]
- 43.Messerli M, Robinson K R. Mol Biol Cell. 1997;8:1296A. [Google Scholar]
- 44.Chan K Y, Patton D L, Cosgrove Y T. Invest Ophthalmol Visual Sci. 1989;30:2488–2498. [PubMed] [Google Scholar]
- 45.Thompson H W, Malter J S, Steinemann T L, Beuerman R W. Invest Ophthalmol Visual Sci. 1991;32:433–436. [PubMed] [Google Scholar]
- 46.Grosshans J, Wieschaus E. Cell. 2000;101:523–531. doi: 10.1016/s0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- 47.Mata J, Curado S, Ephrussi A, Rorth P. Cell. 2000;101:511–522. doi: 10.1016/s0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]