Abstract
Epithelial proliferation of the ventral prostate in rodents peaks between 2 and 4 weeks of age, and by week 8, proliferating cells are rare. We have used ERβ−/− and CYP7B1−/− mice to investigate the role of ERβ and one of its ligands, 5α-androstane-3β,17β-diol (3βAdiol), in growth of the ventral prostate. Before puberty, ERβ was found in quiescent but not in proliferating cells, and proliferating cells occurred more frequently in ventral prostates of ERβ−/− mice than in wild-type littermates. Treatment with 3βAdiol decreased proliferation in wild-type but not in ERβ−/− mice. In rats, treatment with 3βAdiol from postnatal day 2 to 28 resulted in reduction in growth of ventral prostates. The prostates of CYP7B1−/− mice were hypoproliferative before puberty and smaller than those of their wild-type littermates after puberty. Because CYP7B1 represents the major pathway for inactivating 3βAdiol in the prostate, we suggest that ERβ, 3βAdiol, and CYP7B1 are the components of a pathway that regulates growth of the rodent ventral prostate. In this pathway, ERβ is an antiproliferative receptor, 3βAdiol is an ERβ ligand, and CYP7B1 is the enzyme that regulates ERβ function by regulating the level of 3βAdiol.
Prostate cancer is the most common malignancy in men, and mortality from this disease is second only to that from lung cancer in Western society (1). Both the etiology and the mechanisms involved in progression of prostate cancer have been, and continue to be, extensively investigated, but to date the causes of this disease remain unknown. Today we are beginning to know more about genetic factors behind development of prostate cancer. Tumor susceptibility genes have been found on both chromosome 1q (two genes), 1p, 16q, and X. One of those on 1q is cloned, and testing strategies are under development (2). The length of CAG repeats at the N terminus of the androgen receptor (3) is also an important factor.
In the 1940s androgen ablation was the first line of treatment (4, 5). Today radical surgery or radical irradiation are the most efficient measures in the localized disease and, in the metastasizing disease, androgen ablation therapy offers palliation. On removal of androgens, prostate cancer may become refractory to androgens and growth may occur in the absence of hormone (6). Except for palliative care, no treatment exists for the disease at this stage.
ERβ is expressed in the normal prostate and levels seem to decrease in prostate cancer (7–9). Because loss of ERβ is associated with proliferation of the prostate epithelium in mice (10), it seems possible that ERβ could be a target for the treatment of proliferative disease of the prostate. We have shown that the endogenous estrogen in the prostate is not estradiol-17β but 5α-androstane-3β,17β-diol (3βAdiol) (10). This steroid, 3βAdiol, is a metabolite of 5α-dihydrotestosterone (DHT) and its estrogenic activity is terminated by hydroxylation in a reaction catalyzed by CYP7B1 (refs. 11–13; Fig. 1). In this study we have investigated the role of ERβ, 3βAdiol, and CYP7B1 in the growth of the prostate epithelium.
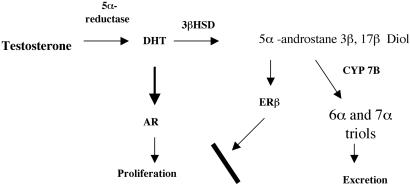
Figure 1.
Diagram showing the endocrine pathway where 3βAdiol and ERβ regulate prostate growth. In this pathway, DHT is derived from testosterone through the action of 5α-reductase, and 3βAdiol is derived from DHT through the activity of the enzyme 3β-hydroxysteroid dehydrogenase (3βHSD). On binding to ERβ, 3βAdiol represses the expression of androgen receptor (AR) and, therefore, inhibits androgen-driven proliferation. The levels of 3βAdiol in the prostate are regulated by the expression of CYP7B1, which inactivates 3βAdiol by converting it to 5α-androstane-3β,6α,17β-triol and to 5α-androstane-3β,7α,17β-triol (6α and 7α triols).
Materials and Methods
Animals.
Sprague–Dawley rats were purchased from Mollegard (Ejby, Denmark). ERβ−/− and CYP7B1−/− mice and their wild-type littermates were bred at the Huddinge University Hospital Animal Facility. Animals were housed in a controlled environment on an illumination schedule of 12 h light/12 h dark, and fed a standard pellet diet with water provided ad libitum. Newborn pups were treated with 3βAdiol dissolved in corn oil at a dosage of 3 mg/kg every other day from postnatal day 2 to 28. Control animals received corn oil. Ventral prostates from control and treated male rats were collected at 1–6 weeks of age. For proliferation studies, 5-bromo-2′-deoxyuridine (BrdUrd; 100 mg/kg body weight) was given by s.c. injection 2 h before tissue collection. All prostates used for immunohistochemical staining were fixed in 4% paraformaldehyde for 12 h, and 6-μm paraffin sections were cut.
Chemicals and Antibodies.
5α-Androstane-3β,17β-diol was purchased from Sigma. 5-Bromo-2′-deoxyuridine was from Roche (Mannheim, Germany). Polyclonal sheep anti-CYP7B1 antibody was produced in the laboratory of R. Lathe, Edinburgh. Polyclonal rabbit anti-AR (N-20) and anti-cyclin A (H-432) antibodies were from Santa Cruz Biotechnology. Monoclonal anti-BrdUrd antibody was from PharMingen. Horseradish peroxidase-conjugated goat anti-rabbit, Cy3-conjugated anti-rabbit, anti-mouse, and FITC-conjugated anti-sheep, and anti-rabbit secondary antibodies raised in the donkey were obtained from Jackson ImmunoResearch. Alkaline phosphatase-conjugated rabbit anti-mouse secondary antibody was from Vector Laboratories.
Immunohistochemical Staining.
Paraffin-embedded sections (6 μm) of ventral prostates were dewaxed and rehydrated. Antigens were retrieved by 40 min of incubation in citrate buffer in a microwave oven, with power set at 300 W. Sections were incubated in 0.5% H2O2 for 30 min at room temperature (RT) to quench endogenous peroxidase. (This step was omitted when fluorescence-conjugated secondary antibody was used.) Samples were then incubated with 0.5% Triton X-100 for 20 min at RT, followed with 20% normal serum (from the animal species in which the secondary antibodies were raised) for 30 min at RT. After washing in PBS, sections were incubated with primary antibodies at RT for 4 h. Antibodies were diluted in 3% BSA as follows: ERβ, BrdUrd, and cyclin A (1:100); AR and CYP7B1 (1:1,000). After washing in PBS, sections were incubated with the corresponding secondary antibodies (all in 1:1,000 dilution) for 1 h at RT. After washing, the sections were either slightly counterstained with hematoxylin, dehydrated, and mounted or mounted directly for fluorescent staining, in Vectashield antifading medium (Vector Laboratories). The sections were examined under a Zeiss fluorescence microscope with filters suitable for selectively detecting the fluorescence of FITC (green), Cy3 (red), or a light microscope. For colocalization, images from the same section but showing different antigen signals were overlaid. Cells where both FITC- and Cy3-conjugated secondary antibodies were present have an orange or yellow color. For colocalization of cyclin A and ERβ, another method was used because the two primary antibodies were both raised in rabbits. After staining for ERβ with FITC-conjugated secondary antibody, pictures were taken under fluorescence microscope and positively stained areas were marked. The slides were then washed in Mild Antibody Stripping Solution (Chemicon) for 15 min at 4°C. After three 10-min washes in PBS, slides were blocked by incubation with 20% normal goat serum for 30 min at RT. This incubation was followed by incubation with rabbit anti-cyclin A (1:100) antibody for 4 h at RT. After three 10-min washes in PBS, horseradish peroxidase-conjugated secondary goat anti-rabbit antibodies diluted in 2% rat serum in a 1:1,000 ratio were applied to the slides and incubated for 1 h at RT. After thorough washing in PBS, sections were developed with diaminobenzidine (Dako), lightly counterstained with Mayer's hematoxylin, dehydrated, and mounted with Permount (HISTOLAB, Gothenburg, Sweden). Pictures of the same areas as those positively staining for ERβ were taken and analyzed under a light microscope. For BrdUrd staining, an alkaline phosphatase-conjugated rabbit anti-mouse secondary antibody was used. The BrdUrd-positive cells were visualized with an alkaline phosphatase substrate kit I (SK-5100, Vector Laboratories) according to the protocols provided by the manufacturer.
Counting of Proliferating Cells.
The rates of epithelial cell proliferation in rat ventral prostate were expressed as percentage of the total cell nuclei that were cyclin A- or BrdUrd-positive. The total nuclear population was visualized with hematoxylin or, for fluorescence staining, 4′,6-diamidino-2-phenylindole dihydrochloride staining. The number of total epithelial cells and cyclin A- or BrdUrd-positive cells were counted from three areas of each sample. χ2 test was used to test the statistical significance.
Western Blot Detection of CYP7B1.
Prostate microsomal fractions were prepared and Western blotting was done according to the protocols described (14). Similar amounts of microsomal proteins from each sample were loaded in each lane of a precast 4–20% acrylamide gradient gel (Invitrogen). After SDS/PAGE, proteins were transferred to poly(vinylidene difluoride) membranes. Membranes were blocked for 1 h at RT in blocking buffer (PBS containing 10% fat-free milk and 0.1% Nonidet P-40), and incubated for 2 h at RT in primary anti-CYP7B1 antibody (1:2,000 in blocking buffer). After another 1-h wash in blocking buffer, membranes were exposed to horseradish peroxidase-conjugated rabbit anti-sheep secondary antibody (1:2,000) for 1 h at RT. After washing in PBS, the signals were visualized by enhanced chemiluminescence kits (Amersham Pharmacia). Sample loading was checked by staining the blot with Coomassie brilliant blue R 250.
RT-PCR Evaluation of AR and Glutathione Peroxidase in Ventral Prostates of ERβ−/− Mice.
Total RNA was isolated from frozen ventral prostates with Trizol LS Reagent according to the protocols provided by the manufacturer (GIBCO/BRL). RT-PCR was done in a one-tube reaction by using SuperScript One-Step RT-PCR system (GIBCO/BRL). In brief, the reaction mixture contained 1 μg of template RNA, 25 μl of 2× reaction buffer, 0.2 μM each primer, and 1 μl of RT/Platinum Taq in a total volume of 50 μl. RT-PCR was performed with a Gene Amp PCR system 2400 (Perkin–Elmer). RNA was reverse transcribed at 50°C for 30 min, followed by PCR with the program 94°C, 30 s, 30 s at the individual annealing temperatures (see below), 72°C, 30 s, 30 cycles for AR and for 25 cycles for glutathione peroxidase (GPx) and GAPDH, and final extension at 72°C for 7 min. GAPDH was used as an internal control. The primer sequences and individual annealing temperatures are listed below: AR (annealing temperature 52°C, product length 499 bp), forward primer, 5′-GTATCT ATGTGCCAGCAGA-3′, reverse primer, 5′-CAAAGTAGAGCATCCT GGA-3′; glutathione peroxidase (annealing temperature 55°C, product length 392 bp), forward, 5′-GTGAGCCCAGGAGTTCTGCAGT-3′, reverse, 5′-TATGAGTGGTAC CATCTACGAGTAT-3′; and GAPDH (annealing temperature 56°C, product length 466 bp), forward, 5′-CAGCCGCATCTTCTTGTG-3′, reverse, 5′-AGTTGTCATAT TTCTCG TGGTTCA-3′. PCR products were run in 1.5% agarose gel and visualized under UV light.
Results
The Relationship between Expression of ERβ, CYP7B1 and Prostate Epithelial Cellular Proliferation.
As shown in Figs. 2, 3, and 4, the peak of epithelial cellular proliferation measured as expression of cyclin A, is between 2 and 4 weeks of age. Thereafter, proliferation decreases and by 8 weeks of age, cells expressing cyclin A are rare. At the peak of proliferation, the proliferating epithelial cells in the ventral prostate expressed high levels of CYP7B1 but had no detectable ERβ, whereas in nonproliferating cells the level of ERβ was high and that of CYP7B1 was low.
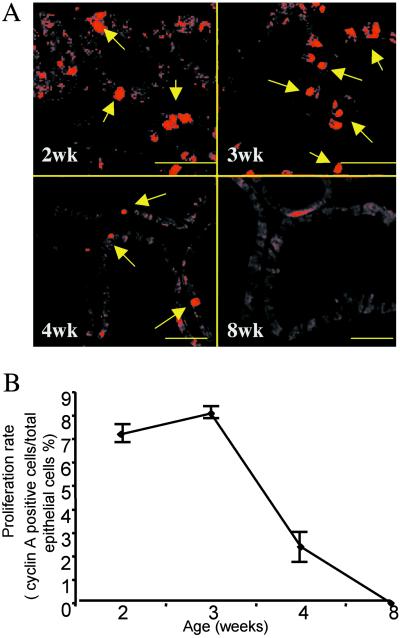
Figure 2.
The window of proliferation during prostate development. Ventral prostates of rats of 2, 3, 4, and 8 weeks of age were examined for cyclin A (A) expression in paraffin-embedded sections. Arrows indicate cyclin A-positive nuclei. In B, the proliferating rates are expressed as the number of cyclin A-positive cells divided by the total number of epithelial cell nuclei in each section. (Bars = 50 μm.) Each age group contains five animals.
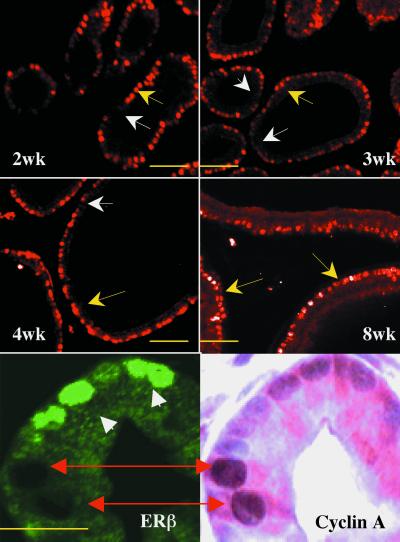
Figure 3.
Expression of ERβ during development and its relation to cellular proliferation in the rat ventral prostate. Ventral prostates of rats of 2, 3, 4, and 8 weeks of age were stained for ERβ. Before puberty (2–4 weeks of age), ERβ expression in the epithelium is heterogeneous, some cells are positive (yellow arrows), and some cells are negative (white arrows). In the mature ventral prostates, ERβ expression is homogeneous (yellow arrows). Double staining for cyclin A and ERβ shows that the ERβ-negative cells are those cells that are cyclin A-positive (red double-head arrows), whereas the ERβ-positive cells (white arrow heads) are cyclin A-negative. (Short bars = 50 μm; long bar = 20 μm.)
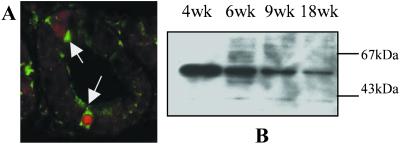
Figure 4.
Expression of CYP7B1 during development and its relation to cell proliferation. Colocalization of CYP7B1 and cyclin A in 3-week-old rat ventral prostates (A). The proliferating cells (cyclin A-positive cells) have red nuclei and are indicated with white arrows. These cells have the highest content of CYP7B1 (green cytoplasmic staining). CYP7B1 expression in prostate microsomes of 4-, 6-, 9-, and 18-week-old rats, detected by Western blotting, is depicted in B. Similar amounts of microsomal protein from each age group of animals were loaded in the lanes, and the accuracy of loading was checked by staining of the blot with Coomassie brilliant blue (data not shown).
Western blot analysis (Fig. 4B) showed that CYP7B1 expression was high in the ventral prostate before puberty (4-week-old rats), and decreased postpubertally (between 6 and 18 weeks of age). In normal 8-week-old rats, when the prostate is well differentiated, all of the epithelial cells had a nonproliferating phenotype, that is, ERβ was highly expressed and CYP7B1 expression was low. These results show a positive correlation between prostate epithelial cellular proliferation and expression of CYP7B1.
Inhibition of Growth of the Developing Ventral Prostate by 3βAdiol.
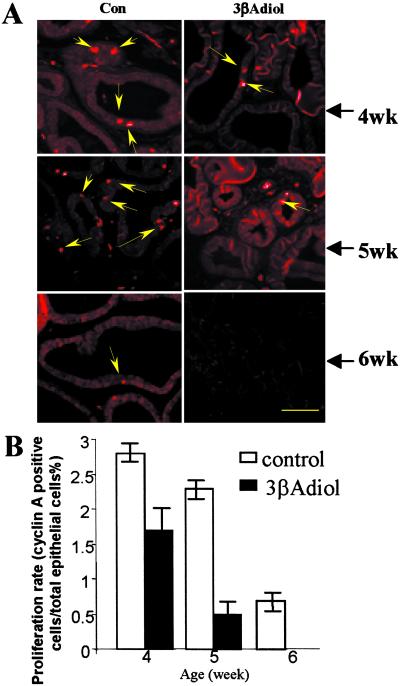
Rats were treated with 3βAdiol from postnatal day 2 to 28 at a dosage of 3 mg/kg body weight. As indicated by the decrease in cyclin A staining, shown in Figs. 5 A and B, these data support the hypothesis that CYP7B1 promotes proliferation by elimination of the antiproliferative ligand, 3βAdiol, and inhibition of growth occurs when the metabolic capacity of CYP7B1 is surpassed.
Figure 5.
The effects of 3βAdiol treatment on the growth of rat ventral prostate. The effects of 3βAdiol treatment on proliferation of epithelial cells assessed by cyclin A staining are shown in A. Arrows indicate where fewer cyclin A-positive nuclei are in the treated rats. In B, the statistical significance of the differences in proliferation rate was tested by using the Student′s t test (n = 5, P < 0.05 in all of the three age groups compared).
Ventral Prostate Phenotypes of ERβ−/− and CYP7B1−/− Mice During Development.
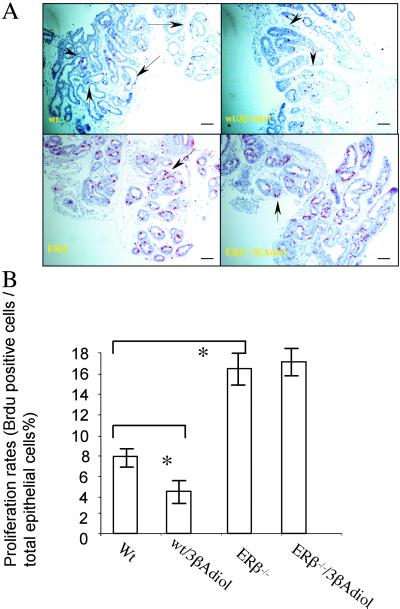
To investigate further the role of ERβ and CYP7B1 in epithelial cellular proliferation, both ERβ−/− (14) and CYP7B1−/− (16) mice were used. Mice were treated with 3βAdiol and proliferation was evaluated with BrdUrd labeling. A short exposure to BrdUrd (2 h before killing) labeled only those cells that were in S phase as visualized by cyclin A staining (data not shown). The proliferation rate of untreated ERβ−/− mouse prostates was significantly higher than that of wild-type littermates, and treatment with 3βAdiol resulted in a decrease in epithelial cell proliferation in wild-type but not in ERβ−/− mice (Fig. 6 A and B). These results indicate that the presence of ERβ is necessary for the antiproliferative effects of 3βAdiol. The high proliferation rate in the ERβ−/− mouse prostate indicates that ERβ is more than just a marker of a more differentiated epithelial cell. It suggests that ERβ normally has a role in inhibiting proliferation.
Figure 6.
Comparison of the effect of 3βAdiol treatment on ventral prostate growth in wild-type and ERβ−/− mice. In A, when vehicle-treated (Wt) and 3βAdiol-treated mice were compared (Wt/3βAdiol), 3βAdiol treatment resulted in a reduction in the number of proliferating cells in the ventral prostates of 3-week-old wild-type mice. Proliferation was detected with BrdUrd staining. (Positive cells are purple and indicated with arrows.) In ERβ−/− mouse prostates higher proportion of BrdUrd-positive cells occurs than in the prostates of their wild-type littermates and treatment with 3βAdiol (ERβ−/−/3βAdiol) did not result in a reduction of growth. In B, the statistical significance of the difference in proliferation rates was evaluated by using the Student′s t test (n = 5 in each group; * indicates significant difference between the line-linked groups, P < 0.05. (Bars = 50 μm.)
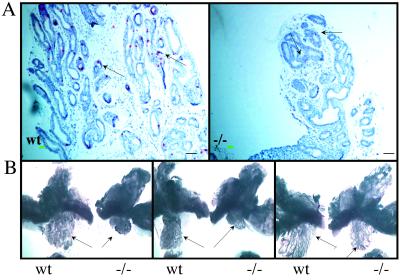
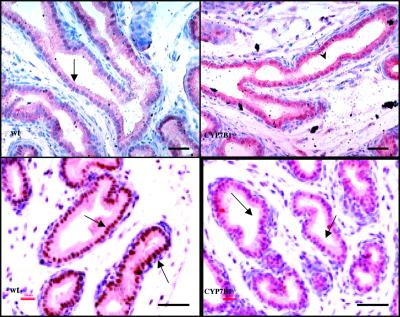
As with ERβ−/− mice, 3-week-old CYP7B1−/− mice were exposed to BrdUrd for 2 h before sacrifice and proliferation in ventral prostate evaluated by immunohistochemical staining. CYP7B1−/− mice had a significantly lower proliferation rate than their wild-type littermates (Fig. 7A). This reduced growth rate, during prepubertal development; influenced the size of the ventral prostates in adult mice; and, at 3 months of age, the ventral prostates, but not the other lobes of prostate, were smaller in CYP7B1−/− mice than in wild-type littermates (Fig. 7B). As might be expected, if the CYP7B1−/− mouse phenotype is caused by an excess of ERβ ligand, the ventral prostates of CYP7B1−/− mice at 3 weeks of age expressed high levels of ERβ and low levels of AR (Fig. 8).
Figure 7.
Reduction in growth of the ventral prostates of CYP7B1−/− mice. BrdUrd staining of ventral prostate of 3-week-old wild-type (Wt) and CYP7B1−/− (−/−) littermates (A) shows that wild-type mouse prostates have significantly more BrdUrd-positive cells than those of the −/− mice (arrows). (B) A central sagittal cut along the middle line of urethra was made to separate the paired lobes of ventral, dorsal, and lateral prostates. Photographs were taken under a dissection microscope with focus on the ventral lobes (arrows). The CYP7B1−/− ventral prostates are smaller than those of their wild-type littermates. (Bars = 50 μm.)
Figure 8.
Evaluation of AR and ERβ expression in the ventral prostates of 3-week-old CYP7B1−/− mice. Immunohistochemical staining with antibodies specific for ERβ and AR shows that in the epithelial cells of the ventral prostate (arrows), the CYP7B1−/− mice have more ERβ and less AR than the wild-type littermates. (Bars = 50 μm.)
The Relationship between AR Level and Proliferation of Prostate Epithelial Cells.
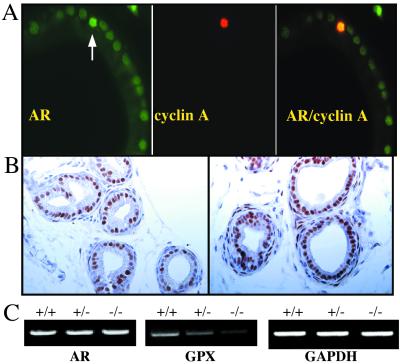
Because the epithelial cells of the mature prostate express AR but do not proliferate despite the presence of a constant level of androgen, it is suggested that levels of AR have to be increased for proliferation to occur. Evidence supporting an increase comes from a correlation between proliferation rate and the AR content of the various prostate lobes (17) and from transgenic mice in which AR is overexpressed in the prostate epithelium (18). In AR-overexpressing transgenic mice, there is a late-developing prostatic epithelial hyperplasia similar to what is seen in ERβ−/− mice (17). In the present study, the relationship between proliferating (cyclin A-expressing) cells and AR levels was evaluated. Proliferating cells in the immature ventral prostate, as shown in Fig. 9A, are the ones whose level of AR is higher that that in the surrounding quiescent cells. Although we and others have shown that loss of ERβ results in up-regulation of AR, detectable both by Western blots and immunohistochemistry (10, 19, 20), no evidence of increase in the level of AR mRNA, detected by RT-PCR, in ERβ−/− mouse prostates (Fig. 9C). Therefore, it seems that the higher level of AR protein, which is evident in Fig. 9B, is due to a change in AR protein turnover, which means that, in the absence of ERβ, either an increase occurs in the translation of the AR mRNA and/or a reduction occurs in degradation of the AR protein. Increased levels of AR and androgen-independent activation of AR can be caused by phosphorylation of the AR itself or by phosphorylation of one of its coactivators (21, 22).
Figure 9.
Correlation of AR expression with cellular proliferation in mouse ventral prostate. Colocalization of AR and cyclin A in the ventral prostates of 3-week-old mice is shown in A. The white arrow illustrates a cell that is cyclin A-positive (red) and which is also the cell with the highest content of AR (green). In B, the immunohistochemical staining for AR in the ventral prostates of 3-week-old WT and ERβ−/− mice is shown. In the WT prostate, AR expression is heterogeneous and AR-negative epithelial cells exist (arrows). In the ERβ−/− prostate, the AR level, in general, is higher than in WT prostate and AR expression is homogeneous throughout the epithelium with a remarkable absence of AR-negative cells. (Bars = 50 μm.) (C) RT-PCR evaluation of AR mRNA in 3-week-old WT (+/+), ERβ +/− (+/−) and ERβ−/− (−/−) mouse ventral prostates. GAPDH is used as an internal control and glutathione peroxidase (GPx) as a positive control. GPx was found with cDNA arrays (unpublished data) to be down-regulated in the ERβ−/− prostate. AR mRNA was not changed in the ERβ+/− or ERβ−/− mouse prostates (in each group, RNA used for RT-PCR was from three animals).
Discussion
Although the prostate is an androgen-dependent tissue, estrogens influence both normal functions and pathological changes in this gland (23). Estrogen has been reported to be both a tumor promoter and suppressor depending on the dose and the type of estrogenic agent used (24). This dual action may be due to the existence of two estrogen receptors, ERα and ERβ. In rodents, ERα is not found in the epithelium of the ventral prostate but is expressed in the anterior prostate or coagulating gland and in prostatic stroma. Stromal ERα is thought to stimulate growth factor secretion and thus to affect epithelial growth indirectly (25). ERα and ERβ have similar affinities for estradiol-17β and can bind to estrogen response elements in the promoter regions of estrogen-regulated genes (26). In addition, they may act oppositely in regulating some target genes by means of response elements other than estrogen response elements (e.g., AP1 site; ref. 27).
3βAdiol is derived from the potent androgen DHT, through the action of 3β-hydroxysteroid dehydrogenase (see Fig. 1). Even though 3βAdiol has been shown to bind to estrogen receptor in pituitary cytosol (28), the high level of 3βAdiol hydroxylase (CYP7B1) in the pituitary (13, 29) makes this gland resistant to the estrogenic actions of 3βAdiol. Thus, unlike estradiol-17β (30), 3βAdiol does not result in chemical castration (31). In this study we have shown that regulation of the levels of 3βAdiol by CYP7B1 is a key factor in regulation of prostatic growth. We provide evidence that proliferating cells in the prostate epithelium have elevated levels of AR and that AR protein but not mRNA levels are regulated by ERβ and its ligand 3βAdiol in the prostate epithelium.
The role of CYP7B1 in the prostate described in the present study permits some insight into the optimal use of some pharmaceuticals in present clinical practice. The first of these is the 5α-reductase inhibitor, Finasteride, a drug useful in the treatment of benign prostatic hyperplasia (32) and now in clinical trials for prevention of prostate cancer (33, 34). According to the scheme depicted in Fig. 1, blocking of 5α-reductase will have two opposite effects on the prostate. It will prevent the formation of the potent androgen DHT and should thus reduce prostate growth. However, it would also prevent the formation of 3βAdiol and, by doing so, would remove the growth-limiting effects of ERβ. In addition, because inhibition of 5α-reductase causes accumulation of testosterone and removal of ERβ action increases the level of AR in the prostate, the overall effect of Finasteride would be to favor proliferation of the prostate epithelium.
The second pharmaceutical is Ketoconazol, a general cytochrome P450 inhibitor that is usually used in second-line androgen-deprivation therapy (35, 36). The rationale behind its use is that it inhibits the P450s involved in androgen synthesis and results in a more complete androgen ablation (37). In view of the role of CYP7B1 in the prostate, it is possible that some of the beneficial effects of Ketoconazol could be due to its inhibition of CYP7B1. If this is the case, 3βAdiol itself or even dehydroepiandrosterone (DHEA) would be of clinical benefit in the prevention of prostate growth. DHEA has been used as adjuvant treatment in prostate cancer with mixed success (38). DHEA is converted in the body to 5-androstene-3β,17β-diol, which is also a ligand for estrogen receptors (25, 39) and a substrate for CYP7B1 (17, 40). According to our model, for DHEA to have repressive effects on prostatic growth, a functional ERβ has to be in the prostate. As cited above (7–9), studies show that ERβ tends to be lost in advanced prostate cancer. The population of patients who might benefit from 3βAdiol or DHEA should first be assessed for the presence of ERβ in the prostate.
In conclusion, we have described a pathway that controls prostate epithelial cellular proliferation. It is composed of CYP7B1, 3βAdiol, and ERβ. This mechanism is summarized in Fig. 1. The data show that exposure to ERβ ligands during early development seems to be a decisive factor in determining prostate size. In addition, the study suggests that ERβ ligands could be used to suppress proliferation of prostatic epithelial proliferation when the prostate is deprived of DHT.
Acknowledgments
The excellent technical participation of Jenny Ekman and Makbule Sagici is gratefully acknowledged. We are grateful to the IngaBritt and Arne Lundberg's Research Foundation and Robert Lundberg's Fund for providing special instrumentation that made this study possible. This research was supported by grants from the Swedish Cancer Fund and KaroBio AB.
Abbreviations
- AR
androgen receptor
- 3βAdiol
5α-androstane-3β,17β-diol
- DHT
5α-dihydrotestosterone
- DHEA
dehydroepiandrosterone
References
- 1.Coffey D S. Cancer. 1993;71:880–886. doi: 10.1002/1097-0142(19930201)71:3+<880::aid-cncr2820711403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Nupponen N N, Visakorpi T. Microsc Res Tech. 2000;51:456–463. doi: 10.1002/1097-0029(20001201)51:5<456::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Chang B L, Zheng S L, Hawkins G A, Isaacs S D, Wiley K E, Turner A, Carpten J D, Bleecker E R, Walsh P C, Trent J M, et al. Hum Genet. 2002;110:122–129. doi: 10.1007/s00439-001-0662-6. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges C V. Cancer Res. 1941;1:293–297. [Google Scholar]
- 5.Huggins C, Stevens R E, Hodges C V. Arch Surg. 1941;43:209–223. [Google Scholar]
- 6.Scher H I, Sternberg C N. Semin Urol. 1985;3:239–280. [PubMed] [Google Scholar]
- 7.Leav I, Lau K M, Adams J Y, McNeal J E, Taplin M E, Wang J, Singh H, Ho S M. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath L G, Henshall S M, Lee C S, Head D R, Quinn D I, Makela S, Delprado W, Golovsky D, Brenner P C, O'Neill G, et al. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 9.Royuela M, de Miguel M P, Bethencourt F R, Sanchez-Chapado M, Fraile B, Arenas M I, Paniagua R. J Endocrinol. 2001;168:447–454. doi: 10.1677/joe.0.1680447. [DOI] [PubMed] [Google Scholar]
- 10.Weihua Z, Makela S, Andersson L C, Salmi S, Saji S, Webster J I, Jensen E V, Nilsson S, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacs J T, McDermott I R, Coffey D S. Steroids. 1979;33:675–692. doi: 10.1016/0039-128x(79)90116-8. [DOI] [PubMed] [Google Scholar]
- 12.Gemzik B, Jacob S, Jennings S, Veltman J, Parkinson A. Arch Biochem Biophys. 1992;296:374–383. doi: 10.1016/0003-9861(92)90587-m. [DOI] [PubMed] [Google Scholar]
- 13.Sundin M, Warner M, Haaparanta T, Gustafsson J-Å. J Biol Chem. 1987;262:12293–12297. [PubMed] [Google Scholar]
- 14.Warner M, Stromstedt M, Moller L, Gustafsson J-Å. Endocrinology. 1989;124:2699–2706. doi: 10.1210/endo-124-6-2699. [DOI] [PubMed] [Google Scholar]
- 15.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J-Å, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose K, Allan A, Gauldie S, Stapleton G, Dobbie L, Dott K, Martin C, Wang L, Hedlund E, Seckl J R, et al. J Biol Chem. 2001;276:23937–23944. doi: 10.1074/jbc.M011564200. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee P P, Banerjee S, Brown T R. Endocrinology. 2001;142:4066–4075. doi: 10.1210/endo.142.9.8376. [DOI] [PubMed] [Google Scholar]
- 18.Stanbrough M, Leav I, Kwan P W, Bubley G J, Balk S P. Proc Natl Acad Sci USA. 2001;98:10823–10828. doi: 10.1073/pnas.191235898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng G, Weihua Z, Makinen S, Makela S, Saji S, Warner M, Gustafsson J-Å, Hovatta O. Biol Reprod. 2002;66:77–84. doi: 10.1095/biolreprod66.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Prins G S, Birch L, Couse J F, Choi I, Katzenellenbogen B, Korach K S. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 21.Moilanen A M, Karvonen U, Poukka H, Janne O A, Palvimo J J. Mol Biol Cell. 1998;9:2527–2543. doi: 10.1091/mbc.9.9.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadar M D, Hussain M, Bruchovsky N. Endocr Relat Cancer. 1999;6:487–502. doi: 10.1677/erc.0.0060487. [DOI] [PubMed] [Google Scholar]
- 23.Ekman P. Prostate Suppl. 2000;10:14–18. [PubMed] [Google Scholar]
- 24.Griffiths K. Prostate. 2000;45:87–100. doi: 10.1002/1097-0045(20001001)45:2<87::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Risbridger G, Wang H, Young P, Kurita T, Wang Y Z, Lubahn D, Gustafsson J-Å, Cunha G, Wong Y Z. Dev Biol. 2001;229:432–422. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper G G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-Å. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 27.Kushner P J, Agard D A, Greene G L, Scanlan T S, Shiau A K, Uht R M, Webb P. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 28.Thieulant M L, Samperez S, Jouan P. Endocrinology. 1981;108:1552–1560. doi: 10.1210/endo-108-4-1552. [DOI] [PubMed] [Google Scholar]
- 29.Guiraud J M, Samperez S, Jouan P. Steroids. 1982;40:625–639. doi: 10.1016/0039-128x(82)90003-4. [DOI] [PubMed] [Google Scholar]
- 30.Verjans H L, Eik-Nes K B. Acta Endocrinol. 1977;84:829–841. doi: 10.1530/acta.0.0840829. [DOI] [PubMed] [Google Scholar]
- 31.Thompson I M, Jr, Kouril M, Klein E A, Coltman C A, Ryan A, Goodman P. Urology. 2001;57, Suppl. 1:230–234. doi: 10.1016/s0090-4295(00)00980-8. [DOI] [PubMed] [Google Scholar]
- 32.Ekman P. Eur Urol. 1998;33:312–317. doi: 10.1159/000019566. [DOI] [PubMed] [Google Scholar]
- 33.Auclerc G, Antoine E C, Cajfinger F, Brunet-Pommeyrol A, Agazia C, Khayat D. Oncologist. 2000;5:36–44. doi: 10.1634/theoncologist.5-1-36. [DOI] [PubMed] [Google Scholar]
- 34.Coltman C A, Jr, Thompson I M, Jr, Feigl P. Eur Urol. 1999;35:544–547. doi: 10.1159/000019895. [DOI] [PubMed] [Google Scholar]
- 35.Peehl D M, Seto E, Feldman D. Urology. 2001;58, Suppl. 1:123–126. doi: 10.1016/s0090-4295(01)01254-7. [DOI] [PubMed] [Google Scholar]
- 36.Blagosklonny M V, Dixon S C, Figg W D. J Urol. 2000;163:1022–1026. [PubMed] [Google Scholar]
- 37.Labrie F, Belanger A, Dupont A, Luu-The V, Simard J, Labrie C. Clin Invest Med. 1993;16:475–492. [PubMed] [Google Scholar]
- 38.Jones J A, Nguyen A, Straub M, Leidich R B, Veech R L, Wolf S. Urology. 1997;50:784–788. doi: 10.1016/S0090-4295(97)00395-6. [DOI] [PubMed] [Google Scholar]
- 39.Simard J, Labrie F. J Steroid Biochem. 1987;26:539–546. doi: 10.1016/0022-4731(87)90005-7. [DOI] [PubMed] [Google Scholar]
- 40.Warner M, Tollet P, Stromstedt M, Carlstrom K, Gustafsson J-Å. J Endocrinol. 1989;122:341–349. doi: 10.1677/joe.0.1220341. [DOI] [PubMed] [Google Scholar]