Abstract
Intestinal glucagon-like peptide–1 (GLP-1) is a hormone released into the hepatoportal circulation that stimulates pancreatic insulin secretion. GLP-1 also acts as a neuropeptide to control food intake and cardiovascular functions, but its neural role in glucose homeostasis is unknown. We show that brain GLP-1 controlled whole-body glucose fate during hyperglycemic conditions. In mice undergoing a hyperglycemic hyperinsulinemic clamp, icv administration of the specific GLP-1 receptor antagonist exendin 9–39 (Ex9) increased muscle glucose utilization and glycogen content. This effect did not require muscle insulin action, as it also occurred in muscle insulin receptor KO mice. Conversely, icv infusion of the GLP-1 receptor agonist exendin 4 (Ex4) reduced insulin-stimulated muscle glucose utilization. In hyperglycemia achieved by i.v. infusion of glucose, icv Ex4, but not Ex9, caused a 4-fold increase in insulin secretion and enhanced liver glycogen storage. However, when glucose was infused intragastrically, icv Ex9 infusion lowered insulin secretion and hepatic glycogen levels, whereas no effects of icv Ex4 were observed. In diabetic mice fed a high-fat diet, a 1-month chronic i.p. Ex9 treatment improved glucose tolerance and fasting glycemia. Our data show that during hyperglycemia, brain GLP-1 inhibited muscle glucose utilization and increased insulin secretion to favor hepatic glycogen stores, preparing efficiently for the next fasting state.
Introduction
Glucose homeostasis depends on signals from endocrine, neural, and metabolic origins. Such signals control endogenous glucose production and utilization to maintain a physiological glycemia. Among the regulatory signals, the neuropeptides generated by the CNS play an essential role in the regulation of important processes such as food intake (1–3). The action of these neuropeptides on energy balance includes control of key regulatory functions of glucose homeostasis via the CNS, including pancreatic and intestinal hormone secretion (4, 5) and hepatic glycogen storage (6, 7). Consequently, defects in the CNS and/or the autonomic nervous system (ANS) may be associated with hyperglycemic episodes contributing to the development of diabetes.
The peptide glucagon-like peptide–1 (GLP-1) is considered a hormone when released by enteroendocrine L cells of the intestine and a neuropeptide when released in the brain (8, 9). When produced in the gut, the main hormonal effect of GLP-1 is to stimulate glucose-induced insulin secretion (10). This effect occurs postprandially when glucose levels are elevated, consequently minimizing development of hypoglycemia. In the brain, a limited number of cerebral cells contain GLP-1 and are mainly located in the nucleus of the tractus solitarius and area postrema (11, 12). In addition, cerebral GLP-1 receptor activation leads to the secretion of catecholamines providing input to sympathetic preganglionic neurons (12). Therefore, GLP-1 is linked to the regulation of the ANS. This link explains the observation that icv administration of a GLP-1 receptor agonist increases blood pressure and heart rate (12). As a neuropeptide, brain GLP-1 (11) regulates several neuroendocrine and ANS-dependent responses such as food and water intake (13, 14). However, while some extrapancreatic effects have been reported, particularly in the enteric area (15, 16), whether central GLP-1 has any role in the control of peripheral glucose metabolism is unknown.
Glucose sensors are specialized cells localized in different tissues including the brain, the pancreas, the peripheral nervous system, and the digestive tract. Glucose sensors detect glycemic variations and produce signals accordingly that trigger different functions in target cells (15, 17–20) through the ANS (7, 21–23). Such regulation is involved in a glucoregulatory reflex loop. We and others previously showed that the sensor in the hepatoportal area controls whole-body glucose utilization independently from insulin action, an effect dependent on the presence of a functional GLP-1 receptor (24–27). However, the regulatory role of GLP-1 in the brain to control central glucose responsiveness remains to be studied. Related to the present hypothesis, previous work showed that pro-opiomelanocortin–derived peptides enhanced the actions of insulin on both uptake and production of glucose (28). Hence, increasing evidence implicates a neuroendocrine network in the coupling of energy balance and insulin action.
The aim of this study was to determine the role of central GLP-1 in the control of whole-body glucose homeostasis. We infused glucose i.v. or intragastrically in awake WT and GLP-1 receptor KO mice to achieve hyperglycemia. Under these conditions, we studied the role of central GLP-1 by infusing the specific GLP-1 receptor antagonist exendin 9–39 (Ex9) or the GLP-1 receptor agonist exendin 4 (Ex4) into the lateral ventricle of the brain. Central Ex4 infusion markedly enhanced hyperglycemia-stimulated insulin secretion but induced whole-body insulin resistance, while hepatic glycogen storage increased. Consequently, insulin-stimulated glucose utilization was blunted to favor redistribution of glucose from muscle toward liver, where glycogen was stored efficiently, consistent with postprandial disposition of ingested carbohydrates.
Results
Brain GLP-1 controls whole-body insulin sensitivity only during hyperglycemia.
To assess the role of brain GLP-1 in the control of glucose fluxes, hyperinsulinemic clamps at different glycemic levels were performed simultaneously with either icv infusion of the GLP-1 receptor modulator Ex9 (antagonist) or Ex4 (agonist) in normal C57BL/6J mice or icv infusion of artificial cerebrospinal fluid (ACF) in GLP-1 receptor KO and WT mice. We infused Ex4 rather than GLP-1 itself because of its longer half life and stability, which would ensure that brain GLP-1 receptors were maximally activated (29). Insulin was infused in the femoral vein at a supraphysiological rate to maximally stimulate glucose utilization, and glycemia was clamped at 5.5, 10, or 20 mM in different sets of mice to activate all glucose sensors.
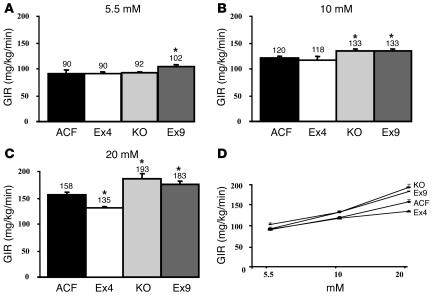
Under euglycemic conditions, the glucose infusion rate (GIR) was similar among control, GLP-1 receptor KO, and Ex4-infused mice (Figure 1A). Higher GIRs were required to achieve hyperglycemia in all groups (Figure 1D), but the GIR was significantly higher in GLP-1 receptor KO and Ex9-infused mice than in controls at 10 and 20 mM (Figure 1, B and C). Conversely, Ex4-infused mice presented a lower GIR than controls, showing a state of insulin resistance only under 20-mM hyperglycemic conditions (Figure 1C).
Figure 1.
Central control by GLP-1 of whole-body GIR. GIR (mg/kg/min) was calculated in steady-state euglycemic hyperinsulinemic conditions (5.5 mM; A) and in hyperglycemic conditions (10 mM, B; and 20 mM, C) in C57BL/6 control mice with ACF, Ex4, or GLP-1 antagonist Ex9 infused into their brains and in GLP-1 receptor KO mice (KO). Values are shown above each bar; error bars indicate ± SEM. (D) Dose response to glucose in all sets of mice plotted against GIRs in order to calculate the r2 of each curve. The mean of 6–11 mice per group is shown. *P < 0.05 versus icv ACF-infused mice.
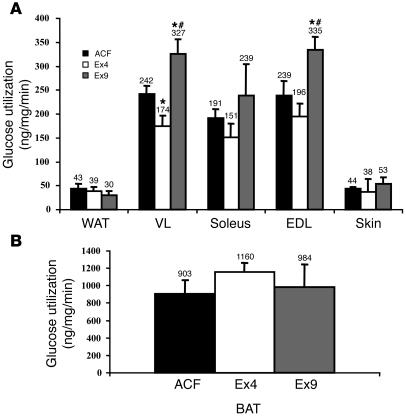
To determine which tissues were responsible for glucose utilization, we injected 3H-2-deoxyglucose (2DG) into mice that had Ex4, Ex9 or ACF infused into their brains during hyperinsulinemic hyperglycemic conditions. The 2DG utilization rate was increased in the vastus lateralis in response to icv Ex9 infusion. This muscle is composed of glycolytic and oxidative fiber types and therefore representative of all body muscles. Similar results were obtained in the extensor digitorum longus muscle, which is considered glycolytic. Conversely, Ex4 reduced the rate of 2DG utilization under the same conditions (Figure 2A). No differences were noted among treatments in white (Figure 2A) or interscapular brown (Figure 2B) adipose depots.
Figure 2.
Central control by GLP-1 of individual tissue glucose utilization. Individual tissue glucose utilization (ng/mg/min) was assessed during 20-mM hyperglycemic hyperinsulinemic conditions in (A) white adipose tissue (WAT), vastus lateralis (VL), soleus, extensor digitorum longus (EDL), skin and in (B) interscapular brown adipose tissue (BAT) of C57BL/6 control mice with ACF, Ex4, or Ex9 infused into their brains. The mean of 4–8 mice per group is shown. *P < 0.05 versus icv ACF-infused mice; #P < 0.05 versus icv Ex4-infused mice.
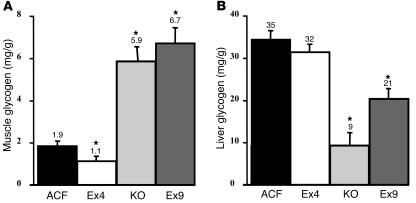
To determine the fate of the glucose absorbed by muscle, we assessed glycogen content at the completion of 20-mM hyperglycemic hyperinsulinemic clamps. Muscle glycogen content was increased more than 3-fold in GLP-1 receptor KO and icv Ex9-infused mice (Figure 3A). Conversely, it was significantly reduced when Ex4 was infused into the brain. A reduction in liver glycogen content was noted with icv Ex9 and in GLP-1 receptor KO mice, while no effect was observed during icv Ex4 treatment (Figure 3B). We next sought to ascertain that the effect of icv Ex4 and Ex9 required a functional GLP-1 receptor. The peptides were separately infused into the brain of GLP-1 receptor KO mice during hyperglycemic hyperinsulinemic clamps. Muscle glycogen content was 5.0 ± 0.3 versus 4.9 ± 0.4 mg/g, and the GIR was 202.4 ± 10.3 versus 203.4 ± 5.6 mg/kg/min, in Ex4- and Ex9-infused mice, respectively (data not shown).
Figure 3.
Central control by GLP-1 of muscle and liver glycogen content. Glycogen content (mg/g wet tissue) was measured in ground hind limb muscles (A) and liver (B) from C57BL/6 control mice with ACF, Ex4, or Ex9 infused into their brains and GLP-1 receptor KO mice during 20-mM hyperglycemic hyperinsulinemic conditions. The mean of 6–11 mice per group is shown. *P < 0.05 versus icv ACF-infused mice.
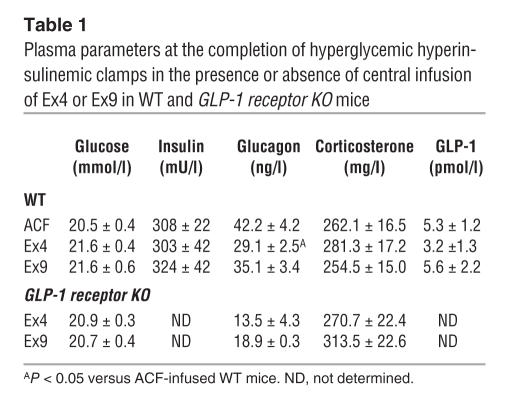
Circulating factors might be regulated by the central action of either GLP-1 agonists and antagonists and may be involved in the control of tissue glycogen content. We assessed plasma corticosterone, glucagon, and GLP-1 concentrations at the completion of the icv peptide infusions. The data show that plasma corticosterone and GLP-1 concentrations were similar across treatments in WT and GLP-1 receptor KO mice (Table 1). Conversely, plasma glucagon concentration was reduced by Ex4 infusion in WT mice, while Ex9 had no effect. No differences were noted in any parameter following Ex4 or Ex9 infusion in GLP-1 receptor KO mice.
Table 1.
Plasma parameters at the completion of hyperglycemic hyperinsulinemic clamps in the presence or absence of central infusion of Ex4 or Ex9 in WT and GLP-1 receptor KO mice
Brain GLP-1 controls insulin-stimulated muscle glucose utilization and glycogen content independently from insulin action.
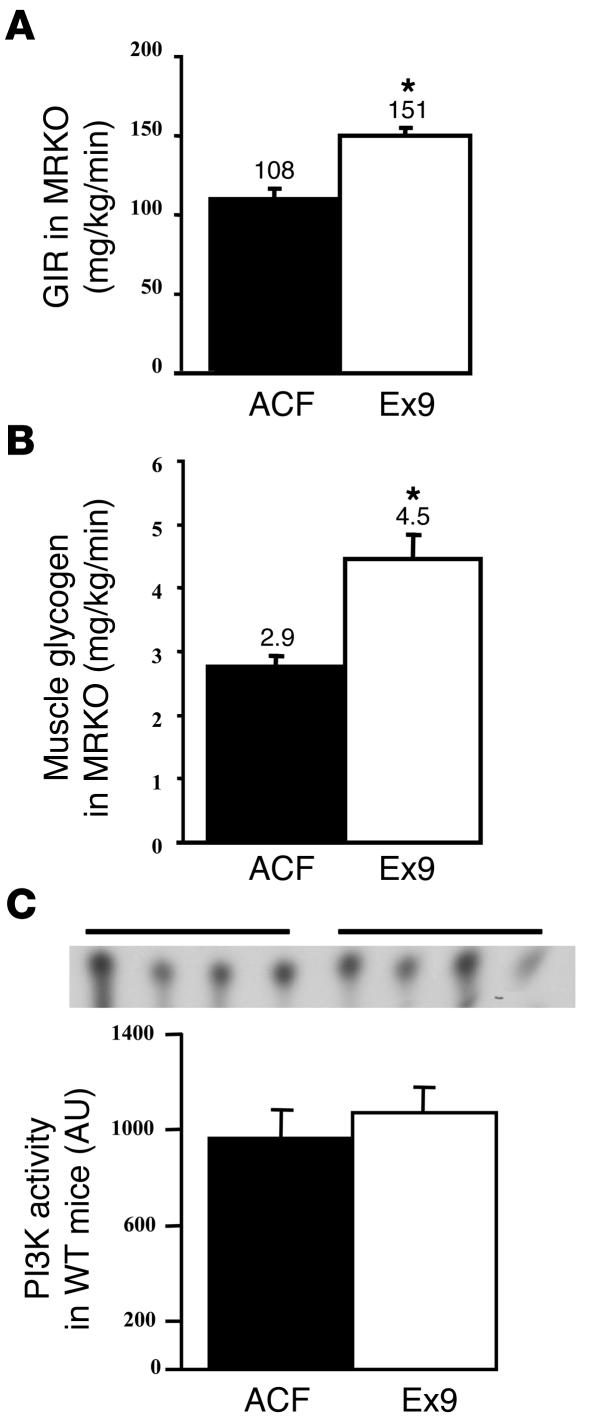
A main question that remained was whether GLP-1 action requires glucose alone or glucose plus insulin to control muscle glycogen content. Indeed, we previously showed that the hepatoportal glucose sensor activated muscle glucose utilization by a mechanism independent from insulin (30). We first assessed whether the stimulatory effect of central Ex9 on muscle glucose metabolism was exerted through the activation of muscle insulin receptor. Hyperglycemic 20-mM hyperinsulinemic clamps were performed in muscle insulin receptor KO (MIRKO) mice, with or without icv Ex9 infusion, and we assessed whole-body GIR and muscle glycogen content. Both parameters were increased when MIRKO mice were infused with Ex9 into the brain (Figure 4, A and B), showing that the central effect of Ex9 is independent of muscle insulin receptor signaling. This observation was further confirmed in normal mice by quantifying IRS-1–dependent PI3K activity at the completion of the hyperglycemic clamp, which showed no difference in PI3K activity between Ex9-infused and ACF mice (Figure 4C). Notably, the GIR of the MIRKO mice was lower than in WT mice, consistent with muscle insulin resistance (compare with Figure 1).
Figure 4.
Central control by GLP-1 of whole-body GIR and muscle glycogen content are independent of muscle insulin action. (A) GIR (mg/kg/min) and (B) muscle glycogen content (mg/g) were measured in MIRKO mice infused with ACF (black bars) or Ex9 (white bars) during 20-mM hyperglycemic hyperinsulinemic conditions. The mean of 5–6 mice per group is represented. (C) PI3K activity (AU) in hind limb muscles of C57BL/6 mice infused with ACF (black bars) or Ex9 (white bars). The mean of 4 mice per group is shown. *P < 0.05 versus icv ACF-infused mice.
Role of brain GLP-1 receptor on muscle glycogen synthase kinase 3β, carbohydrate-responsive element–binding protein, and hexokinase II.
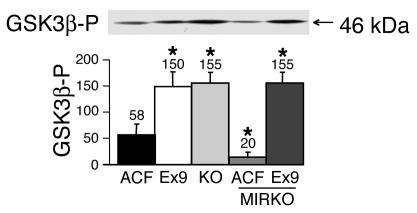
We next assessed whether the changes in muscle glycogen content observed with Ex9 infusion during hyperglycemia depended on the activity of glycogen synthase kinase 3β (GSK3β), which is inactivated by phosphorylation to promote glycogen synthesis. The amount of phosphorylated GSK3β (GSK3β-P) in muscle was significantly increased in icv Ex9-infused mice and in GLP-1 receptor KO mice (Figure 5). Importantly, Ex9 infusion also increased GSK3β-P in the muscles of MIRKO mice, consistent with the lack of requirement of muscle insulin receptor signaling for the central effect of Ex9. It is noteworthy that GSK3β-P was reduced in MIRKO compared with normal control mice, consistent with the status of insulin resistance of MIRKO mice, which can be bypassed by brain GLP-1 receptor blockade.
Figure 5.
Central control by GLP-1 of muscle GSK3β phosphorylation. Muscle content in 46-kDa GSK3β-P in C57BL/6 mice with ACF or Ex9 infused into their brains, GLP-1 receptor KO mice, and MIRKO mice infused with ACF or Ex9 into their brains during a 20-mM hyperglycemic hyperinsulinemic clamp. The mean of 4 mice per group is shown. *P < 0.05 versus ACF-infused WT mice.
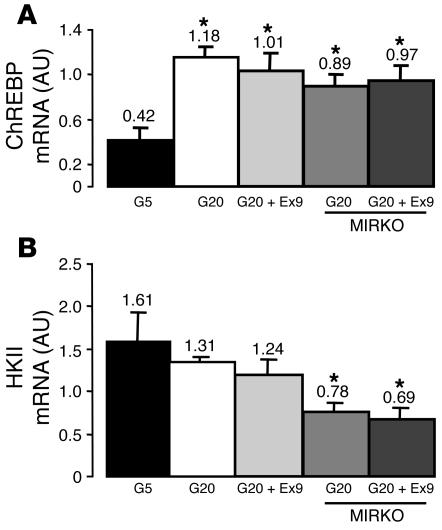
We then determined whether central Ex9 infused during hyperglycemia could also modify the expression of glucose-regulated genes in muscle. We quantified the concentration of carbohydrate-responsive element–binding protein (ChREBP) mRNA by quantitative RT-PCR (31). This gene is under the control of insulin and glucose in liver (32). We also quantified the concentration of hexokinase II (HKII) mRNA, the expression of which is under the control of insulin (33, 34). Hyperglycemia increased ChREBP mRNA content, while no further effect of central Ex9 was observed, showing that the regulation of GSK3β was not a general effect of glucose and Ex9 on muscle (Figure 6A). In addition, in insulin-resistant MIRKO mice, hyperglycemia increased ChREBP levels, whereas icv Ex9 had no effect. HKII mRNA levels did not vary in response to hyperglycemia or icv Ex9 (Figure 6B). Conversely, HKII mRNA concentration was lower in insulin-resistant MIRKO mice than in WT mice. These data show that despite their resistance to insulin, changes in muscle glucose utilization and glycogen synthesis in MIRKO mice specifically reflect the actions of icv Ex9 and are not simply generalized responses to hyperglycemia.
Figure 6.
Central control by central GLP-1 of ChREBP and HKII mRNA in muscles. (A) ChREBP and (B) HKII mRNA concentrations (AU) were assessed in muscles of C57BL/6 and MIRKO mice during hyperinsulinemic euglycemic (5.5 mM, G5) or hyperglycemic (20 mM, G20) clamps. Some hyperglycemic C57BL/6 and MIRKO mice had Ex9 simultaneously infused into their brains. The mean of 4 mice per group is shown. *P < 0.05 versus euglycemic WT mice.
Muscle innervation is required for regulation of muscle glycogen synthesis by the central GLP-1 receptor.
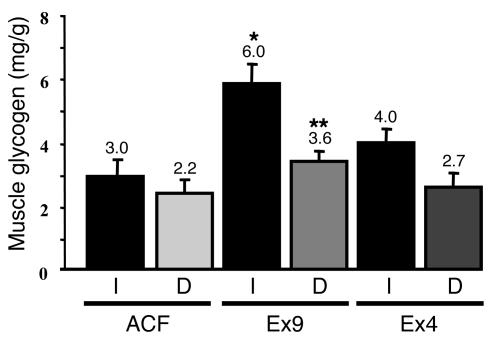
We and others previously showed that the central effect of leptin on muscle glucose and lipid metabolism was prevented when the nerves from the leg were disrupted (35, 36). Therefore, we disrupted the innervation to the left leg muscles by cutting the branches of the sciatic nerve and disrupting the connective and neural tissues surrounding the femoral artery and vein. We then assessed muscle glycogen content in hyperglycemic hyperinsulinemic conditions in the presence or absence of Ex4 and Ex9 infused into the brain. The data show that denervation prevented the Ex9-mediated increase in muscle glycogen content, although the chronic denervation itself slightly reduced the basal glycogen content (Figure 7).
Figure 7.
Central control by GLP-1 of glycogen content in denervated muscles. Glycogen content (mg/g wet tissue) was measured in ground hind limb denervated (D) or normal (I) muscles from C57BL/6 control mice with ACF, Ex4, or Ex9 infused into their brains during 20-mM hyperglycemic hyperinsulinemic conditions. The mean of 5–6 mice per group is shown. *P < 0.05 versus icv ACF-infused mice; **P < 0.05 versus normal muscle.
Ex9 treatment ameliorates insulin sensitivity in fat-fed diabetic mice.
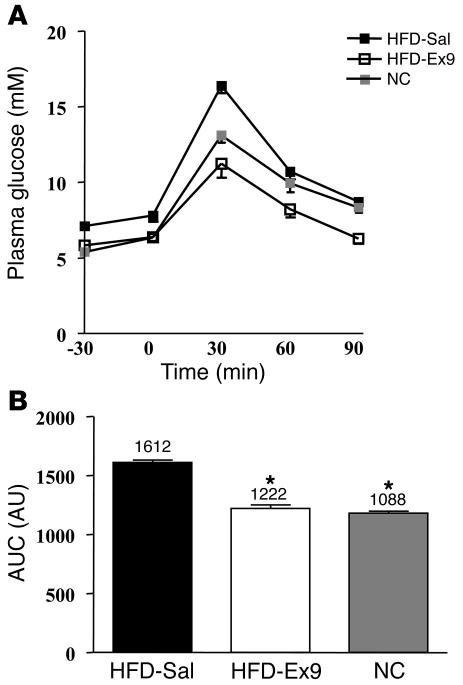
The above results show that whole-body glucose utilization was increased by a mechanism that did not require the muscle insulin receptor when Ex9 was infused into the brain. We tested whether Ex9 could have a putative therapeutic effect in mice with impaired insulin action and glucose tolerance following high-fat feeding. Since chronic icv infusion is not an appropriate therapeutic approach, we examined whether continuous i.p. infusion of Ex9 could reduce the impact of diet on the development of glucose intolerance and diabetes. After 1 month of continuous i.p. Ex9 infusion with osmotic minipumps, the fasting glycemia of mice fed a high-fat diet was normalized when compared with normal, chow-fed mice (P < 0.05; Figure 8A, time 0), whereas plasma insulin levels remained similar (23.3 ± 3.2 versus 28.8 ± 6.0 mU/l in control and Ex9-treated mice, respectively; data not shown). However, i.p. glucose tolerance was improved, as shown by reduced area under curve (Figure 8B) and increased plasma insulin concentration 15 minutes following the glucose challenge (46.6 ± 3.1 versus 91.1 versus 17.0, P < 0.05, in high-fat diet saline-treated, high-fat diet Ex9-treated, and control mice, respectively; data not shown). In addition, with Ex9 treatment, body weight was unchanged (32.5 ± 2.4 versus 33.5 ± 1.8 g; NS), whereas energy intake was slightly increased (335 ± 6 versus 422 ± 10 kcal in control and Ex9-treated mice, respectively; P < 0.05; data not shown).
Figure 8.
Glucose tolerance test in mice with type 2 diabetes induced by a high-fat diet and infused with Ex9 for 4 weeks into the peritoneal cavity. (A) Plasma glucose (mM) during an i.p. glucose tolerance test in C57BL/6 mice fed a high-fat diet (HFD) and infused with Ex9 or saline (Sal) through an osmotic pump for 4 weeks and in mice fed normal chow (NC). (B) Index of area under curve (AUC), expressed in AU, in the 3 tested conditions. The mean of 9 mice per group is shown. *P < 0.05 versus saline-infused HFD mice.
Brain GLP-1 controls liver glycogen synthesis, insulin secretion, and whole-body insulin action.
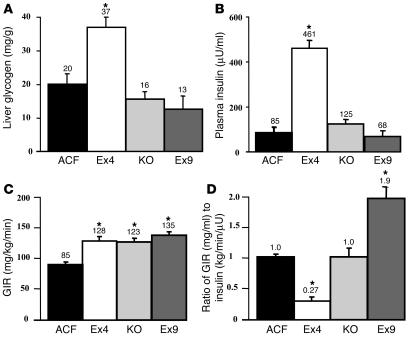
The above data showed that central Ex9 increased and Ex4 reduced muscle glycogen content in the presence of hyperglycemia through a mechanism that does not require the muscle insulin receptor. This suggests that in a physiological situation of hyperglycemia and central GLP-1 secretion, glucose would be utilized by tissues other than muscle. The liver is an important organ for the storage of glucose as glycogen, an effect that depends on hyperinsulinemia. To determine whether the pharmacological manipulation of central GLP-1 receptor regulates hepatic glycogen content, we infused mice for 3 hours with glucose alone (without exogenous insulin) to induce hyperglycemia at 20 mM and stimulate insulin secretion. Hepatic glycogen content was increased when Ex4 was infused into the brain during hyperglycemia (Figure 9A) in association with a large increase in plasma insulin concentration (Figure 9B). Both hepatic glycogen content and plasma insulin concentration were slightly increased or reduced compared with controls when the GLP-1 receptor was blocked either by icv Ex9 or by GLP-1 receptor KO (Figure 9, A and B). However, such differences did not reach statistical significance and were considered to be unchanged. The whole-body GIR was increased in all groups compared with control mice (Figure 9C). However, since plasma insulin was not similar in all groups, we calculated the GIR per plasma insulin unit in each individual. This ratio, as an index of insulin action, was dramatically reduced by Ex4 infusion and increased in Ex9-infused mice (Figure 9D), again confirming the insulin resistance and sensitization observed by Ex4 and Ex9 infusion, respectively, during the hyperinsulinemic hyperglycemic clamp (Figure 1).
Figure 9.
Central control by GLP-1 of liver glycogen content and insulin secretion during hyperglycemic clamp. (A) Liver glycogen content (mg/g); (B) plasma insulin concentration (μU/ml); (C) GIR (mg/kg/min); and (D) the ratio of GIR (mg/ml) to insulin (kg/min/μU) during a 20-mM hyperglycemic clamp in C57BL/6 mice infused simultaneously with ACF, Ex4, or Ex9 and in GLP-1 receptor KO mice. The mean of 6–8 mice per group is shown. *P < 0.05 versus icv ACF-infused mice.
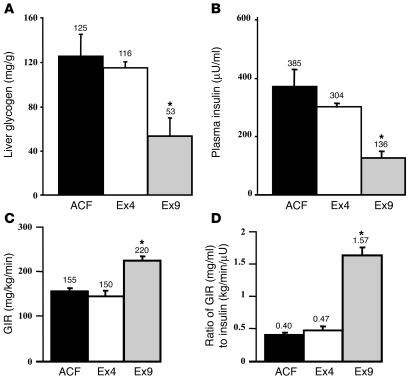
In addition to the role of hyperglycemia and hyperinsulinemia on the regulation of liver glycogen synthesis, we studied whether a more physiological route of glucose administration, i.e., intragastric glucose, would lead to similar regulation of glucose metabolism. Hyperglycemia was produced by infusing glucose directly into the stomach for 3 hours, while ACF, Ex9, or Ex4 was infused into the brain. Hepatic glycogen content was elevated in Ex4-infused mice, but not in Ex9-infused mice (Figure 10A). In control mice, intragastric glucose infusion increased plasma insulin concentration compared with i.v. infusion (intragastric, 385 ± 104 μU/ml, Figure 10B; i.v., 85 ± 22 μU/ml, Figure 9B). During intragastric glucose infusion, icv Ex4 did not change plasma insulin concentration, while Ex9 dramatically reduced hyperinsulinemia. Compared with control mice, the whole-body GIR was unchanged in Ex4-treated mice but increased in Ex9-infused mice (Figure 10C). Consequently, the ratio of the GIR to plasma insulin concentration was unchanged by Ex4 treatment but increased by Ex9 infusion (Figure 10D).
Figure 10.
Central control by GLP-1 of liver glycogen content and insulin secretion during hyperglycemic clamp when glucose was infused into the stomach. (A) Liver glycogen content (mg/g), (B) plasma insulin concentration (μU/ml), (C) GIR (mg/kg min), and (D) the ratio of GIR (mg/ml) to insulin (kg/min/μU) during a 20-mM hyperglycemic clamp achieved by intragastric infusion of glucose was performed in C57BL/6 mice simultaneously infused with ACF, Ex4, or Ex9. The mean of 6–8 mice per group is shown. *P < 0.05 versus icv ACF-infused mice.
The last 2 experiments (i.v. and intragastric glucose infusions) together show that brain GLP-1 promotes insulin secretion and hepatic glycogen synthesis during hyperglycemia, particularly when glucose has a gastric origin.
Discussion
The present study shows for the first time to our knowledge that brain GLP-1 has a role in controlling whole-body and tissue-specific glucose metabolism in hyperglycemic conditions. The data show that blockade of the central GLP-1 receptor, during hyperglycemia only, increased muscle glycogen deposition by a mechanism that did not require a functional muscle insulin receptor. Whole-body blockade of the GLP-1 receptor with i.p. Ex9 was sufficient to prevent the fasted hyperglycemia and glucose intolerance induced by a high-fat diet. In addition, we also found that activation of GLP-1 receptor in the brain by Ex4 induced insulin resistance and increased insulin secretion, promoting the storage of glycogen in the liver. Together, our data suggest that enteric glucose absorption modulates glucose disposal through central GLP-1–dependent mechanisms.
A teleological interpretation of this evidence could be that during a meal, an enteric glucose sensor triggers the release of central GLP-1 in order to favor hepatic glycogen deposition by increasing insulin secretion and muscle insulin resistance. The liver is the main tissue to provide glucose in postabsorptive situations. Therefore, central GLP-1 would restrict the amount of glucose taken up by the large muscle mass, sparing glucose for the liver. Replenishing hepatic rather than muscle glycogen stores is a physiological mechanism to prevent fasting-induced hypoglycemia during the next fasting period.
Central GLP-1 inhibits muscle glycogen accumulation in hyperglycemic conditions.
GLP-1 is an intestinal hormone also considered a neuropeptide because it is produced in the brain (37). However, its role as a neuropeptide regulating the control of glucose homeostasis has not previously been determined. To answer this key question, we studied glucose homeostasis in GLP-1 receptor KO mice and WT mice infused with the GLP-1 receptor agonist Ex4 or the receptor antagonist Ex9 into the brain. Our data show that during hyperglycemia, the whole-body GIR, which is an index of glucose utilization, and muscle glycogen content were increased in GLP-1 receptor KO mice and WT mice in which central GLP-1 receptors were blocked by Ex9 infusion. In addition, the phosphorylation state of GSK3β was augmented. In this form, the enzyme is inhibited and cannot deactivate glycogen synthase, the rate-limiting step of glycogen synthesis (38, 39). The opposite effect, insulin resistance during hyperglycemia, was observed upon stimulation of central GLP-1 receptor with Ex4. The factors regulated by brain GLP-1 signaling remain to be determined but could be related to circulating hormones. It has been shown that both GLP-1 agonists and GLP-1 antagonists can have a potent, acute effect on stress-activated hormones like adrenocorticotropic hormone and corticosterone (40). In our experimental conditions, corticosterone concentrations were similar among all groups. While we cannot rule out a role for the hypothalamic-adrenal gland axis (HPA) in the regulation of tissue glycogen content, we did not detect any correlation between HPA activation and tissue glycogen content in our experimental conditions. Conversely, plasma glucagon concentration was reduced by Ex4 treatment, while no effect was detected when Ex9 was used. This effect was not present in GLP-1 receptor KO mice, demonstrating the GLP-1 receptor–dependent specificity of action of Ex4. Glucagon has no direct effect on muscle but strongly controls liver glycogen stores. Therefore, the decreased levels of glucagon in response to central Ex4 administration under hyperglycemic conditions may contribute to regulated hepatic glycogen stores.
The enhancing effect of central Ex9 on muscle glycogen storage was mostly dependent on hyperglycemia, suggesting that glucose sensors were involved in the regulatory mechanism. The GLP-1 receptor is expressed in hypothalamic nuclei, particularly in the ventromedial nucleus (41–43) and in the posterior pituitary lobe (44), while the actual hormone is mainly present in nucleus of the tractus solitarius and in brain nuclei (42, 45–47). Recently, GLP-1 has been shown to control central glucose metabolism in human subjects, as revealed by positron emission tomography analysis using 2-[F-18]-deoxy-D-glucose (48). In addition, the GLP-1 receptor was colocalized in the human brain by in situ hybridization with glucose transporter GLUT2 and glucokinase (48). All 3 molecules |are important regulators of glucose-stimulated insulin secretion and therefore considered to be part of the molecular mechanisms of glucose-sensing systems (49, 50). Our study implicates a role for GLP-1 in the regulation of glucose-sensing systems in the brain, as we show for the first time to our knowledge that central glucose sensing requires the GLP-1 receptor for the regulation of muscle glycogen storage.
The blockade by central GLP-1 of muscle glycogen storage depends on muscle innervation but not on muscle insulin receptors and may prevent hyperglycemia induced by a high-fat diet.
By infusing the antagonist Ex9, we found that inhibition of central GLP-1 receptor signaling during hyperglycemic conditions increased muscle glucose storage. Previously, we showed that leptin in the brain controlled leg muscle glucose utilization by a mechanism requiring innervation of the leg (35). Similarly, Minokoshi et al. showed that central leptin administration increased fatty acid oxidation by a mechanism depending on the ANS (36). In the present study, innervation of the leg was necessary to transmit central GLP-1 signaling to the leg muscles. However, nerves from the ANS are not directly attached to muscles, but mostly associated with blood vessels (51). Peripheral nerves and their products, including vasoactive peptides and neurotransmitters, control endothelial function and the generation of nitric oxide (51). Our data suggest that mediators released by an intermediary tissue like the endothelium could relay the information to the muscles. Furthermore, we showed that the mechanism by which central GLP-1 controls insulin action on muscle was independent of the muscle insulin receptor, since in MIRKO mice, central Ex9 infusion still increased muscle glycogen deposition and GSK3β phosphorylation. In addition, IRS-1–dependent PI3K activity was unchanged by Ex9 infusion during clamps but was still 3-fold higher than mice not infused with insulin (data not shown). However, we cannot rule out the possibility that central insulin action accounts for changes in muscle glucose utilization. We previously showed that when infused into the brain, insulin increased muscle glucose utilization (52). Similarly, it has been recently shown that central insulin signaling was important for the peripheral response to hypoglycemia or hepatic glucose production (53, 54). The effect of central Ex9 was specific to the GSK3β signaling pathway, since expression of the insulin-sensitive gene HK2 (33) was unchanged by hyperglycemia or Ex9 treatment. Similarly, the expression of ChREBP, a glucose-sensitive gene (32), was increased by hyperglycemia but not further modified by Ex9 treatment.
We then further wondered whether Ex9 treatment could exert its effects in the presence of muscle insulin resistance. We studied mice fed a high-fat diet, which are characterized by impaired glucose tolerance, defective insulin action, and fasting hyperglycemia (55). Infusion of Ex9 into the peritoneal cavity for 1 month markedly improved fasting hyperglycemia, glucose tolerance, and insulin secretion. In addition, food intake and body weight were slightly increased. The relative contributions of improved insulin secretion, muscle glycogen deposition, inhibition of liver glycogen storage, and increased food intake to the phenotype remain to be determined.
Central GLP-1 increases glucose-stimulated insulin secretion.
What would be the physiological relevance of the inhibitory effect of central GLP-1 on the brain’s glucose-sensing system for the regulation of whole-body glucose fluxes? Since insulin is the major hormone controlling glucose homeostasis, we assessed whether central GLP-1 would also control glucose-stimulated insulin secretion. Mice were clamped in a hyperglycemic state to induce insulin secretion while Ex4 was infused in their brains. Plasma insulin concentration and liver glycogen were dramatically increased by Ex4 treatment compared with controls. The glycogen increase is probably the consequence of GLP-1 receptor–induced insulin secretion, since hyperglycemia in the other groups modified neither insulin secretion nor hepatic glycogen storage. Therefore, we speculate that central GLP-1 drives glucose toward the liver, favoring hepatic rather than muscle glycogen deposition. It is noteworthy that under normal conditions, insulin is released into the portal vein toward the liver and would fully activate liver glycogen deposition before reaching the muscle. Brain GLP-1 receptor activation would also favor glycogen storage in liver rather than muscle. Supporting this interpretation is the fact that only one-third of insulin passes the hepatic filter, which could be a physiological regulator to prevent excessive muscle glucose utilization and to spare glucose and insulin for the liver.
Gastric glucose is a stimulatory signal for central GLP-1 in the control of whole-body glucose homeostasis.
In addition to hyperglycemia and hyperinsulinemia, the physiological entry route of glucose into the blood via the intestine could be an important regulator of glucose homeostasis. The enteric glucose sensors are connected to the brain via the ANS. We performed hyperglycemic clamps by infusing glucose into the stomach and Ex4 into the brain. In such conditions, plasma insulin concentration was not further increased by Ex4 treatment. Hyperinsulinemia was considerably higher during gastric glucose infusion than when glucose was administered i.v., indicating that an enteric factor may be stimulating insulin secretion. This glucoincretin effect has been largely described and is attributable to the glucoincretins GLP-1 and gastric inhibitory peptide. For instance, we previously showed that genetic ablation of both incretin hormone receptors totally blunted the ability of oral glucose to induce insulin secretion (56). However, in that study we could not determine whether intestinally or centrally released glucoincretins were responsible for insulin secretion. In the present study, we also infused Ex9 icv during an enteric glucose challenge and showed that plasma insulin was reduced to similar values as when glucose was infused into the femoral vein. In these conditions, blockade of central GLP-1 receptor totally blunted the glucoincretin effect, showing the importance of brain GLP-1 in the stimulation of insulin secretion.
In summary, our data show that brain GLP-1 controls whole-body glucose distribution during hyperglycemia and that an enteric-derived glucose signal is required to enhance glucose-stimulated insulin secretion and insulin resistance. Consequently, upon central GLP-1 receptor activation, hepatic glycogen deposition is favored over excessive muscle glycogen storage. The main physiological consequence of replenishing liver glycogen stores is to ensure that there is enough glucose stored to be released during the next fasting period.
Methods
Animals
Twelve-week-old C57BL/6J (Janvier), GLP-1 receptor KO (in a C57BL/6 background), and MIRKO (57) male mice were housed in a controlled environment (inverted 12-hour daylight cycle), with free access to food and water. All animal experimental procedures were approved by the Rangueil Hospital Committee of the Rangueil Hospital, Toulouse, France.
In a subset of C57BL/6 mice (n = 10), diabetes was induced by feeding them a high-fat, carbohydrate-free diet (55) for 1 month. The diet’s energy content consisted of 72% fat (corn oil and lard), 28% protein, and <1% carbohydrate. The mice were simultaneously treated with Ex9. Body weight and energy intake were recorded weekly. Glucose tolerance was tested after 4 weeks on fasted animals. A 10-μl drop of blood was sampled from the tail vein, and plasma was separated to assess insulin concentration 30 minutes before and 15 minutes after glucose challenge.
Surgical procedures
When required, 1 or more of the following surgical procedures was performed.
Indwelling intragastric catheter.
Under anesthesia (ketamine and xylazine i.p., 100 and 10 mg/kg, respectively) and after hair shaving, a 4-mm laparotomy was performed on the left side of the mouse at the level of the stomach. The stomach was gently extracted, and a 5-mm Teflon catheter was inserted at the proximal part of the stomach through a needle-sized hole. It was secured in place with surgical glue (Histoacryl; 3M), and the other end of the catheter was tunneled under the skin and exteriorized at the back of the neck.
Indwelling icv catheter.
Under similar anesthesia, a 1-cm midline incision was made in the skin over the skull, the animal was placed on a stereotaxic apparatus, and the periosteum was cleaned as described previously (52). A hole 1 mm in diameter was made 0.1 mm lateral and 0.22 mm anteroposterior from the bregma, and a cannulae (Alzet) was inserted 1.7 mm deep. Two supporting screws were placed bilaterally, one in the posterior quadrants and the other in the anterior part of the skull, and secured in place with acrylic dental cement (Magasin Général Dentaire). The cannulae was filled with ACF (Harvard Apparatus) and connected to a sealed tygon catheter.
Indwelling intrafemoral catheter.
Under similar anesthesia, a catheter was indwelled into the femoral vein as previously described (55).
Minipump implantation.
Mice fed the high-fat diet were implanted subcutaneously with an osmotic pump (Alzet) delivering either normal saline at a rate of 0.25 μl/h or Ex9 at a rate of 2 pmol/kg/min through a catheter indwelled into the abdominal cavity.
Denervation of the left leg.
During the indwelling of the femoral catheter, 5 mm of the 2 sciatic nerve branches were cut out from the left leg. In addition, since nerves from the ANS mostly run on blood vessels, the surrounding tissues of left leg femoral artery and veins were disrupted, and the thread securing the catheter to the femoral vein was kept taut.
All mice were allowed to recover for 3 weeks before ongoing infusions. Only animals that fully recovered their presurgery body weight were used for the studies.
Infusions
When indicated, the following infusion protocols were performed.
Glucose clamps.
To assess the effect of glycemia and hyperinsulinemia on glucose turnover, euglycemic or hyperglycemic glucose clamps were performed for 3 hours as described previously (58). Briefly, insulin was infused through the intrafemoral catheter at 18 mU/kg/min to induce a maximal GIR. Blood glycemia was clamped at 5.5, 10, or 20 mM by adjusting the intrafemoral glucose infusion.
Intracerebroventricular infusions.
To determine the role of central GLP-1, we performed icv infusions of the GLP-1 receptor antagonist Ex9 or agonist Ex4 (Bachem) at a rate of 0.5 pmol/kg/min or ACF as a control at a rate of 0.25 μl/h. We previously showed that the infusion rate of Ex9 was unable to modulate whole-body glucose sensors when infused through the femoral vein (25), and therefore Ex9 should only affect the central GLP-1 receptors. To ensure the filling of the tubing connected to the brain, icv infusions were started 30 minutes before the beginning of other intrafemoral or intragastric infusions and continued throughout the whole study.
Intragastric glucose infusion to induce hyperglycemia.
Fasted mice were infused with glucose into the stomach. A 30% glucose solution was infused for 3 hours at a rate maintaining glycemia around 20 mM. The proper indwelling of the catheter was visualized at the completion of the infusions.
Individual tissue glucose utilization
Individual tissue glucose utilization was assessed as previously described (55, 59). Briefly, mice were injected with 2DG (PerkinElmer) through the intrafemoral catheter 1 hour before completion of the infusion procedure. Tail blood was sampled at 5, 10, 15, 20, 30, 45, and 60 minutes after the injection to determine the time course of 2DG disappearance. Then the tissues were rapidly harvested, frozen in liquid nitrogen, and stored at –80°C until processed. The 2DG-6-phosphate content was determined from NaOH hydrolysed tissues by the Somogyi procedure (60).
Biochemical analyses
Plasma parameters.
Plasma glucagon and corticosterone concentrations were assessed by RIA using the Glucagon RIA Kit (Linco Research Inc.) and Corticosterone RIA Kit (MP Biomedicals), respectively, according to the manufacturer’s instructions. Total plasma GLP-1 was assessed after plasma alcohol extraction. Since 300 μl were needed per assay, we pooled plasma from 2–4 mice from the same experimental condition. Five determinations per group were performed. Briefly, 1.1 ml of 95% ethyl alcohol was added to 300 μl of plasma in 1.5-ml microfuge tubes, and the mixture was vortexed, incubated in an ice bath for 30 minutes, and centrifuged at 10,000 g for 10 minutes. The supernatant was decanted and dried in a centrifugal vacuum evaporator for 8 hours. Dry plasma extract was rehydrated and measured according to the manufacturer’s instructions (GLP-1 Total RIA Kit; Linco Research Inc.).
Tissue glycogen content.
After extraction, the tissue glycogen was transformed into free glucose using amyloglucosidase (Roche Diagnostics Corp.). Muscles were harvested from the hind limb. Liver and muscles were then ground into powder in liquid nitrogen. Aliquots were then sampled, and glycogen was assessed. The free glucose was assessed by the glucose 6-phosphate oxidase method and used to determine the tissue glycogen content as described previously (61). Plasma insulin concentration was assessed by ELISA (Mercodia) performed on 1 μl of plasma extracted from blood collected at the tip of the tail every 20 minutes during the last hour of infusion.
Molecular analyses
GSK3β was quantified in its phosphorylated form by Western blot analyses. Briefly, the muscles were dissected and immediately ground in 6 volumes 50 mM Tris buffer (pH 7.5) containing 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 50 mM NaF, 5 mM Na3PO4, 1% Triton, and a mixture of protease inhibitors. The tissue extract was centrifuged, the supernatant was isolated, and the protein concentration was determined using the Bio-Rad Laboratories protein assay. Proteins (60 μg) were resolved by 12% acrylamide SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Primary antibodies against GSK3β-P (rabbit polyclonal antibodies; Cell Signaling Technology) were diluted 1:1,000, then incubated overnight at 4°C. Secondary antibodies were obtained from DakoCytomation and used according to the manufacturer’s instructions. Visualization of bound antibodies was performed by incubation with horseradish peroxidase–conjugated secondary antibodies followed by enhanced chemiluminescence and exposure to X-ray film, and the results were quantified using the ImageQuant system (version 1.1; Amersham Biosciences).
Analysis of mRNA expression by real-time quantitative PCR
Total RNA (500 ng) was reverse transcribed for 1 hour at 42°C in a 20-μl final volume reaction containing 50 mM Tris-HCl, 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 250 mM random hexamers (Promega), 250 ng oligo(dT) (Promega), 2 mM of each dNTP and 100 U of superscript II reverse transcriptase (Invitrogen Corp.). Real-time quantitative PCR analysis was performed starting with 6.25 ng of reverse-transcribed total RNA in a final volume of 10 μl PCR reaction with 0.5 μM of each primer (Invitrogen Corp.) and 2 mM MgCl2, using 1× light-cycler DNA Master SYBR Green I mix in a light-cycler instrument (Roche Diagnostics Corp.). Samples were incubated in the light-cycler apparatus for an initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 58°C for 7 seconds, and 72°C for 15 seconds. Primers used to detect HK2 and ChREBP gene expression were previously described (32). SYBR Green I fluorescence emission was determined after each cycle. The relative amounts of the different mRNAs were quantified using the second derivative maximum method of the light-cycler software. Cyclophilin was used as an invariant control, and the relative quantification for a given gene was corrected to the cyclophilin mRNA value.
PI3K assay
Muscles (20 mg) were solubilized for 1 hour at 4°C in 1 ml of buffer A (20 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 150 mM NaF, 2 mM Na3VO4, 1 % NP-40, and protease inhibitors; Roche Diagnostics Corp.). Lysates were centrifuged for 10 minutes at 13,000 g at 4°C. Supernatants were incubated for 4 hours at 4°C with anti–IRS-1 antibodies (5 μg/ml) coupled to protein A sepharose beads. Antibodies against IRS-1 were raised in rabbit against a synthetic peptide (YASINFQKQPEDRQ) corresponding to the carboxyterminal end of rat IRS-1 (Eurogentec). Immune pellets were washed twice with each of the following buffers: (a) PBS containing 1% NP-40 and 200 μM Na3VO4; (b) 100 mM Tris, pH 7.4, 0.5 M LiCl, and 200 μM Na3VO4; and (c) 10 mM Tris pH 7.4, 100 mM NaCl, 1 mM EDTA, and 200 μM Na3VO4. PI3K activity was measured on the immune pellets using [–32P]-ATP (Amersham Biosciences) as previously described (62).
Calculations and statistics
Whole-body glucose utilization was calculated from the mean GIR during the last hour of the infusion. Plasma insulin levels were calculated from the mean insulinemias determined during the last hour of infusion.
Results are presented as mean ± SEM. Statistical significance was analyzed by 2-tailed Student’s t test for unpaired bilaterally distributed values of unequal variance. Values were considered significantly different when P < 0.05.
Acknowledgments
We would like to thank André Pech (Université Paul Sabatier) for animal care. R. Burcelin is a recipient of Action THÉmatique Incitative Programmée and Action Concertée Incitative grants from the CNRS, Université Paul Sabatier, and the Fondation pour la Recherche Médicale. This work was supported in part by a grant from the Juvenile Diabetes Research Foundation to D.J. Drucker. C. Knauf is a recipient of a grant from the Club d’Étude du Système Nerveux Autonome (CESNA). R. Burcelin and C. Knauf are members of CESNA.
Footnotes
See the related Commentary beginning on page 3406.
Nonstandard abbreviations used: ACF, artificial cerebrospinal fluid; ANS, autonomic nervous system; ChREBP, carbohydrate-responsive element–binding protein; 2DG, 3H-2-deoxyglucose; Ex4, exendin 4; Ex9, exendin 9–39; GIR, glucose infusion rate; GLP-1, glucagon-like peptide–1; GSK3β, glycogen synthase kinase 3β; GSK3β-P, phosphorylated GSK3β; HKII, hexokinase II; MIRKO, muscle insulin receptor KO.Conflict of interest: D.J. Drucker has served as an advisor or consultant within the past 12 months to Abbott Laboratories, Amylin Pharmaceuticals, Bayer Inc., Conjuchem Inc., Eli Lilly Inc., GlaxoSmithKline, Merck Research Laboratories, Novartis Pharmaceuticals, NPS Pharmaceuticals Inc., PPD Inc., Syrrx Inc., and Triad Pharmaceuticals Inc.Work in D.J. Drucker’s laboratory is sponsored in part by grants from Merck Research Laboratories, Novartis Pharmaceuticals, Eli Lilly Inc., and Novo Nordisk.
References
- 1.Szekely M, Szelenyi Z. Regulation of energy balance by peptides: a review. Curr. Protein Pept. Sci. 2005;6:327–353. doi: 10.2174/1389203054546343. [DOI] [PubMed] [Google Scholar]
- 2.Kalra S. Appetite and body weight regulation: is it all in the brain? Neuron. 1997;19:220–230. doi: 10.1016/s0896-6273(00)80934-4. [DOI] [PubMed] [Google Scholar]
- 3.Kalra S, et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 4.Gyires K. Neuropeptides and gastric mucosal homeostasis. Curr. Top Med. Chem. 2004;4:63–73. doi: 10.2174/1568026043451609. [DOI] [PubMed] [Google Scholar]
- 5.Lustig R. Autonomic dysfunction of the beta-cell and the pathogenesis of obesity. Rev. Endocr. Metab. Disord. 2003;4:23–32. doi: 10.1023/a:1021819318484. [DOI] [PubMed] [Google Scholar]
- 6.Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J. Auton. Nerv. Syst. 1984;10:359–372. doi: 10.1016/0165-1838(84)90033-x. [DOI] [PubMed] [Google Scholar]
- 7.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab. Rev. 1987;3:185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- 8.Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 9.Holst J. Glucagon like peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology. 1994;107:1848–1855. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 10.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 11.Jin SL, et al. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J. Comp. Neurol. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Invest. 2002;110:43–52. doi:10.1172/JCI200215595. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turton MD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 14.Tang-Christensen M, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Seino S, Kirchgessner AL. Identification and characterization of glucoresponsive neurons in the enteric nervous system. J. Neurosci. 1999;19:10305–10317. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer J, Vayro S, Shirazi-Beechey SP. Mechanism of glucose sensing in the small intestine. Biochem. Soc. Trans. 2003;31:1140–1142. doi: 10.1042/bst0311140. [DOI] [PubMed] [Google Scholar]
- 17.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 18.Orsini JC, Himmi T, Wiser AK, Perin J. Local versus indirect action of glucose on the lateral hypothalamic neurons sensitive to glycemic level. Brain Res. Bull. 1990;25:49–53. doi: 10.1016/0361-9230(90)90251-t. [DOI] [PubMed] [Google Scholar]
- 19.Himmi T, Boyer A, Orsini JC. Changes in lateral hypothalamic neuronal activity accompanying hyper and hypoglycemias. Physiol. Behav. 1998;44:347–354. doi: 10.1016/0031-9384(88)90036-4. [DOI] [PubMed] [Google Scholar]
- 20.Koyama Y, et al. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 21.Shimazu T, Amakawa A. Regulation of glycogen metabolism in liver by the autonomic nervous system. Biochim. Biophys. Acta. 1975;385:242–256. doi: 10.1016/0304-4165(75)90352-9. [DOI] [PubMed] [Google Scholar]
- 22.Hevener A, Bergman R, Donovan C. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes. 2000;49:8–12. doi: 10.2337/diabetes.49.1.8. [DOI] [PubMed] [Google Scholar]
- 23.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46:1521–1525. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- 24.Nishizawa M, et al. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J. Auton. Nerv. Syst. 2000;80:14–21. doi: 10.1016/s0165-1838(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 25.Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–1728. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- 26.Nishizawa M, et al. Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am. J. Physiol. Endocrinol. Metab. 2003;284:E1027–E1036. doi: 10.1152/ajpendo.00503.2002. [DOI] [PubMed] [Google Scholar]
- 27.Dardevet D, et al. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am. J. Physiol. Endocrinol. Metab. 2004;287:E75–E81. doi: 10.1152/ajpendo.00035.2004. [DOI] [PubMed] [Google Scholar]
- 28.Obici S, et al. Central melanocortin receptors regulate insulin action. J. Clin. Invest. 2001;108:1079–1085. doi:10.1172/JCI200112954. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deacon CF, et al. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia. 1998;41:271–278. doi: 10.1007/s001250050903. [DOI] [PubMed] [Google Scholar]
- 30.Burcelin R, et al. GLUT4, AMP kinase, but not the insulin receptor, are required for hepatoportal glucose sensor–stimulated muscle glucose utilization. J. Clin. Invest. 2003;111:1555–1562. doi:10.1172/JCI200316888. doi: 10.1172/JCI16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita H, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dentin R, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J. Biol. Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 33.Postic C, et al. The effects of hyperinsulinemia and hyperglycemia on GLUT4 and hexokinase II mRNA and protein in rat skeletal muscle and adipose tissue. Diabetes. 1993;42:922–929. doi: 10.2337/diab.42.6.922. [DOI] [PubMed] [Google Scholar]
- 34.Printz RL, Magnuson MA, Granner DK. Mammalian glucokinase. Annu. Rev. Nutr. 1993;13:463–496. doi: 10.1146/annurev.nu.13.070193.002335. [DOI] [PubMed] [Google Scholar]
- 35.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 36.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 37.Blazquez E, et al. Glucagon-like peptide-1 (7–36) amide as a novel neuropeptide. Mol. Neurobiol. 1998;18:157–173. doi: 10.1007/BF02914270. [DOI] [PubMed] [Google Scholar]
- 38.Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J. Appl. Physiol. 2002;93:384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez JE, et al. The anti-diabetic agent sodium tungstate activates glycogen synthesis through an insulin receptor-independent pathway. J. Biol. Chem. 2003;278:42785–42794. doi: 10.1074/jbc.M308334200. [DOI] [PubMed] [Google Scholar]
- 40.Kinzig KP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J. Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J. Neurochem. 1996;66:920–927. doi: 10.1046/j.1471-4159.1996.66030920.x. [DOI] [PubMed] [Google Scholar]
- 42.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology. 1987;121:1076–1082. doi: 10.1210/endo-121-3-1076. [DOI] [PubMed] [Google Scholar]
- 44.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Identification of specific binding sites for glucagon-like peptide-1 on the posterior lobe of the rat pituitary. Neuroendocrinology. 1995;62:130–134. doi: 10.1159/000126997. [DOI] [PubMed] [Google Scholar]
- 45.Mercer JG, Moar KM, Findlay PA, Hoggard N, Adam CL. Association of leptin receptor (OB-Rb), NPY and GLP-1 gene expression in the ovine and murine brainstem. Regul. Pept. 1998;75–76:271–278. doi: 10.1016/s0167-0115(98)00078-0. [DOI] [PubMed] [Google Scholar]
- 46.Goldstone AP, et al. Effect of leptin on hypothalamic GLP-1 peptide and brain-stem pre-proglucagon mRNA. Biochem. Biophys. Res. Commun. 2000;269:331–335. doi: 10.1006/bbrc.2000.2288. [DOI] [PubMed] [Google Scholar]
- 47.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl. 5):S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez E, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 49.Matschinsky FM. A lesson in metabolic regulation inspired by the glucokinase paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 50.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med. Res. Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Perrin C, Knauf C, Burcelin R. Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology. 2004;145:4025–4033. doi: 10.1210/en.2004-0270. [DOI] [PubMed] [Google Scholar]
- 53.Fisher SJ, Bruning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes. 2005;54:1447–1451. doi: 10.2337/diabetes.54.5.1447. [DOI] [PubMed] [Google Scholar]
- 54.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 55.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2002;282:E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 56.Preitner F, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J. Clin. Invest. 2004;113:635–645. doi:10.1172/JCI200420518. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruning J, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 58.Burcelin R, Thorens B. Glucagon secretion is predominantly controlled by extrapancreatic GLUT2-dependent glucose sensors. Diabetes. 2001;50:1282–1289. doi: 10.2337/diabetes.50.6.1282. [DOI] [PubMed] [Google Scholar]
- 59.Burcelin R, Dolci W, Thorens B. Portal glucose infusion in the mouse induces hypoglycemia. Evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes. 2000;49:1635–1642. doi: 10.2337/diabetes.49.10.1635. [DOI] [PubMed] [Google Scholar]
- 60.Somogyi M. Determination of blood sugar. J. Biol. Chem. 1945;160:69–73. [Google Scholar]
- 61.Burcelin R, et al. Impaired glucose homeostasis in mice lacking the alpha1b-adrenergic receptor subtype. J. Biol. Chem. 2004;279:1108–1115. doi: 10.1074/jbc.M307788200. [DOI] [PubMed] [Google Scholar]
- 62.Heydrick SJ, et al. Defect in skeletal muscle phosphatidylinositol-3-kinase in obese insulin-resistant mice. J. Clin. Invest. 1993;91:1358–1366. doi: 10.1172/JCI116337. [DOI] [PMC free article] [PubMed] [Google Scholar]