Abstract
Platelets, derived from megakaryocytes, have an essential role in thrombosis and hemostasis. Over the past 10 years, a great deal of new information has been obtained concerning the various aspects of hematopoiesis necessary to maintain a steady-state platelet level to support physiologic hemostasis. Here we discuss the differentiation of HSCs into megakaryocytes, with emphasis on the key cytokine signaling pathways and hematopoietic transcription factors. Recent insight into these processes elucidates the molecular bases of numerous acquired and inherited hematologic disorders. It is anticipated that the growing knowledge in these areas may be exploited for new therapeutic strategies to modulate both platelet numbers and their thrombogenicity.
Development of the megakaryocyte lineage from HSCs
Like all terminally differentiated hematopoietic cells, megakaryocytes arise from common HSCs, which are responsible for lifelong production of all circulating blood cells (1). Hematopoietic cells are classified by 3 means: (a) by surface markers that are mainly detected by flow cytometry, (b) by their developmental potential assessed ex vivo in colony assays, and (c) by their ability to reconstitute host animals in vivo. Individual cells that reconstitute multilineage hematopoiesis for at least 6 months, termed long-term repopulating HSCs, are rare, constituting less than 0.1% of total nucleated marrow cells. In mice, these are highly enriched within a population of cells with surface markers Lin–Sca-1+c-kithigh (2–5) (Figure 1). This population has been referred to as the LSK long-term HSC population, but for ease of reading we will refer to it below as the “HSC” population. The production of mature blood cells from HSCs involves a series of successive differentiation steps in which the developmental and proliferative capacities of progenitors become increasingly restricted.
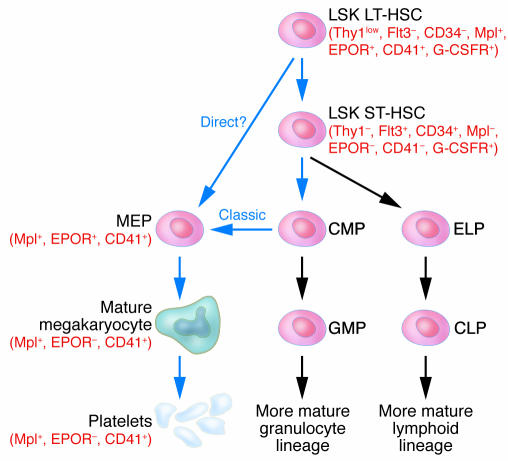
Figure 1.
Megakaryopoiesis pathways. The figure extends from the HSC to platelets and offers a combination of the more “classical” pathway, leading to the common megakaryocyte-erythroid progenitor (MEP), and a proposed “direct” route from the HSC. Pathways leading to platelet production are indicated by blue arrows and other pathways by gray arrows. Surface markers of importance are noted in parentheses in red. LT-HSC, long-term HSC; ST-HSC, short-term HSC; Thy1, thymus 1 (“low” indicates low surface antigen and “–” indicates none detectable); Flt3, FMS-like tyrosine kinase 3; EPOR, erythropoietin receptor; CD41, glycoprotein IIb/IIIa or αIIbβ3 integrin receptor; G-CSFR, G-CSF receptor; CMP, common myeloid progenitor; ELP, early lymphoid progenitor; GMP, granulocyte/monocyte progenitor; CLP, common lymphoid progenitor.
The differentiation of HSCs has been tracked by the expression of cell surface markers including the tyrosine kinase cytokine receptor Flt3 (6), which is absent on the HSC (7). The classical model for hematopoiesis is that committed HSCs give rise to 2 lineages, a common lymphoid progenitor capable of producing lymphocytes, and a common myeloid progenitor with developmental potential restricted to myeloid, macrophage, eosinophil, erythroid, and megakaryocyte lineages (Figure 1) (8–10). Erythroid and megakaryocyte lineages arise from a common megakaryocyte-erythroid progenitor (MEP) derived from the common myeloid progenitor (11). However, recent studies reveal that progenitors that have surface markers similar to those of HSCs, but have become Flt3-positive, upon further differentiation into lymphoid and myeloid lineages do not produce megakaryocytes or erythrocytes in vitro or in vivo (Figure 1) (12). Thus FLT3-negative HSCs express markers of committed megakaryocytes and erythroid precursors and may directly give rise to MEPs (see below and Figure 1). When early stem cells become Flt3+, erythromegakaryocytic marker expression is lost, while lymphoid and myeloid marker expression and developmental potential are retained. These findings deviate from the classical model for hematopoiesis and indicate that loss of erythromegakaryocytic potential may represent a relatively early event in HSC differentiation in certain study models. This surprising finding highlights our incomplete understanding of hematopoiesis and the plasticity of the process. The model presented in Figure 1 takes into account both the classical pathway, which predicts that HSCs split into common myeloid and lymphoid progenitors (8–10), and the newer findings suggesting a direct pathway from the HSC to the MEP (12). Improvement in fine mapping of cell lineages by flow cytometry analysis and the development of new approaches for studies of the earliest stages of hematopoiesis may further delineate the steps involved in lineage commitment under varied circumstances.
While erythroid and megakaryocyte lineages are believed to share a common MEP (8–10) (Figure 1), the signals that regulate the final separation of these lineages are not well understood. Erythroid and megakaryocytic precursors express both common and unique hematopoietic transcription factors. Among the latter, no single unique factors have been identified to determine lineage choice of the MEP. It is also possible that the final lineage of the MEP is determined by the combinatorial action of multiple nuclear proteins.
The first cells fully committed to the megakaryocyte lineage, termed CFU-Meg, are characterized by a unique cell surface phenotype (13) and form a small cluster of pure megakaryocytes in culture. CFU-Meg cells give rise to 2N megakaryocytes, which, in turn, undergo endomitosis and cytoplasmic differentiation, resulting in a pool of mature megakaryocytes recognized by their large size and characteristic morphology. In the normal human marrow, approximately 1 in 10,000 nucleated cells is a megakaryocyte, while in disorders associated with increased peripheral platelet destruction, such as immune thrombocytopenia purpura, the number increases about 10-fold (14).
Megakaryopoiesis is first noted in the embryonic yolk sac, although studies of animals with severe quantitative and qualitative platelet deficiencies, such as NF-E2–/– mice, showed that platelets are not critical for prenatal survival (15). In mice, fetal megakaryocytes appear to represent a distinct lineage with biologic features different from those of their adult counterparts (16). Several human disorders also support this point. One example is the thrombocytopenia and absent radius (TAR) syndrome, a disorder of unknown etiology characterized by moderate to severe thrombocytopenia in infancy that is typically outgrown in early childhood (17). Another example is transient myeloproliferative disorder and acute megakaryoblastic leukemia in Down syndrome, which develop nearly exclusively in the neonatal period and the first years of life, respectively (18). Interestingly, recent studies demonstrate that fetal megakaryocyte progenitors are uniquely sensitive to mutations in the transcription factor GATA-1, which accompany these disorders (19). Perhaps another example of the distinct nature of fetal/infant megakaryopoiesis is the well-known propensity of severely ill neonates to develop prolonged thrombocytopenia with slow marrow recovery of platelet production (20).
Unique aspects of megakaryocyte maturation
The hallmark of megakaryocyte development is the formation of a large cell (∼50–100 μm diameter) containing a single, large, multilobulated, polyploid nucleus (21). Eventually, each megakaryocyte releases approximately 104 platelets (22). Unlike other cells, megakaryocytes undergo an endomitotic cell cycle during which they replicate DNA but do not undergo anaphase or cytokinesis; as a result, they acquire a DNA content of up to 256N per cell (23). The mechanisms regulating endomitosis are not fully understood. Clearly cyclins are involved, though a combined knockout of cyclins D1, D2, and D3, while specifically affecting hematopoiesis and causing late midgestation fetal loss due in part to anemia, was not noted to affect megakaryopoiesis (24). On the other hand, the cyclin E–null mouse clearly had a defect in megakaryopoiesis and in development of trophoblasts, another cell line dependent on endomitosis (25). Other studies on chromosomal passenger proteins Aurora-B, survivin, and inner centromere protein showed normal levels overall in megakaryocytes (26), although one report suggests that survivin and Aurora-B may be mislocalized or absent during an important phase of endomitosis (27). The biologic importance of endoreduplication is unclear in terms of its necessity for cell size and for platelet release.
Cellular maturation of megakaryocytes is distinguished by accumulation of characteristic surface markers including GPIbα,β/GPIX/GPV receptors, a cytoplasmic demarcation system believed to participate in platelet formation, distinctive platelet organelles such as the α- and dense granules, and organelle granular proteins that participate in platelet function, such as platelet factor 4 (PF4) and vWF (28). Of note, the extent of polyploidization is not closely synchronized with cellular maturation, so that different degrees of ploidy are present at each stage. For this reason it has been hard to distinguish “early-onset” megakaryocyte-specific genes from “late-onset” ones (29).
Despite their close relationship in hematopoietic phylogeny and numerous common hematopoietic transcription factors, erythroid and megakaryocyte lineages do not share many specific proteins or organelles. However, it is interesting to note that both lineages circulate in anucleate forms. The platelet equivalent in fish is called a thrombocyte and is a circulating nucleated diploid cell (30, 31). The purpose of platelet and erythrocyte enucleation in mammals is unclear. One possibility is that loss of nuclei increases flexibility and distensibility of circulating cells, optimizing delivery of specialized functions within small-caliber capillary beds.
Cytokines involved in megakaryopoiesis
In humans, homeostatic mechanisms regulate the normal platelet count within an approximately 3-fold range (150 × 103 to 450 × 103 per cubic micrometer). Disorders that consume platelets increase their production. Numerous hematopoietic growth factors regulate different aspects of megakaryocyte biology (Figure 2). Certain cytokines, including GM-CSF, IL-3, IL-6, IL-11, IL-12, and erythropoietin (EPO), stimulate proliferation of megakaryocytic progenitors (32). Other cytokines, including IL-1α and leukemia inhibitory factor (LIF), modulate megakaryocyte maturation and platelet release (32, 33). Many of these cytokines have broad effects on all hematopoietic lineages. Presently, the multilineage cytokine IL-11 (Neumega) is the only clinically approved drug for treating thrombocytopenia (34).
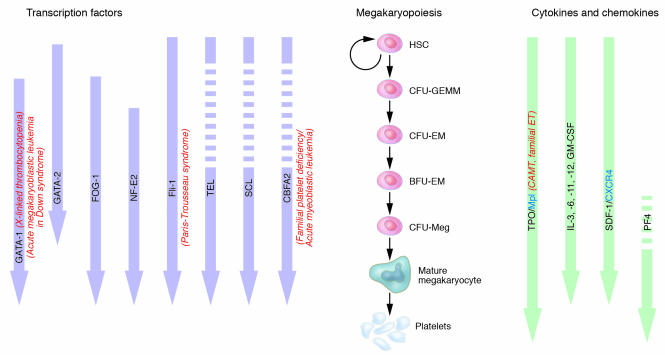
Figure 2.
Regulation of megakaryopoiesis by cytokines, chemokines, and transcription factors. In the middle panel, a scheme based on the classical pathway of megakaryopoiesis is shown. Cytokines and chemokines that influence that process are shown on the right side as green arrows to indicate the approximate level of development at which they have their influence. Open white areas in arrows indicate levels at which the cytokine is not known to act. Blue text refers to cytokine receptors of significance in megakaryopoiesis. Transcription factors that affect megakaryopoiesis are shown on the left side, and the lilac-colored arrows indicate the approximate point of their influence. Open white areas in arrows indicate levels at which the transcription factor is not known to act. Clinically relevant diseases linked to defects of these regulators are noted in red, italicized text. CFU-GEMM, CFU, granulocyte, erythrocyte, macrophage, megakaryocyte; CFU-EM, CFU, erythrocyte, megakaryocyte; BFU-EM, burst-forming unit, erythrocyte, megakaryocyte; CFU-Meg, CFU, megakaryocyte.
More than 10 years ago, a more potent and relatively specific megakaryocyte/platelet cytokine, termed Mpl ligand or thrombopoietin (TPO), was identified. This cytokine is discussed below, along with 2 chemokines, stromal cell–derived factor-1 (SDF-1; CXCL12) and PF4 (CXCL4), that have important effects on megakaryopoiesis and platelet production. A detailed Review by Kenneth Kaushansky that focuses on the role of TPO and its receptor in thrombopoiesis is part of this series on the biology of megakaryocytes and platelets (35).
TPO.
Mpl is a GP130 family member previously identified as important for megakaryocyte formation in vitro (36). This pivotal observation led to identification of the Mpl ligand, termed TPO, which was determined to markedly stimulate megakaryocyte production (37–40). TPO is highly homologous to EPO in its N-terminal half, reflecting a close evolutionary relationship between their respective receptor signaling pathways. Abrogation of either Mpl or TPO in mice decreases megakaryocyte numbers in the marrow and circulating platelets by approximately 85% (41–43). Clearly the TPO:Mpl axis is important but not essential for megakaryopoiesis. Furthermore, studies of Mpl knockout animals showed that the TPO:Mpl axis functions in early hematopoietic progenitors, including HSCs (44, 45) (Figure 1). Thus, the TPO:Mpl axis appears to be important for hematopoiesis in general and megakaryopoiesis specifically. The discovery of TPO has contributed greatly to platelet biology, because it permits relatively large and pure cultures of megakaryocytes to be generated in vitro. TPO remains under development as a potential clinical thrombopoietic and/or hematopoietic agent and as a drug to stimulate ex vivo expansion of HSCs (46).
In adults, humoral regulation of thrombopoiesis differs from that of erythropoiesis, where the kidneys produce EPO in response to tissue hypoxia. In contrast, TPO is produced constitutively, and its circulating levels are regulated by its end product, platelets. Circulating TPO is believed to control endogenous megakaryocyte numbers and platelet count. In the steady state, TPO is synthesized predominantly and constitutively in the liver (47). Mpl receptors on circulating platelets absorb TPO to negatively regulate its availability for stimulating hematopoietic progenitor cells in the marrow (48). TPO is also produced by bone marrow stromal cells (49). The relative impact of circulating versus paracrine TPO production on platelet numbers is unclear. It is possible that these different modes of production satisfy distinct hematopoietic pools in different niches.
Defects in TPO:Mpl signaling occur in several human disorders. For example, Mpl mutations, mostly causing frameshifts and early termination, occur in congenital amegakaryocytic thrombocytopenia, a rare disorder of life-threatening thrombocytopenia and megakaryocyte deficiency in infancy (50–52) (Figure 2). Given the role of Mpl in HSC development, it is also possible that congenital amegakaryocytic thrombocytopenia patients are at risk for developing more diffuse hematopoietic defects, including aplastic anemia (53). Activating mutations in the TPO gene promoter (54) and the Mpl protein (55) occur in a subset of patients with familial essential thrombocythemia (ET) (Figure 2), a disorder characterized by increased numbers of hyperaggregable platelets. In contrast, the majority of patients with the more common acquired adult, myeloproliferative form of ET harbor somatic activating mutations in the JAK2 gene (56). These mechanistic differences could explain why familial ET carries an excellent long-term prognosis that differs from the high incidence of leukemic transformation in acquired ET (57).
SDF-1.
SDF-1 enhances both megakaryopoiesis and homing of HSCs to the bone marrow during fetal development (58). SDF-1 stimulates megakaryopoiesis via TPO-independent CXCR4 receptor pathways by enhancing the chemotactic activity of their progenitors (59, 60). This activity of SDF-1 may be important for the movement of megakaryocyte progenitors from the proliferative “osteoblastic niche” to the “vascular niche” for platelet formation (61). Indeed, both in TPO–/– and in Mpl–/– mice, infusions of SDF-1 can rescue platelet production (61). There may be clinical utility for SDF-1 infusions to improve platelet production. For example, HIV may cause thrombocytopenia by infecting megakaryocyte precursors through interactions with their CXCR4 receptors (62). Thus, SDF-1 mimetic drugs may improve HIV-related thrombocytopenia by competing with the virus for its megakaryocyte receptor (63).
PF4 and other chemokines.
PF4 is an α-granule protein that inhibits megakaryocyte development and maturation in vitro (64), as other CXC and CC subfamily chemokines have subsequently been shown to do (65). The in vitro findings for PF4 are corroborated by altered platelet counts in PF4–/– mice and transgenic PF4-overexpressing mice (66). Platelet α-granules contain large stores not only of the platelet-specific chemokines PF4 and the closely related protein platelet basic protein (PBP; CXCL7), but also of other chemokines, especially RANTES (CCL5) and ENA-78 (CXCL5) (67, 68). They negatively regulate megakaryopoiesis and are mild platelet agonists through cognate receptors on developing megakaryocytes. These are weak agonists of platelet activation and also may be important in linking thrombosis and inflammation. Release of α-granular contents in the marrow could affect platelet numbers in pathologic states. For example, the discharge of chemokines during chemotherapy or radiation therapy may contribute to thrombocytopenia that occurs during these treatment modalities. In this case, strategies to inhibit this process could be used preemptively to prevent thrombocytopenia.
Transcription factors involved in megakaryopoiesis
Megakaryopoiesis is regulated by multiple cytokines influencing the survival and proliferation of increasingly committed progenitors as they transition from one hematopoietic niche to another in an organized fashion (61). During this process, a series of transcription factors coordinately regulate the chromatin organization of megakaryocyte-specific genes and prime them for expression en route to platelet formation. Some of these events are beginning to be understood, especially the hematopoietic-specific transcription factor complexes involved in terminal differentiation. Numerous nuclear proteins with important roles in megakaryocyte formation, growth regulation, and platelet release have been identified, mainly through loss-of-function studies in mice and analysis of human diseases. Important examples are discussed below.
GATA-1/FOG-1 complex.
GATA-1 was first isolated as an essential 2–zinc finger, erythroid transcription factor that binds the DNA sequence WGATAR (69) (Figure 3). GATA-1 is also expressed and of functional consequence in megakaryocytes, mast cells, and eosinophils (70, 71). Transient expression reporter gene studies of megakaryocyte-specific proximal promoters defined several functionally important GATA-binding sites (72–74). A point mutation in a GATA-binding site of the GP1bb proximal promoter region causes a form of Bernard-Soulier syndrome (74).
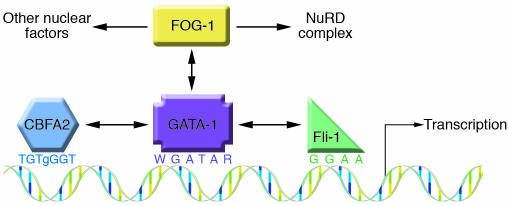
Figure 3.
Assembly of transcription factor complex at a megakaryocyte-specific gene. A schematic representation of the proximal promoter region of a hypothetical megakaryocyte-specific gene modeled after the Itga2b gene. GATA-1 is shown in the middle binding to its known consensus sequence (69) and interacting with FOG-1, Fli-1, and CBFA2 of the RUNX complex (each interaction is indicated by a 2-headed arrow). Fli-1 and CBFA2 bind to their adjacent cognate on the DNA (89, 122), while FOG-1 is recruited through its interactions with the N-terminal zinc finger of GATA-1. In turn, FOG-1 recruits the NuRD complex and other nuclear factors.
While targeted disruption of the GATA-1 gene in mice causes embryonic lethality due to anemia (75), a megakaryocyte-specific knock down of GATA-1 expression results in significant thrombocytopenia and increased numbers of immature and dysmorphic megakaryocytes (76). GATA-2 is a closely related transcription factor and is also hematopoietic-specific, but it is expressed earlier and participates in maintenance of HSCs and multipotential progenitors (77). GATA-1 and GATA-2 are believed to have both overlapping and unique functions (69, 78). Continued GATA-2 expression during early megakaryopoiesis may explain the partial ability for platelet formation in the GATA-1 knockdown mouse (Figure 2).
FOG-1 (Friend of GATA-1, or Zfpm1) is a 9–zinc finger, hematopoietic-specific transcription factor isolated as a GATA-1 binding partner (79) (Figure 3). Targeted disruption of the FOG-1 gene markedly inhibits erythroid development, causing embryonic death from severe anemia. Detailed study of these animals and FOG-1–/– ES cells also demonstrated an early block to megakaryocytic development with no identifiable precursors. FOG-1 does not appear to bind DNA directly but, rather, associates with target genes indirectly through interactions with GATA proteins (Figure 3). FOG-1 is the only protein of its kind expressed in the erythromegakaryocytic lineages. The severity of the megakaryocyte defect in the FOG-1–/– mouse suggests that most or all critical GATA-1– and GATA-2–related activities require interactions with FOG-1. In support of this, GATA-1 and FOG-1 synergistically enhance the expression of the megakaryocyte-specific αIIb gene (80, 81). These observations have been expanded to show that direct contact is needed between the N-terminal zinc finger of GATA-1 and FOG-1. This synergism applies to multiple megakaryocyte-specific genes and also involves a specific Ets family transcription factor (see below). The N-terminus of FOG-1 plays a unique, nonredundant role in megakaryocyte-specific expression (82), possibly through its ability to recruit the corepressor complex NuRD (83). The clinical importance of the GATA-1/FOG-1 interaction in megakaryopoiesis is demonstrated by the identification of patients with X-linked thrombocytopenia and variable anemia who have GATA-1 mutations that impair FOG-1 binding (84–86) (Figure 2).
An important role for GATA-1 in regulating the maturation and proliferation of megakaryocyte progenitors is further evidenced by the recent discovery of acquired somatic mutations associated with both megakaryoblastic leukemia and transient myeloproliferative syndrome in infants with Down syndrome (87, 88). These mutations occur in the first coding exon, causing early termination and production of a truncated protein, termed GATA-1Short, via translation initiation from a downstream internal methionine. This mutant form of GATA-1 may act as a dominant oncogene by specifically stimulating the proliferation of fetal megakaryocyte progenitors (19).
Fli-1 and TEL.
The proximal promoters of many megakaryocyte-specific genes contain tandem-binding sites for GATA and Ets proteins, suggesting functional interactions between these 2 classes of transcription factors (71–73) (Figure 3). The Ets family is diverse with at least 30 members, all sharing an Ets-binding domain that recognizes a GGAA core sequence (89). Numerous Ets members are present in primary megakaryocytes and/or megakaryocytic cell lines (90–92). While a number of reports suggest a function for Ets-1 in megakaryopoiesis (93), the clearest story of a common transcriptional regulator appears to be that for Fli-1, an Ets transcription factor initially recognized to be important for T cell differentiation (94) and early hematopoiesis/vasculogenesis (95) (Figure 2). GATA-1/FOG-1 synergy for many megakaryocyte-specific genes appears to involve Fli-1 (81), and Fli-1 binds to the proximal promoter of these genes in vivo. The molecular basis of this synergy is still not fully known, but Fli-1 does bind GATA-1 (96) (Figure 3). In undifferentiated hematopoietic cell lines, overexpressed Fli-1 can induce megakaryocytic features (91). Moreover, Fli1 gene–disrupted mice either have abnormal megakaryocytes with thrombocytopenia (97) or fail to develop recognizable megakaryocytes (98), depending on the size of the Fli-1 gene deletion. Fli-1 expression also inhibits erythroid differentiation (99). Thus, Fli-1 may be a lineage-determining factor for megakaryocyte development. Hemizygous deficiency of Fli-1 expression causes thrombocytopenia associated with abnormal megakaryocytes in patients with Paris-Trousseau syndrome (100).
TEL, or ETV6, another Ets protein in the pointed domain subfamily, is closely related to Fli-1 and may also function in megakaryocytopoiesis (101). The pointed domain of TEL is a short N-terminal domain involved in self-oligomerization. Like Fli-1, TEL is important in early HSCs (102) (Figure 2), and TEL overexpression can drive megakaryocyte differentiation of hematopoietic cell lines (92). Remarkably, conditional disruption of the TEL gene demonstrated a unique, nonredundant role for TEL in megakaryopoiesis (103). Specifically, loss of TEL in the erythromegakaryocyte lineage results in large, highly proliferative megakaryocytes and mild thrombocytopenia. This phenotype resembles that of GATA-1 deficiency and also has overlapping features with the NF-E2 knockout phenotype described below.
NF-E2.
NF-E2 is a hematopoietic-specific transcription factor consisting of a tissue-specific p45 leucine zipper–containing subunit dimerized with a ubiquitous p18 subunit (104). In vitro studies suggested an important role for NF-E2 erythroid gene expression. Surprisingly, Nfe2-null mice do not develop anemia but, rather, exhibit severe thrombocytopenia with a marrow containing excessive immature, dysplastic megakaryocytes (15) (Figure 2). The molecular basis for this effect on megakaryocyte differentiation and platelet release has yet to be resolved. It may be that intracellular signaling pathways related to Rab27b (105) or cytoskeletal proteins (106) are underexpressed in the absence of p45, causing impaired proplatelet formation and platelet release.
SCL.
SCL, or TAL1, is a basic helix-loop-helix transcription factor, initially identified in human T cell leukemias with multilineage characteristics (107), and has also been implicated in the earliest stages of hematopoiesis/vasculogenesis in the mouse embryo (108, 109) (Figure 2). LacZ knock-in studies suggest that SCL is expressed in myeloid, lymphoid, erythroid, and megakaryocytic lineages (110). In spite of this widespread expression, conditional disruption of the TAL1 gene in late-stage hematopoiesis demonstrated an absolute requirement only in the erythromegakaryocytic lineages of the yolk sac and fetal liver (111).
RUNX1.
RUNX1 is a hematopoietic/vasculogenic–specific protein first noted because of its involvement in several leukemic chromosomal translocations, particularly t(8;21), which generates the AML1-ETO fusion protein (112). RUNX1 is a heterodimer of core-binding factor α-2 (CBFA2) that binds DNA and the β subunit CBFB, which does not directly bind DNA (113). RUNX1 was initially thought to be solely involved in myeloid differentiation (114), but studies on targeting of the CBFA2 gene demonstrated an essential role for RUNX1 in early hematopoiesis and vasculogenesis (115, 116). A unique role for RUNX1 in adult megakaryopoiesis was established when a rare, dominantly inherited thrombocytopenia associated with an increased risk of developing acute myeloblastic leukemia was shown to be due to haploinsufficiency of CBFA2 (117, 118) (Figure 2). A role for CBFA2 in adult megakaryopoiesis was also confirmed in mice (119). RUNX1 appears to interact with GATA-1 (120) (Figure 3), and overexpression of RUNX1 can drive hematopoietic cell lines into a megakaryocytic phenotype (121), suggesting a role in lineage determination.
Conclusions
Mechanisms that regulate formation of the erythromegakaryocytic precursor and its commitment to unilineage megakaryocyte development are active areas of investigation. The discovery of new cytokines and transcription factors associated with megakaryopoiesis has enhanced our understanding of normal platelet development and human thrombocytopenias. One current challenge is to better define the developmental pathways though which MEPs and megakaryocytes arise from HSCs. For example, a recent finding that the onset of Flt3 receptor expression in early hematopoiesis coincides with loss of erythromegakaryocytic capacity suggests novel pathways for MEP formation.
Additionally, comparative studies of developmental hematopoiesis in embryos and adults should extend our understanding of normal and pathologic megakaryopoiesis at distinct developmental stages. In addition, recent studies support a central role for multifactor transcriptional complexes containing GATA-1, FOG-1, and Fli-1 in terminal megakaryocytic differentiation. Formation and regulation of these complexes may illustrate a mechanism that initiates megakaryocyte commitment from the MEP. Further studies into all of these various areas of megakaryopoiesis promises to provide new insights into numerous hematopoietic disorders and may also have broader clinical applications by elucidating novel strategies to regulate platelet count and/or platelet thrombogenicity.
Footnotes
Nonstandard abbreviations used: CBFA2, core-binding factor α-2; EPO, erythropoietin; ET, essential thrombocythemia; MEP, megakaryocyte-erythroid progenitor; PF4, platelet factor 4; SDF-1, stromal cell–derived factor-1; TPO, thrombopoietin.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 2.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 3.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CL, Johnson GR. Murine hematopoietic stem and progenitor cells. I. Enrichment and biologic characterization. Blood. 1995;85:1472–1479. [PubMed] [Google Scholar]
- 5.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 6.Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991;6:1641–1650. [PubMed] [Google Scholar]
- 7.Adolfsson J, et al. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 8.Kanz L, Straub G, Bross KG, Fauser AA. Identification of human megakaryocytes derived from pure megakaryocytic colonies (CFU-M), megakaryocytic-erythroid colonies (CFU-M/E), and mixed hemopoietic colonies (CFU-GEMM) by antibodies against platelet associated antigens. Blut. 1982;45:267–274. doi: 10.1007/BF00320194. [DOI] [PubMed] [Google Scholar]
- 9.Nakahata T, Gross AJ, Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J. Cell. Physiol. 1982;113:455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- 10.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 11.Debili N. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood. 1996;88:1284–1296. [PubMed] [Google Scholar]
- 12.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:205–210. doi: 10.1073/pnas.262655099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branehog I, Ridell B, Swolin B, Weinfeld A. Megakaryocyte quantifications in relation to thrombokinetics in primary thrombocythaemia and allied diseases. Scand. J. Haematol. 1975;15:321–332. doi: 10.1111/j.1600-0609.1975.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 15.Shivdasani RA, et al. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 16.Palis J, Koniski A. Analysis of hematopoietic progenitors in the mouse embryo. Methods Mol. Med. 2004;105:289–302. doi: 10.1385/1-59259-826-9:289. [DOI] [PubMed] [Google Scholar]
- 17.al-Jefri AH, Dror Y, Bussel JB, Freedman MH. Thrombocytopenia with absent radii: frequency of marrow megakaryocyte progenitors, proliferative characteristics, and megakaryocyte growth and development factor responsiveness. Pediatr. Hematol. Oncol. 2000;17:299–306. doi: 10.1080/088800100276280. [DOI] [PubMed] [Google Scholar]
- 18.Crispino JD. GATA1 mutations in Down syndrome: implications for biology and diagnosis of children with transient myeloproliferative disorder and acute megakaryoblastic leukemia. Pediatr. Blood Cancer. 2005;44:40–44. doi: 10.1002/pbc.20066. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, et al. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat. Genet. 2005;37:613–619. doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 20.Sola MC. Evaluation and treatment of severe and prolonged thrombocytopenia in neonates. Clin. Perinatol. 2004;31:1–14. doi: 10.1016/j.clp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Cajano A, Polosa P. Contribution to the study of the morphology of megakaryocytes and blood platelets with Feulgen’s test. Haematologica. 1950;34:1113–11121. [PubMed] [Google Scholar]
- 22.Long MW. Megakaryocyte differentiation events. Semin. Hematol. 1998;35:192–199. [PubMed] [Google Scholar]
- 23.Odell TT, Jr, Jackson CW, Gosslee DG. Maturation of rat megakaryocytes studied by microspectrophotometric measurement of DNA. Proc. Soc. Exp. Biol. Med. 1965;119:1194–1199. doi: 10.3181/00379727-119-30412. [DOI] [PubMed] [Google Scholar]
- 24.Kozar K. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Geng Y. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 26.Geddis AE, Kaushansky K. Megakaryocytes express functional Aurora-B kinase in endomitosis. Blood. 2004;104:1017–1024. doi: 10.1182/blood-2004-02-0419. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Aberrant quantity and localization of Aurora-B/AIM-1 and survivin during megakaryocyte polyploidization and the consequences of Aurora-B/AIM-1-deregulated expression. Blood. 2004;103:3717–3726. doi: 10.1182/blood-2003-09-3365. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt A, Guichard J, Masse JM, Debili N, Cramer EM. Of mice and men: comparison of the ultrastructure of megakaryocytes and platelets. Exp. Hematol. 2001;29:1295–1302. doi: 10.1016/s0301-472x(01)00733-0. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre P, Winter JN, Meng Y, Cohen I. Ex vivo expansion of early and late megakaryocyte progenitors. J. Hematother. Stem Cell Res. 2000;9:913–921. doi: 10.1089/152581600750062363. [DOI] [PubMed] [Google Scholar]
- 30.Jagadeeswaran P, Sheehan JP, Craig FE, Troyer D. Identification and characterization of zebrafish thrombocytes. Br. J. Haematol. 1999;107:731–738. doi: 10.1046/j.1365-2141.1999.01763.x. [DOI] [PubMed] [Google Scholar]
- 31.Gregory M, Jagadeeswaran P. Selective labeling of zebrafish thrombocytes: quantitation of thrombocyte function and detection during development. Blood Cells Mol. Dis. 2002;28:418–427. doi: 10.1006/bcmd.2002.0527. [DOI] [PubMed] [Google Scholar]
- 32.Gordon MS, Hoffman R. Growth factors affecting human thrombocytopoiesis: potential agents for the treatment of thrombocytopenia. Blood. 1992;80:302–307. [PubMed] [Google Scholar]
- 33.Vainchenker W, Debili N, Mouthon MA, Wendling F. Megakaryocytopoiesis: cellular aspects and regulation. Crit. Rev. Oncol. Hematol. 1995;20:165–192. doi: 10.1016/1040-8428(94)00159-q. [DOI] [PubMed] [Google Scholar]
- 34.Orazi A, et al. Effects of recombinant human interleukin-11 (Neumega rhIL-11 growth factor) on megakaryocytopoiesis in human bone marrow. Exp. Hematol. 1996;24:1289–1297. [PubMed] [Google Scholar]
- 35.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J. Clin. Invest. 2005;115:3339–3347. doi:10.1172/JCI26674. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Methia N, Louache F, Vainchenker W, Wendling F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood. 1993;82:1395–1401. [PubMed] [Google Scholar]
- 37.Bartley TD, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77:1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 38.Lok S, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 39.Kaushansky K, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 40.de Sauvage FJ, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 41.Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 42.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 43.Murone M, Carpenter DA, de Sauvage FJ. Hematopoietic deficiencies in c-mpl and TPO knockout mice. Stem Cells. 1998;16:1–6. doi: 10.1002/stem.160001. [DOI] [PubMed] [Google Scholar]
- 44.Debili N, et al. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood. 1995;86:2516–2525. [PubMed] [Google Scholar]
- 45.Young JC, et al. Thrombopoietin stimulates megakaryocytopoiesis, myelopoiesis, and expansion of CD34+ progenitor cells from single CD34+Thy-1+Lin- primitive progenitor cells. Blood. 1996;88:1619–1631. [PubMed] [Google Scholar]
- 46.Basser R. The impact of thrombopoietin on clinical practice. Curr. Pharm. Des. 2002;8:369–377. doi: 10.2174/1381612023395989. [DOI] [PubMed] [Google Scholar]
- 47.Jelkmann W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur. J. Gastroenterol. Hepatol. 2001;13:791–801. doi: 10.1097/00042737-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Kaushansky K. Thrombopoietin: understanding and manipulating platelet production. Annu. Rev. Med. 1997;48:1–11. doi: 10.1146/annurev.med.48.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Guerriero A, et al. Thrombopoietin is synthesized by bone marrow stromal cells. Blood. 1997;90:3444–3455. [PubMed] [Google Scholar]
- 50.Ihara K, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3132–3136. doi: 10.1073/pnas.96.6.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Oudenrijn S, et al. Mutations in the thrombopoietin receptor, Mpl, in children with congenital amegakaryocytic thrombocytopenia. Br. J. Haematol. 2000;110:441–448. doi: 10.1046/j.1365-2141.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- 52.Ballmaier M, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97:139–146. doi: 10.1182/blood.v97.1.139. [DOI] [PubMed] [Google Scholar]
- 53.Ballmaier M, Germeshausen M, Krukemeier S, Welte K. Thrombopoietin is essential for the maintenance of normal hematopoiesis in humans: development of aplastic anemia in patients with congenital amegakaryocytic thrombocytopenia. Ann. N. Y. Acad. Sci. 2003;996:17–25. doi: 10.1111/j.1749-6632.2003.tb03228.x. [DOI] [PubMed] [Google Scholar]
- 54.Ghilardi N, Skoda RC. A single-base deletion in the thrombopoietin (TPO) gene causes familial essential thrombocythemia through a mechanism of more efficient translation of TPO mRNA. Blood. 1999;94:1480–1482. [PubMed] [Google Scholar]
- 55.Ding J, et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood. 2004;103:4198–4200. doi: 10.1182/blood-2003-10-3471. [DOI] [PubMed] [Google Scholar]
- 56.Kaushansky K. On the molecular origins of the chronic myeloproliferative disorders: it all makes sense. Blood. 2005;105:4187–4190. doi: 10.1182/blood-2005-03-1287. [DOI] [PubMed] [Google Scholar]
- 57.Barbui T. The leukemia controversy in myeloproliferative disorders: is it a natural progression of disease, a secondary sequela of therapy, or a combination of both? Semin. Hematol. 2004;41:15–17. doi: 10.1053/j.seminhematol.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Wang JF, Liu ZY, Groopman JE. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–764. [PubMed] [Google Scholar]
- 59.Majka M, et al. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. Blood. 2000;96:4142–4151. [PubMed] [Google Scholar]
- 60.Kowalska MA, et al. Megakaryocyte precursors, megakaryocytes and platelets express the HIV co-receptor CXCR4 on their surface: determination of response to stromal-derived factor-1 by megakaryocytes and platelets. Br. J. Haematol. 1999;104:220–229. doi: 10.1046/j.1365-2141.1999.01169.x. [DOI] [PubMed] [Google Scholar]
- 61.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 62.Lee B, Ratajczak J, Doms RW, Gewirtz AM, Ratajczak MZ. Coreceptor/chemokine receptor expression on human hematopoietic cells: biological implications for human immunodeficiency virus-type 1 infection. Blood. 1999;93:1145–1156. [PubMed] [Google Scholar]
- 63.Seibert C, Sakmar TP. Small-molecule antagonists of CCR5 and CXCR4: a promising new class of anti-HIV-1 drugs. Curr. Pharm. Des. 2004;10:2041–2062. doi: 10.2174/1381612043384312. [DOI] [PubMed] [Google Scholar]
- 64.Gewirtz AM, Calabretta B, Rucinski B, Niewiarowski S, Xu WY. Inhibition of human megakaryocytopoiesis in vitro by platelet factor 4 (PF4) and a synthetic COOH-terminal PF4 peptide. J. Clin. Invest. 1989;83:1477–1486. doi: 10.1172/JCI114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gewirtz AM, et al. Chemokine regulation of human megakaryocytopoiesis. Blood. 1995;86:2559–2567. [PubMed] [Google Scholar]
- 66.Eslin DE, et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104:3173–3180. doi: 10.1182/blood-2003-11-3994. [DOI] [PubMed] [Google Scholar]
- 67.Kowalska MA, et al. Stromal cell-derived factor-1 and macrophage-derived chemokine: 2 chemokines that activate platelets. Blood. 2000;96:50–57. [PubMed] [Google Scholar]
- 68.Clemetson KJ, et al. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- 69.Weiss MJ, Orkin SH. GATA transcription factors: key regulators of hematopoiesis. Exp. Hematol. 1995;23:99–107. [PubMed] [Google Scholar]
- 70.Zon LI, et al. Expression of mRNA for the GATA-binding proteins in human eosinophils and basophils: potential role in gene transcription. Blood. 1993;81:3234–3241. [PubMed] [Google Scholar]
- 71.Lemarchandel V, Ghysdael J, Mignotte V, Rahuel C, Romeo PH. GATA and Ets cis-acting sequences mediate megakaryocyte-specific expression. Mol. Cell. Biol. 1993;13:668–676. doi: 10.1128/mcb.13.1.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin F, Prandini MH, Thevenon D, Marguerie G, Uzan G. The transcription factor GATA-1 regulates the promoter activity of the platelet glycoprotein IIb gene. J. Biol. Chem. 1993;268:21606–21612. [PubMed] [Google Scholar]
- 73.Deveaux S, et al. Analysis of the thrombopoietin receptor (MPL) promoter implicates GATA and Ets proteins in the coregulation of megakaryocyte-specific genes. Blood. 1996;87:4678–4685. [PubMed] [Google Scholar]
- 74.Ludlow LB, et al. Identification of a mutation in a GATA binding site of the platelet glycoprotein Ibbeta promoter resulting in the Bernard-Soulier syndrome. J. Biol. Chem. 1996;271:22076–22080. doi: 10.1074/jbc.271.36.22076. [DOI] [PubMed] [Google Scholar]
- 75.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai FY, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 78.Fujiwara Y, Chang AN, Williams AM, Orkin SH. Functional overlap of GATA-1 and GATA-2 in primitive hematopoietic development. Blood. 2004;103:583–585. doi: 10.1182/blood-2003-08-2870. [DOI] [PubMed] [Google Scholar]
- 79.Tsang AP, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 80.Gaines P, Geiger JN, Knudsen G, Seshasayee D, Wojchowski DM. GATA-1- and FOG-dependent activation of megakaryocytic alpha IIB gene expression. J. Biol. Chem. 2000;275:34114–34121. doi: 10.1074/jbc.M006017200. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, et al. Control of megakaryocyte-specific gene expression by GATA-1 and FOG-1: role of Ets transcription factors. EMBO J. 2002;21:5225–5234. doi: 10.1093/emboj/cdf527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantor AB, Katz SG, Orkin SH. Distinct domains of the GATA-1 cofactor FOG-1 differentially influence erythroid versus megakaryocytic maturation. Mol. Cell. Biol. 2002;22:4268–4279. doi: 10.1128/MCB.22.12.4268-4279.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong W, et al. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 2005;24:2367–2378. doi: 10.1038/sj.emboj.7600703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nichols KE, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat. Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu C, et al. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood. 2002;100:2040–2045. doi: 10.1182/blood-2002-02-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehaffey MG, Newton AL, Gandhi MJ, Crossley M, Drachman JG. X-linked thrombocytopenia caused by a novel mutation of GATA-1. Blood. 2001;98:2681–2688. doi: 10.1182/blood.v98.9.2681. [DOI] [PubMed] [Google Scholar]
- 87.Wechsler J, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat. Genet. 2002;32:148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 88.Greene ME, et al. Mutations in GATA1 in both transient myeloproliferative disorder and acute megakaryoblastic leukemia of Down syndrome. Blood Cells Mol. Dis. 2003;31:351–356. doi: 10.1016/j.bcmd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 90.Terui K, et al. Expression of transcription factors during megakaryocytic differentiation of CD34+ cells from human cord blood induced by thrombopoietin. Tohoku J. Exp. Med. 2000;192:259–273. doi: 10.1620/tjem.192.259. [DOI] [PubMed] [Google Scholar]
- 91.Athanasiou M, et al. Increased expression of the ETS-related transcription factor FLI-1/ERGB correlates with and can induce the megakaryocytic phenotype. Cell Growth Differ. 1996;7:1525–1534. [PubMed] [Google Scholar]
- 92.Sakurai T, et al. Effects of overexpression of the Ets family transcription factor TEL on cell growth and differentiation of K562 cells. Int. J. Oncol. 2003;22:1327–1333. [PubMed] [Google Scholar]
- 93.Jackers P, Szalai G, Moussa O, Watson DK. Ets-dependent regulation of target gene expression during megakaryopoiesis. J. Biol. Chem. 2004;279:52183–52190. doi: 10.1074/jbc.M407489200. [DOI] [PubMed] [Google Scholar]
- 94.Anderson MK, Hernandez-Hoyos G, Diamond RA, Rothenberg EV. Precise developmental regulation of Ets family transcription factors during specification and commitment to the T cell lineage. Development. 1999;126:3131–3148. doi: 10.1242/dev.126.14.3131. [DOI] [PubMed] [Google Scholar]
- 95.Brown LA, et al. Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech. Dev. 2000;90:237–252. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 96.Eisbacher M, et al. Protein-protein interaction between Fli-1 and GATA-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative DNA binding. Mol. Cell. Biol. 2003;23:3427–3441. doi: 10.1128/MCB.23.10.3427-3441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hart A, et al. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–177. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 98.Kawada H, et al. Defective megakaryopoiesis and abnormal erythroid development in Fli-1 gene-targeted mice. Int. J. Hematol. 2001;73:463–468. doi: 10.1007/BF02994008. [DOI] [PubMed] [Google Scholar]
- 99.Athanasiou M, Mavrothalassitis G, Sun-Hoffman L, Blair DG. FLI-1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia. 2000;14:439–445. doi: 10.1038/sj.leu.2401689. [DOI] [PubMed] [Google Scholar]
- 100.Raslova H, et al. FLI1 monoallelic expression combined with its hemizygous loss underlies Paris-Trousseau/Jacobsen thrombopenia. J. Clin. Invest. 2004;114:77–84. doi:10.1172/JCI200421179. doi: 10.1172/JCI21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mackereth CD, et al. Diversity in structure and function of the Ets family PNT domains. J. Mol. Biol. 2004;342:1249–1264. doi: 10.1016/j.jmb.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 102.Wang LC, et al. The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow. Genes Dev. 1998;12:2392–2402. doi: 10.1101/gad.12.15.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hock H, et al. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–2341. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993;362:722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- 105.Tiwari S, et al. A role for Rab27b in NF-E2-dependent pathways of platelet formation. Blood. 2003;102:3970–3979. doi: 10.1182/blood-2003-03-0977. [DOI] [PubMed] [Google Scholar]
- 106.Lecine P, Italiano JE, Jr, Kim SW, Villeval JL, Shivdasani RA. Hematopoietic-specific beta 1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood. 2000;96:1366–1373. [PubMed] [Google Scholar]
- 107.Aplan PD, Lombardi DP, Kirsch IR. Structural characterization of SIL, a gene frequently disrupted in T-cell acute lymphoblastic leukemia. Mol. Cell. Biol. 1991;11:5462–5469. doi: 10.1128/mcb.11.11.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 109.Visvader JE, Fujiwara Y, Orkin SH. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 1998;12:473–479. doi: 10.1101/gad.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elefanty AG, et al. Characterization of hematopoietic progenitor cells that express the transcription factor SCL, using a lacZ “knock-in” strategy. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11897–11902. doi: 10.1073/pnas.95.20.11897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schlaeger TM, Mikkola HK, Gekas C, Helgadottir HB, Orkin SH. Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood. 2005;105:3871–3874. doi: 10.1182/blood-2004-11-4467. [DOI] [PubMed] [Google Scholar]
- 112.Nucifora G, Rowley JD. AML1 and the 8;21 and 3;21 translocations in acute and chronic myeloid leukemia. Blood. 1995;86:1–14. [PubMed] [Google Scholar]
- 113.Speck NA, et al. Core-binding factor: a central player in hematopoiesis and leukemia. Cancer Res. 1999;59(Suppl. 7):1789s–1793s. [PubMed] [Google Scholar]
- 114.Zhang DE, et al. Function of PU.1 (Spi-1), C/EBP, and AML1 in early myelopoiesis: regulation of multiple myeloid CSF receptor promoters. Curr. Top. Microbiol. Immunol. 1996;211:137–147. doi: 10.1007/978-3-642-85232-9_14. [DOI] [PubMed] [Google Scholar]
- 115.Takakura N, et al. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 116.North T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 117.Song WJ, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat. Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 118.Michaud J, et al. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 2002;99:1364–1372. doi: 10.1182/blood.v99.4.1364. [DOI] [PubMed] [Google Scholar]
- 119.Ichikawa M, et al. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat. Med. 2004;10:299–304. doi: 10.1038/nm997. [DOI] [PubMed] [Google Scholar]
- 120.Elagib KE, et al. RUNX1 and GATA-1 coexpression and cooperation in megakaryocytic differentiation. Blood. 2003;101:4333–4341. doi: 10.1182/blood-2002-09-2708. [DOI] [PubMed] [Google Scholar]
- 121.Niitsu N, et al. AML1a but not AML1b inhibits erythroid differentiation induced by sodium butyrate and enhances the megakaryocytic differentiation of K562 leukemia cells. Cell Growth Differ. 1997;8:319–326. [PubMed] [Google Scholar]
- 122.Westendorf JJ, Hiebert SW. Mammalian runt-domain proteins and their roles in hematopoiesis, osteogenesis, and leukemia [review] J. Cell. Biochem. 1999;75(Suppl.):51–58. doi: 10.1002/(sici)1097-4644(1999)75:32+<51::aid-jcb7>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]