Abstract
The platelet integrin αIIbβ3 is required for platelet aggregation. Like other integrins, αIIbβ3 resides on cell surfaces in an equilibrium between inactive and active conformations. Recent experiments suggest that the shift between these conformations involves a global reorganization of the αIIbβ3 molecule and disruption of constraints imposed by the heteromeric association of the αIIb and β3 transmembrane and cytoplasmic domains. The biochemical, biophysical, and ultrastructural results that support this conclusion are discussed in this Review.
Integrins are ubiquitous transmembrane α/β heterodimers that mediate diverse processes requiring cell-matrix and cell-cell interactions such as tissue migration during embryogenesis, cellular adhesion, cancer metastases, and lymphocyte helper and killer cell functions (1). Eighteen integrin α subunits and 8 integrin β subunits have been identified in mammals that combine to form 24 different heterodimers. The resulting heterodimers can then be grouped into subfamilies according to the identity of their β subunit (1). Platelets express 3 members of the β1 subfamily (αIIβ1, αvβ1, and αvIβ1) that support platelet adhesion to the ECM proteins collagen, fibronectin, and laminin, respectively (2–5), and both members of the β3 subfamily (αvβ3 and αIIbβ3). Although αvβ3 mediates platelet adhesion to osteopontin and vitronectin in vitro (6, 7), it is uncertain whether it plays a role in platelet function in vivo. By contrast, αIIbβ3, a receptor for fibrinogen, vWF, fibronectin, and vitronectin, is absolutely required for platelet aggregation. Consequently, inherited abnormalities in αIIbβ3 number or function preclude platelet aggregation, resulting in the bleeding disorder Glanzmann thrombasthenia (8). Conversely, thrombi that arise in the arterial circulation result from the αIIbβ3-mediated formation of platelet aggregates (9). Because αIIbβ3 plays an indispensable role in hemostasis and thrombosis, it is among the most intensively studied integrins. Thus, there is a wealth of new information relating αIIbβ3 structure and function, the subject of this Review.
Expression of αIIbβ3 is restricted to cells of the megakaryocyte lineage. In megakaryocytes, αIIbβ3 is assembled from αIIb and β3 precursors in the endoplasmic reticulum (10) and undergoes posttranslational processing in the Golgi complex, where αIIb is cleaved into heavy and light chains (11). There are approximately 80,000 copies of αIIbβ3 on the surface of unstimulated platelets (12), and additional heterodimers in the membranes of platelet granules are translocated to the platelet surface during platelet secretion (13). A critical feature of αIIbβ3 function is that it is modulated by platelet agonists. Thus, while αIIbβ3 can support the adhesion of unstimulated platelets to many of its ligands when they are immobilized in vitro, platelet stimulation is required to enable αIIbβ3 to mediate platelet aggregation by binding soluble fibrinogen and vWF (14). EM images of rotary-shadowed αIIbβ3 reveal that the heterodimer consists of an 8-by-12-nm nodular head containing its ligand-binding site and two 18-nm flexible stalks containing its transmembrane (TM) and cytoplasmic domains (15).
Crystal structures for the extracellular portions of αvβ3 and αIIbβ3
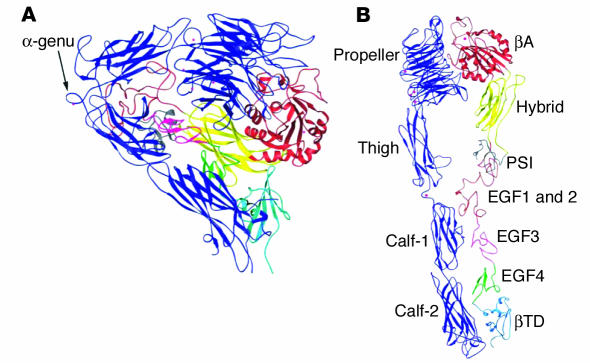
A major advance in understanding the structure and function of αIIbβ3 resulted from the reports of crystal structures for the extracellular portions of αIIbβ3 (16) and the closely related integrin αvβ3 (17). Xiong and coworkers prepared crystals of a presumably activated conformation of the αvβ3 extracellular region grown in the presence of Ca2+ (17). Surprisingly, the crystals revealed that the head region was severely bent over 2 nearly parallel tails (Figure 1). When the structure was extended, its appearance and dimensions were consistent with rotary-shadowed EM images of αIIbβ3. The structure itself revealed that the amino terminus of αv was folded into a β-propeller configuration, followed by a “thigh” and 2 “calf” domains, constituting the extracellular portion of the αv stalk. The αv “knee” or “genu,” the site at which the head region bends, was located between the thigh and first calf domain. The β3 head consists of a βA domain whose fold resembles that of integrin α subunit “I-domains” and contains a metal ion–dependent adhesion site (MIDAS) motif, as well as a hybrid domain whose fold is similar to that of I-set Ig domains. The interface between the αv β-propeller and the β3 βA domain, the site at which the αv head interacts with the β3 head, resembles the interface between the Gα and Gβ subunits of G proteins. The β3 stalk consists of a PSI (plexin, semaphorin, integrin) domain, 4 tandem EGF repeats, and a unique carboxyterminal βTD domain. A cyclic Arg-Gly-Asp–containing (RGD-containing) pentapeptide, soaked into the crystal in the presence of Mn2+ (18), inserted into a crevice between the β-propeller and βA domains with the Arg side chain located in a groove on the upper surface of the propeller and the Asp carboxylate protruding into a cleft between loops on the βA surface, implying that the crevice constitutes at least a portion of the binding site for RGD-containing αvβ3 ligands.
Figure 1.
Ribbon diagram of the structure of the extracellular portion of αvβ3. (A) Bent conformation of αvβ3 as it was present in the crystal. (B) Extension of the structure to reveal its domains. Adapted with permission from Annual Review of Cell and Developmental Biology (97).
Subsequently, Xiao et al. reported 2 crystal structures of a complex consisting of the αIIb β-propeller and the β3 βA, hybrid, and PSI domains (16). The structures revealed an open, presumably high-affinity conformation, similar to EM images of the αvβ3 extracellular domain–containing ligand, with a 62° angle of separation between the α and β subunits due in part to a 10-A downward movement of the α7 helix of the βA domain relative to the hybrid and the PSI domain. Reorganization of hydrogen bonds in the interface between the α7 helix and βC strand of the hybrid domain allowed the hybrid domain and the rigidly connected PSI domain to swing out, causing a 70-Å separation of the αIIb and β3 stalks at their “knees,” a feature noted in EM images of active forms of αIIbβ3 in the presence or absence of ligand (19).
Ligand binding to αIIbβ3
Fibrinogen, the major αIIbβ3 ligand, is composed of pairs of Aα, Bβ, and γ chains folded into 3 nodular domains. Although peptides corresponding to either the carboxyterminal 10–15 amino acids of the γ chain (20) or the 2 α chain RGD motifs inhibit fibrinogen binding to αIIbβ3 (21), only the γ chain sequence is required for fibrinogen binding to αIIbβ3 (22). Nonetheless, RGD-based peptides and peptidomimetics inhibit αIIbβ3 function in vitro and are clinically effective antagonists of αIIbβ3 function in vivo (23). The structural basis for these observations is not entirely clear, but competitive binding measurements indicate that γ chain and RGD peptides cannot bind to αIIbβ3 at the same time (24), implying that RGD peptides inhibit fibrinogen binding by preventing the interaction of the γ chain with αIIbβ3.
Ligand binding to αIIbβ3 involves specific regions of the aminoterminal portions of both αIIb and β3. In the crystal structure of the αIIbβ3 head domain, ligand binds to a “specificity-determining” loop in the β3 βA domain and to a “cap” composed of 4 loops on the upper surface of the αIIb β-propeller domain (16). The αIIb β-propeller results from the folding of 7 contiguous aminoterminal repeats (17, 25). Each blade of the propeller is formed from 4 antiparallel β strands located in each repeat; loops connecting the strands are located on either the upper or the lower surface of the propeller. A number of naturally occurring and laboratory-induced mutations distributed between αIIb residues 145 and 224 and located in loops on the upper surface of the propeller impair αIIbβ3 function, implying that these residues interact with ligand (26–28). Further, Kamata et al. replaced each of the 27 loops in the αIIb propeller with the corresponding loops from α4 or α5 (29). They found that 8 replacements, all located on the upper surface of the second, third, and fourth repeats, abrogated fibrinogen binding to αIIbβ3, suggesting that fibrinogen binds to the upper surface of the propeller in a region centered around the third repeat. Previous chemical cross-linking experiments suggested that the fibrinogen γ chain binds to αIIb in the vicinity of its second calmodulin-like motif near amino acids 294–314 (30), but these residues are located on the lower surface of the propeller and are unlikely to interact with ligands such as fibrinogen (16). It is noteworthy that ligand binding itself induces conformational changes in αIIbβ3, most often detected by the appearance of neoepitopes for mAbs. In fact, such ligand-induced changes or LIBSs (ligand-induced binding sites) may be responsible for the immune-mediated thrombocytopenia associated with the clinical use of αIIbβ3 antagonists (31).
Ligand binding to αIIbβ3 requires divalent cations (32). Eight divalent cation-binding sites were identified in the αvβ3 crystal structure (17, 18). Four were located in the αv β-propeller domain, 1 at the αv genu, and 3 in the β3 βA domain, but only those located in the βA domain appeared to participate in ligand binding. In the absence of ligand, only the βA ADMIDAS (adjacent to the metal Ion–dependent adhesion site) motif was occupied, but when Mn2+ and a cyclic RGD ligand were present, each of the βA sites contained a cation. One site was the βA MIDAS; Mn2+ present at this site was in direct contact with ligand. A second Mn2+, located 6 Å from the MIDAS, was bound to a site designated ligand-induced metal-binding site (LIMBS), but the cation at this site did not interact with ligand. It had been postulated that Mn2+ induces integrin activation by antagonizing inhibitory effects of Ca2+ (33), but the αvβ3 crystal structure suggests that cations bound to the MIDAS and LIMBS motifs act by stabilizing the ligand-occupied conformation of the βA domain (18).
Regulation of αIIbβ3 ligand-binding activity
Integrins reside on cell surfaces in an equilibrium between inactive and active conformations (34). In experiments where the cytoplasmic domains of αLβ2 and α5β1 were replaced by acidic and basic peptides (35, 36), purified integrins were inactive when their stalks were in proximity and active when the stalks were farther apart. This was corroborated by measurements of fluorescence resonance energy transfer (FRET) efficiency between cyan and yellow fluorescent proteins fused to the cytoplasmic domains of αL and β2 expressed in K562 cells (37). FRET efficiency decreased when αLβ2 interacted with immobilized or soluble ligand, implying that bidirectional signaling resulted from the coupling of conformational changes in the αLβ2 extracellular domain to the spatial separation of the αL and β2 cytoplasmic domains, a result consistent with EM images of αIIbβ3 in which scissor-like movements of the αIIb and β3 stalks differentiate active and inactive molecules (19).
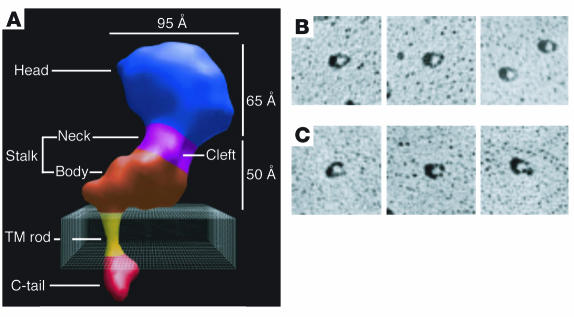
Nonetheless, the relationship of these observations to the αIIbβ3 and αvβ3 crystal structures is controversial. Takagi et al., supported by negatively stained EM images of active and inactive integrins, suggested that the bent conformation of αvβ3 in crystals corresponds to low-affinity αvβ3 and the shift to a high-affinity conformation occurs when the integrin undergoes a global reorganization characterized by a “switchblade-like” opening to an extended structure and scissor-like separation of the α and β subunit stalks (34). Xiong et al., however, suggested that the bent conformation resulted from flexibility at the αv and β3 genua and from crystal contacts not likely to occur in nature (17). This possibility was supported by cryo-EM reconstructions of intact inactive αIIbβ3 molecules, which revealed a collapsed but unbent structure consisting of a large globular head and an L-shaped stalk whose axis was rotated approximately 60° with respect to the head and was connected at an angle of approximately 90° to a rod containing the TM domains of the integrin (Figure 2A) (38). They also suggested that extension at the “knees” may be a post-ligand-binding “outside-in” signaling event and that the transition of αvβ3 from its inactive to its active conformation results when the CD loop of the β3 βTD domain moves away from the βA domain, allowing the latter to assume its active conformation (39). How to reconcile each of these models with the rotary-shadowed EM images of demonstrably inactive and active αIIbβ3 shown in Figure 2, B and C, is not obvious.
Figure 2.
Cryo-EM reconstruction and rotary-shadowed EM images of αIIbβ3. (A) Cryo-EM reconstruction. The resolution is 20 Å. Adapted with permission from Proceedings of the National Academy of Sciences of the United States of America (38). (B and C) Rotary-shadowed EM images. The images in B were obtained in the presence of 1 mM Ca2+ and the images in C in the presence of 1 mM Mn2+. Reproduced with permission from Blood (19).
The αIIb and β3 cytoplasmic domains constrain αIIbβ3 function
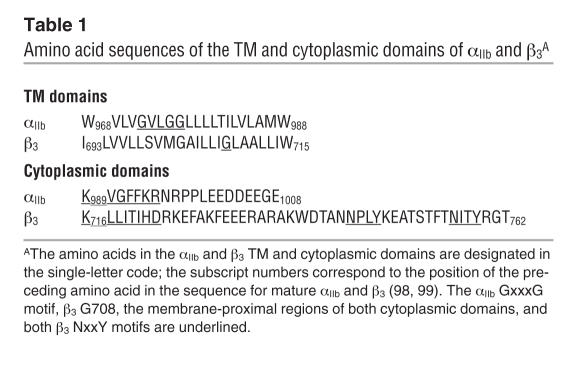
Cytoplasmic domain sequences, most convincingly demonstrated for conserved membrane-proximal sequences, constrain integrins in their low-affinity (inactive) conformations. Thus, truncation of the αIIb cytoplasmic domain at Gly991 or the β3 cytoplasmic domain at Leu717 or deletion of the conserved membrane-proximal αIIb GFFKR or β3 LLITIHD motifs (Table 1) shifts αIIbβ3 to its active state (40). Similarly, constitutive αIIbβ3 function can be induced by replacement of αIIb residue F992, F993, or R995 or β3 residue D723 with alanine, whereas heterodimers containing simultaneous R995→D and D723→R substitutions are inactive (41). These observations led to the suggestion that the membrane-proximal sequences form an activation-constraining “clasp,” an essential feature of which is a salt bridge between αIIb R995 and β3 D723. Paradoxically, replacing the αIIb cytoplasmic domain with the cytoplasmic domain of α2, α5, α6A, or α6B, each of which contains a GFFKR motif, activates αIIbβ3 (40). This implies that additional cytoplasmic domain sequences modulate αIIbβ3 function, consistent with the inhibitory effects observed for the β3 mutation Ser752Pro (42), β3 truncation at Arg724 (43), and mutations involving the β3 sequences EFAKFEEE, NPLY, and NITY (44–46) and the αIIb sequence Pro998/Pro999 (47, 48).
Table 1.
Amino acid sequences of the TM and cytoplasmic domains of αIIb and β3A
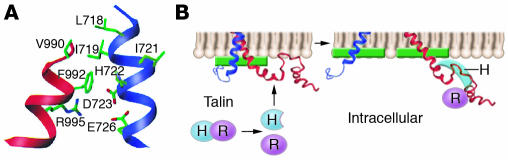
Interaction between the αIIb and β3 cytoplasmic domains has been studied experimentally using peptides dissolved in aqueous buffer or anchored to phospholipid micelles via aminoterminal myristoylation. Using terbium luminescence and electrospray ionization mass spectroscopy, Haas and Plow observed the formation of a cation-containing complex involving αIIb residues 999–1,008 and β3 residues 721–740 (49). Similarly, Vallar et al. used surface plasmon resonance to detect a weak (Kd ∼50 μM) KVGFFKR-dependent, calcium-stabilized complex between soluble αIIb cytoplasmic domain and immobilized β3 cytoplasmic domain peptides (50). Further, Weljie et al. determined an NMR structure for a heterodimer that formed at low ionic strength between an 11-residue GFFKR-containing αIIb peptide and a 25-residue LLITIHD-containing β3 peptide (51). They identified 2 conformers differing in the conformation of the β3 backbone: one had an elongated β3 structure; the other was bent back at D723–A728, causing the peptide to adopt a closed L shape. Nonetheless, both conformers were predominantly helical with significant hydrophobic interactions between V990 and F993 of αIIb and L717–I721 of β3. Although there was no NMR evidence of an R995–D723 salt bridge, modeling suggested that a salt bridge was possible if the β3 backbone was elongated. Vinogradova et al. also used NMR to characterize complexes between full-length αIIbβ3 cytoplasmic domain peptides (48, 52, 53). Despite low affinity, they identified interfaces for the complexes that included hydrophobic and electrostatic interactions between membrane-proximal helices (Figure 3A) (52). When the experiments were repeated in the presence of diphosphocholine micelles, αIIb residues 989–993 and β3 residues 716–721 were embedded in lipid and there was interaction between β3 residues 741 and 747 and micelle lipid (53). Talin binding to β3 disrupted the complex of αIIb with β3 as well as β3 interaction with lipid (Figure 3B). On the other hand, Li et al. were unable to detect heteromeric interaction between proteins corresponding to the αIIb and β3 TM and cytoplasmic domains in diphosphocholine micelles at physiologic salt concentrations using a number of biophysical techniques, perhaps because heteromeric interaction is substantially weaker than homomeric interaction (54). Similarly, Ulmer et al. did not detect heteromeric interactions of αIIb with β3 in an NMR analysis of a coiled-coil construct containing the αIIb and β3 cytoplasmic domains (55).
Figure 3.
Interaction of the αIIb and β3 cytoplasmic domains. (A) Backbone ribbon diagram of the αIIbβ3 membrane-proximal cytoplasmic domain clasp showing hydrophobic and electrostatic interactions. Reproduced with permission from Cell (52). (B) Model of the changes that may occur in the clasp following talin binding to the β3 cytoplasmic domain. Adapted with permission from Proceedings of the National Academy of Sciences of the United States of America (53).
Proteins that interact with the αIIb and β3 cytoplasmic domains
Proteins have been identified, most often using yeast 2-hybrid screens, that bind to the cytoplasmic domains of integrin α and β subunits. These proteins include CIB (calcium- and integrin-binding protein) (56), Aup1 (ancient ubiquitous protein 1) (57), ICln (a chloride channel regulatory protein) (58), and PP1c (the catalytic subunit of protein phosphatase 1) (59), each of which binds to the membrane-proximal αIIb sequence KVGFFKR. However, because a substantial portion of this sequence is likely embedded in the plasma membrane (60, 61), the physiologic importance of these interactions is uncertain. Proteins that interact with the β3 cytoplasmic domain include the cytoskeletal proteins talin, α-actinin, filamin, myosin, and skelemin; various members of the Src family of kinases; the kinases integrin-linked kinase (ILK), Syk, and Shc; the adaptor Grb2; the scaffold RACK1; CD98 (62); and β3-endonexin (63). Binding of myosin, Shc, and Grb2 requires platelet aggregation and spreading, as well as the Fyn-mediated phosphorylation of β3 tyrosines 747 and 759, and has been implicated in post-receptor-binding cytoskeleton-mediated events such as clot retraction (64).
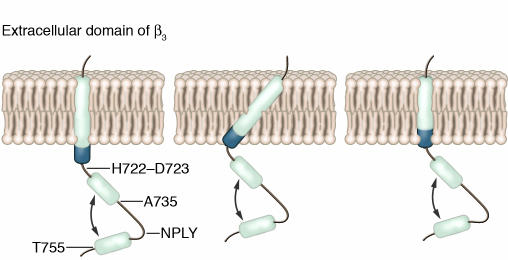
Binding of β3-endonexin or talin to the β3 cytoplasmic domain is noteworthy because it can activate αIIbβ3. β3-Endonexin, a 14-kDa protein of unknown function, induces αIIbβ3 activation when coexpressed with αIIbβ3 in tissue culture cells by interacting with residues located in both the aminoterminal and the carboxyterminal regions of the β3 cytoplasmic domain, in particular the carboxyterminal NITY motif (65–67). Nonetheless, there is no evidence as yet that β3-endonexin regulates αIIbβ3 function in platelets. One explanation for the presence of 2 discontinuous β3-endonexin–binding sites in the β3 cytoplasmic domain has been provided by an NMR analysis of a protein encompassing the β3 TM and cytoplasmic domains (Figure 4) (68). This analysis revealed that the β3 TM helix extended into the membrane-proximal region of the cytoplasmic domain, ending at an apparent hinge at residues H722–D723 (Table 1). Two additional helical stretches, extending from residues 725 to 735 and 748 to 755, were also present (Figure 4). Because the latter helices can interact with each other, they can place the proximal and distal regions of the β3 cytoplasmic domain in proximity.
Figure 4.
Model of the structure of the β3 TM and cytoplasmic domains. Helices are shown as cylinders. Three different orientations of the β3 TM domain in the plasma membrane are shown. The membrane-proximal region of the cytoplasmic domain is shaded. Arrows indicate possible interactions between helices. Adapted with permission from Biochemistry (68).
Talin, an abundant 250-kDa cytoskeletal protein, forms antiparallel homodimers that bind to the cytoplasmic domain of integrin β subunits as well as to other cytoskeletal proteins such as actin and vinculin (69). Talin is composed of a 50-kDa head domain containing its principal integrin-binding site and a 220-kDa rod domain that binds to integrins with lesser affinity (69). The talin head itself contains an approximately 300-residue FERM (four-point-one, ezrin, radixin, moesin) domain that folds into F1, F2, and F3 subdomains (69). F2 and F3 bind to the β3 cytoplasmic domain, although the affinity of F3 binding is substantially greater (70). A crystal structure for a fusion protein composed of the F2 and F3 subdomains and a contiguous aminoterminal peptide corresponding to the midportion of the β3 cytoplasmic domain, including its NPLY motif, revealed that the interaction of the β3 peptide with F3 was mainly hydrophobic and that NPLY interacted with F3 in a manner that resembled that of canonical PTB domain ligands (71). However, studies using NMR also revealed that F3 and F2-F3 interact with the membrane-proximal region of the β3 cytoplasmic domain (71, 72), consistent with previous observations that talin binds to peptides corresponding to this portion of β3 (73).
Overexpressing the talin head domain in αIIbβ3-expressing CHO cells induces αIIbβ3 activation (74), either directly because talin disrupts the clasp between αIIb and β3 (Figure 3B) or indirectly via conformational changes induced by F3 binding to the β3 NPLY motif (70). Conversely, reducing talin expression using short hairpin RNAs decreases ligand binding to αIIbβ3 in CHO cells and in ES cell–derived agonist-stimulated megakaryocytes (75). Taken together, these results imply that talin binding to the β3 cytoplasmic domain may be a final step in αIIbβ3 activation. Nonetheless, how talin binding to the β3 cytoplasmic domain is regulated remains to be determined. The integrin-binding domain in intact talin appears to be masked (76). Although the enzyme calpain can cleave talin, releasing its head domain (77), calpain activation in platelets is a relatively late step after platelet stimulation (78) and would be unlikely to contribute to integrin-activating inside-out signaling. On the other hand, talin binds to membrane-associated phosphoinositol 4,5-bisphosphate, inducing a conformational change that enables it to bind to the β1 cytoplasmic domain (79). By analogy, talin binding to phosphoinositol 4,5-bisphosphate may enable it to bind to β3.
Regulation of αIIbβ3 function by TM domain interaction
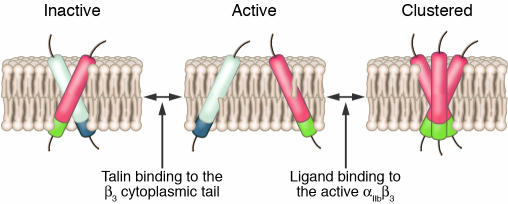
TM domain–mediated protein oligomerization is a common mechanism for the assembly of membrane proteins and regulation of protein function (80). Specificity is achieved via specific sequence motifs superimposed on more general oligomerization frameworks (81–83). For example, the sequence motif GxxxG, first recognized as a framework for the homomeric association of the glycophorin A (GpA) TM helix (81), has been identified as the most overrepresented sequence motif in TM domain databases (82).
With regard to integrin TM domains, Li and coworkers reported that peptides corresponding to the αIIb and β3 TM domains readily undergo homodimeric and homotrimeric association, respectively, in phospholipid micelles (54), and Schneider and Engelman found that fusion proteins containing the α2β1, α4β7, and αIIbβ3 TM domains undergo integrin-specific TM domain–mediated homomeric and heteromeric association in bacterial membranes (84). Subsequently, Li et al. reported that facilitating the homomeric association of the β3 TM helix by replacing either G708 or M701 with a polar asparagine induced αIIbβ3 activation and clustering when the mutants were expressed in CHO cells (85). They also found that mutation of the αIIb GxxxG motif located at residues 972–975 disrupted the homomeric association of αIIb TM helix (86) and paradoxically induced αIIbβ3 activation and clustering (87). These observations suggested the “push-pull” mechanism for αIIbβ3 activation shown in Figure 5. Processes that destabilize the association of the αIIb and β3 TM helices, such as talin binding to the β3 cytoplasmic domain, would be expected to promote dissociation of the helices with concomitant αIIbβ3 activation. Conversely, intermolecular interactions that either require separation of the αIIb and β3 TM helices, such as homo-oligomerization, or are more favorable when they separate, such as ligand-induced αIIbβ3 clustering (88), would be expected to pull the equilibrium toward the activated state.
Figure 5.
Diagram illustrating the “push-pull” hypothesis for regulation of the αIIbβ3 activation state. The white and blue cylinders represent the αIIb TM and membrane-proximal cytoplasmic domain helices, respectively. The red and green cylinders represent the β3 TM and membrane-proximal cytoplasmic domain helices, respectively.
The ability of homomeric TM helix interactions to induce αIIbβ3 activation and clustering remains controversial (89, 90), but there is compelling evidence that heterodimeric interactions constrain αIIbβ3 in a low-affinity state. By simultaneously scanning the αIIb and β3 TM helices with cysteine residues, Luo et al. detected the formation of disulfide bonds with a helical periodicity in a region corresponding to αIIb residues 966–974 and β3 residues 693–702, consistent with the presence of a unique αIIbβ3 TM heterodimer (91). They also scanned the αIIb and β3 helices with leucines, confirming that mutation of the αIIb GxxxG motif induces αIIbβ3 activation (90). Partridge et al. used random mutagenesis of the β3 TM and cytoplasmic domains to search for interactions constraining αIIbβ3 activation (92). They detected 12 activating mutations in the membrane-proximal cytoplasmic domain and 13 activating mutations in the β3 TM helix. Nine of the latter were predicted to shorten the helix, perhaps activating αIIbβ3 by altering the tilt of the helix in the membrane (Figure 4). The remaining mutations were located in the carboxyterminal half of the helix and were postulated to activate αIIbβ3 by disrupting the packing of an αIIbβ3 TM heterodimer.
Despite the biochemical evidence supporting the presence of αIIb and β3 TM domain oligomers, their existence has not been confirmed by NMR spectroscopy or x-ray crystallography because of difficulty in obtaining high-resolution structures for TM proteins using these techniques. However, computational methods have been used to construct TM domain models incorporating the constraints imposed by mutational data. Based on cryo-EM images (Figure 2A), Adair and Yeager proposed that the TM domains of inactive αIIbβ3 associate in a parallel α-helical coiled coil (38). Using the R995–D723 salt bridge as the primary constraint, they found that a right-handed coiled coil based on the GpA TM dimer (93) placed more conserved residues in the helix-helix interface than a coiled coil based on the canonical left-handed leucine zipper. Gottschalk and coworkers proposed that the αIIb and β3 TM helices remain in close contact in the activated state and that the helix-helix interface is a GpA-like structure containing the αIIb G972xxxG975 and β3 S699xxxA703 motifs (94). Moreover, simulated annealing and molecular dynamics supported a model in which the αIIb and β3 TM domains interact weakly in a right-handed coiled coil when the integrin is in its low-affinity conformation (95). Subsequently, in order to account for both aminoterminal and carboxyterminal restraints, Gottschalk proposed that the αIIbβ3 TM and membrane-proximal cytoplasmic domains form a right-handed coiled coil in which the helices interact over their entire length, placing the αIIb GxxxG motif, but not β3 S699xxxA703, in the helix-helix interface (96). By contrast, Luo et al. used their disulfide cross-linking data to construct a model based on the GpA TM dimer; however, in this model, the αIIb GxxxG-like motif corresponded to residues 968–972, rather than 972–975 (91). DeGrado and coworkers used a Monte Carlo–simulated annealing algorithm to obtain atomic models for an αIIb TM homodimer (86) and an αIIbβ3 heterodimer (87). In each case, a family of structures was found that satisfied mutational constraints. For the αIIb homodimer, all structures had right-handed crossing angles ranging from 40° to 60°, but with an interface rotated by 50° relative to the GpA homodimer. In the case of the αIIbβ3 heterodimer, initial docking identified local minima with both right- and left-handed crossing angles. However, the right-handed structures had lower energies and more extensive interactions, and the αIIb GxxxG motif was in intimate contact with the β3 TM domain. Lastly, Partridge et al., using a Monte Carlo simulation, obtained 2 structures for an αIIbβ3 TM heterodimer with helix packing near either the amino or the carboxyl termini of the helices, respectively; of the 2 models, carboxyterminal helix packing was more consistent with their mutational data (92). It is obvious that there is wide disparity among these models, making it clear that obtaining actual structures for αIIb and β3 TM domain hetero- and homo-oligomers will be the next major advance in our understanding of the structural basis for the regulation of platelet integrin function.
Footnotes
Nonstandard abbreviations used: GpA, glycophorin A; MIDAS, metal ion–dependent adhesion site; PSI, plexin, semaphorin, integrin; RGD, Arg-Gly-Asp; TM, transmembrane.
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Piotrowicz RS, Orchekowski RP, Nugent DJ, Yamada KY, Kunicki TJ. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J. Cell Biol. 1988;106:1359–1364. doi: 10.1083/jcb.106.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ill CR, Engvall E, Ruoslahti E. Adhesion of platelets to laminin in the absence of activation. J. Cell Biol. 1984;99:2140–2145. doi: 10.1083/jcb.99.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnenberg A, Modderman P, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–488. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 5.Staatz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J. Cell Biol. 1989;108:1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett JS, Chan C, Vilaire G, Mousa SA, DeGrado WF. Agonist-activated αvβ3 on platelets and lymphocytes binds to the matrix protein osteopontin. J. Biol. Chem. 1997;272:8137–8140. doi: 10.1074/jbc.272.13.8137. [DOI] [PubMed] [Google Scholar]
- 7.Paul BZS, Vilaire G, Kunapuli SP, Bennett JS. Concurrent signaling from Gαq- and Gαi-coupled pathways is essential for agonist-induced αvβ3 activation on human platelets. J. Thromb. Haemost. 2003;1:814–820. doi: 10.1046/j.1538-7836.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 8.George JN, Caen JP, Nurden AT. Glanzmann’s thrombasthenia: the spectrum of clinical disease. Blood. 1990;75:1383–1395. [PubMed] [Google Scholar]
- 9.Lefkovits J, Plow E, Topol E. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N. Engl. J. Med. 1995;332:1553–1559. doi: 10.1056/NEJM199506083322306. [DOI] [PubMed] [Google Scholar]
- 10.Duperray A, et al. Biosynthesis and processing of platelet GPIIb-IIIa in human megakaryocytes. J. Cell Biol. 1987;104:1665–1673. doi: 10.1083/jcb.104.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolodziej MA, Vilaire G, Gonder D, Poncz M, Bennett JS. Study of the endoproteolytic cleavage of platelet glycoprotein IIb using oligonucleotide-mediated mutagenesis. J. Biol. Chem. 1991;266:23499–23504. [PubMed] [Google Scholar]
- 12.Wagner CL, et al. Analysis of GPIIb/IIIa receptor number by quantitation of 7E3 binding to human platelets. Blood. 1996;88:907–914. [PubMed] [Google Scholar]
- 13.Niiya K, et al. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987;70:475–483. [PubMed] [Google Scholar]
- 14.Bennett JS. Structural biology of glycoprotein IIb-IIIa. Trends Cardiovasc. Med. 1996;6:31–37. doi: 10.1016/1050-1738(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 15.Weisel JW, Nagaswami C, Vilaire G, Bennett JS. Examination of the platelet membrane glycoprotein IIb/IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J. Biol. Chem. 1992;267:16637–16643. [PubMed] [Google Scholar]
- 16.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong JP, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong JP, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 19.Litvinov RI, et al. Functional and structural correlations of individual alphaIIbbeta3 molecules. Blood. 2004;104:3979–3985. doi: 10.1182/blood-2004-04-1411. [DOI] [PubMed] [Google Scholar]
- 20.Kloczewiak M, Timmons S, Lukas TJ, Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationships of peptides corresponding to the carboxy-terminal segment of the γ chain. Biochemistry. 1984;23:1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- 21.Gartner TK, Bennett JS. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J. Biol. Chem. 1985;260:11891–11894. [PubMed] [Google Scholar]
- 22.Farrell DH, Thiagarajan P, Chung DW, Davie EW. Role of fibrinogen α and γ chain sites in platelet aggregation. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10729–10732. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett JS. Novel platelet inhibitors. Annu. Rev. Med. 2001;52:161–184. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- 24.Bennett JS, Shattil SJ, Power JW, Gartner TK. Interaction of fibrinogen with its platelet receptor. Differential effects of α and γ chain fibrinogen peptides on the glycoprotein IIb-IIIa complex. J. Biol. Chem. 1988;263:12948–12953. [PubMed] [Google Scholar]
- 25.Springer TA. Folding of the N-terminal, ligand-binding region of integrin α-subunits into a β-propeller domain. Proc. Natl. Acad. Sci. U. S. A. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamata T, Irie A, Tokuhira M, Takada Y. Critical residues of integrin alphaIIb subunit for binding of alphaIIbbeta3 (glycoprotein IIb-IIIa) to fibrinogen and ligand-mimetic antibodies (PAC-1, OP-G2, and LJ-CP3) J. Biol. Chem. 1996;271:18610–18615. doi: 10.1074/jbc.271.31.18610. [DOI] [PubMed] [Google Scholar]
- 27.Tozer EC, Baker EK, Ginsberg MH, Loftus JC. A mutation in the α subunit of the platelet integrin αIIbβ3 identifies a novel region important for ligand binding. Blood. 1999;93:918–924. [PubMed] [Google Scholar]
- 28.Basani RB, et al. A naturally occurring mutation near the amino terminus of αIIb defines a new region involved in ligand binding to αIIbβ3. Blood. 2000;95:180–188. [PubMed] [Google Scholar]
- 29.Kamata T, Tieu KK, Irie A, Springer TA, Takada Y. Amino acid residues in the alpha IIb subunit that are critical for ligand binding to integrin alpha IIbbeta 3 are clustered in the beta-propeller model. J. Biol. Chem. 2001;276:44275–44283. doi: 10.1074/jbc.M107021200. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza SE, Ginsberg MH, Burke TA, Plow EF. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its α subunit. J. Biol. Chem. 1990;265:3440–3446. [PubMed] [Google Scholar]
- 31.Bougie DW, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100:2071–2076. [PubMed] [Google Scholar]
- 32.Bennett JS, Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J. Clin. Invest. 1979;64:1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J, Piotrowicz R, Mathis D. A mechanism for divalent cation regulation of beta 3-integrins. J. Biol. Chem. 1994;269:960–967. [PubMed] [Google Scholar]
- 34.Takagi J, Petre B, Walz T, Springer T. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 35.Lu C, Takagi J, Springer TA. Association of the membrane proximal regions of the alpha and beta subunit cytoplasmic domains constrains an integrin in the inactive state. J. Biol. Chem. 2001;276:14642–14648. doi: 10.1074/jbc.M100600200. [DOI] [PubMed] [Google Scholar]
- 36.Takagi J, Erickson HP, Springer TA. C-terminal opening mimics ‘inside-out’ activation of integrin α5β1. Nat. Struct. Biol. 2001;8:412–416. doi: 10.1038/87569. [DOI] [PubMed] [Google Scholar]
- 37.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 38.Adair BD, Yeager M. Three-dimensional model of the human platelet integrin alpha IIbbeta 3 based on electron cryomicroscopy and x-ray crystallography. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14059–14064. doi: 10.1073/pnas.212498199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong JP, Stehle T, Goodman SL, Arnaout MA. New insights into the structural basis of integrin activation. Blood. 2003;102:1155–1159. doi: 10.1182/blood-2003-01-0334. [DOI] [PubMed] [Google Scholar]
- 40.O’Toole TE, et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes PE, et al. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, et al. Ser752→Pro mutation in the cytoplasmic domain of integrin β3 subunit and defective activation of platelet integrin αIIbβ3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R, Shattil SJ, Ambruso DR, Newman PJ. Truncation of the cytoplasmic domain of β3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin αIIbβ3 complex. J. Clin. Invest. 1997;100:2393–2403. doi: 10.1172/JCI119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J. Biol. Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- 45.Ylanne J, et al. Mutation of the cytoplasmic domain of the integrin beta 3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J. Biol. Chem. 1995;270:9550–9557. doi: 10.1074/jbc.270.16.9550. [DOI] [PubMed] [Google Scholar]
- 46.Xi X, Bodnar RJ, Li Z, Lam SC, Du X. Critical roles for the COOH-terminal NITY and RGT sequences of the integrin beta3 cytoplasmic domain in inside-out and outside-in signaling. J. Cell Biol. 2003;162:329–339. doi: 10.1083/jcb.200303120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leisner TM, Wencel-Drake JD, Wang W, Lam SC-T. Bidirectional transmembrane modulation of integrin αIIbβ3 conformations. J. Biol. Chem. 1999;274:12945–12949. doi: 10.1074/jbc.274.18.12945. [DOI] [PubMed] [Google Scholar]
- 48.Vinogradova O, Haas T, Plow EF, Qin J. A structural basis for integrin activation by the cytoplasmic tail of the alpha IIb-subunit. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1450–1455. doi: 10.1073/pnas.040548197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas TA, Plow EF. The cytoplasmic domain of alphaIIb beta3. A ternary complex of the integrin alpha and beta subunits and a divalent cation. J. Biol. Chem. 1996;271:6017–6026. doi: 10.1074/jbc.271.11.6017. [DOI] [PubMed] [Google Scholar]
- 50.Vallar L, et al. Divalent cations differentially regulate integrin αIIb cytoplasmic tail binding to β3 and to calcium- and integrin-binding protein. J. Biol. Chem. 1999;274:17257–17266. doi: 10.1074/jbc.274.24.17257. [DOI] [PubMed] [Google Scholar]
- 51.Weljie AM, Hwang PM, Vogel HJ. Solution structures of the cytoplasmic tail complex from platelet integrin alpha IIb- and beta 3-subunits. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5878–5883. doi: 10.1073/pnas.092515799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinogradova O, et al. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 53.Vinogradova O, et al. Membrane-mediated structural transitions at the cytoplasmic face during integrin activation. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4094–4099. doi: 10.1073/pnas.0400742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R, et al. Oligomerization of the integrin alphaIIbbeta3: roles of the transmembrane and cytoplasmic domains. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12462–12467. doi: 10.1073/pnas.221463098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulmer TS, Yaspan B, Ginsberg MH, Campbell ID. NMR analysis of structure and dynamics of the cytosolic tails of integrin alpha IIb beta 3 in aqueous solution. Biochemistry. 2001;40:7498–7508. doi: 10.1021/bi010338l. [DOI] [PubMed] [Google Scholar]
- 56.Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin αIIb cytoplasmic domain. J. Biol. Chem. 1997;272:4651–4654. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- 57.Kato A, et al. Ancient ubiquitous protein 1 binds to the conserved membrane-proximal sequence of the cytoplasmic tail of the integrin alpha subunits that plays a crucial role in the inside-out signaling of alpha IIbbeta 3. J. Biol. Chem. 2002;277:28934–28941. doi: 10.1074/jbc.M204340200. [DOI] [PubMed] [Google Scholar]
- 58.Larkin D, et al. ICln, a novel integrin alphaIIbbeta3-associated protein, functionally regulates platelet activation. J. Biol. Chem. 2004;279:27286–27293. doi: 10.1074/jbc.M402159200. [DOI] [PubMed] [Google Scholar]
- 59.Vijayan KV, Liu Y, Li TT, Bray PF. Protein phosphatase 1 associates with the integrin alphaIIb subunit and regulates signaling. J. Biol. Chem. 2004;279:33039–33042. doi: 10.1074/jbc.C400239200. [DOI] [PubMed] [Google Scholar]
- 60.Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J. Biol. Chem. 1999;274:37030–37034. doi: 10.1074/jbc.274.52.37030. [DOI] [PubMed] [Google Scholar]
- 61.Stefansson A, Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of N- and C-terminal borders of the transmembrane domain of integrin subunits. J. Biol. Chem. 2004;279:21200–21205. doi: 10.1074/jbc.M400771200. [DOI] [PubMed] [Google Scholar]
- 62.Feral CC, et al. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buensuceso CS, Arias-Salgado EG, Shattil SJ. Protein-protein interactions in platelet alphaIIbbeta3 signaling. Semin. Thromb. Hemost. 2004;30:427–439. doi: 10.1055/s-2004-833478. [DOI] [PubMed] [Google Scholar]
- 64.Phillips DR, Prasad KS, Manganello J, Bao M, Nannizzi-Alaimo L. Integrin tyrosine phosphorylation in platelet signaling. Curr. Opin. Cell Biol. 2001;13:546–554. doi: 10.1016/s0955-0674(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 65.Shattil SJ, et al. β3-Endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin β3 subunit. J. Cell Biol. 1995;131:807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kashiwagi H, et al. Affinity modulation of platelet integrin αIIbβ3 by β3-endonexin, a selective binding partner of the β3 integrin cytoplasmic tail. J. Cell Biol. 1997;137:1433–1443. doi: 10.1083/jcb.137.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eigenthaler M, Hofferer L, Shattil SJ, Ginsberg MH. A conserved sequence motif in the integrin β3 cytoplasmic domain is required for its specific interaction with β3-endonexin. J. Biol. Chem. 1997;272:7693–7698. doi: 10.1074/jbc.272.12.7693. [DOI] [PubMed] [Google Scholar]
- 68.Li R, et al. Characterization of the monomeric form of the transmembrane and cytoplasmic domains of the integrin beta 3 subunit by NMR spectroscopy. Biochemistry. 2002;41:15618–15624. doi: 10.1021/bi026822l. [DOI] [PubMed] [Google Scholar]
- 69.Calderwood DA. Talin controls integrin activation. Biochem. Soc. Trans. 2004;32:434–437. doi: 10.1042/BST0320434. [DOI] [PubMed] [Google Scholar]
- 70.Calderwood DA, et al. The phosphotyrosine binding (PTB)-like domain of talin activates integrins. J. Biol. Chem. 2002;277:21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Alvarez B, et al. Structural determinants of integrin recognition by talin. Mol. Cell. 2003;11:49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 72.Ulmer TS, Calderwood DA, Ginsberg MH, Campbell ID. Domain-specific interactions of talin with the membrane-proximal region of the integrin beta3 subunit. Biochemistry. 2003;42:8307–8312. doi: 10.1021/bi034384s. [DOI] [PubMed] [Google Scholar]
- 73.Patil S, et al. Identification of a talin-binding site in the integrin beta(3) subunit distinct from the NPLY regulatory motif of post-ligand binding functions. J. Biol. Chem. 1999;274:28575–28583. doi: 10.1074/jbc.274.40.28575. [DOI] [PubMed] [Google Scholar]
- 74.Calderwood DA, et al. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 75.Tadokoro S, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 76.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 77.Yan B, Calderwood DA, Yaspan B, Ginsberg MH. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 2001;276:28164–28170. doi: 10.1074/jbc.M104161200. [DOI] [PubMed] [Google Scholar]
- 78.Fox JE, Taylor RG, Taffarel M, Boyles JK, Goll DE. Evidence that activation of platelet calpain is induced as a consequence of binding of adhesive ligand to the integrin, glycoprotein IIb-IIIa. J. Cell Biol. 1993;120:1501–1507. doi: 10.1083/jcb.120.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martel V, et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 2001;276:21217–21227. doi: 10.1074/jbc.M102373200. [DOI] [PubMed] [Google Scholar]
- 80.Popot JL, Engelman DM. Helical membrane protein folding, stability, and evolution. Annu. Rev. Biochem. 2000;69:881–922. doi: 10.1146/annurev.biochem.69.1.881. [DOI] [PubMed] [Google Scholar]
- 81.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 82.Senes A, Gerstein M, Engelman DM. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J. Mol. Biol. 2000;296:921–936. doi: 10.1006/jmbi.1999.3488. [DOI] [PubMed] [Google Scholar]
- 83.Dawson JP, Melnyk RA, Deber CM, Engelman DM. Sequence context strongly modulates association of polar residues in transmembrane helices. J. Mol. Biol. 2003;331:255–262. doi: 10.1016/s0022-2836(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 84.Schneider D, Engelman DM. Involvement of transmembrane domain interactions in signal transduction by alpha/beta integrins. J. Biol. Chem. 2004;279:9840–9846. doi: 10.1074/jbc.M312749200. [DOI] [PubMed] [Google Scholar]
- 85.Li R, et al. Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science. 2003;300:795–798. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 86.Li R, Bennett JS, Degrado WF. Structural basis for integrin alphaIIbbeta3 clustering. Biochem. Soc. Trans. 2004;32:412–415. doi: 10.1042/BST0320412. [DOI] [PubMed] [Google Scholar]
- 87.Li W, et al. A push-pull mechanism for regulating integrin function. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1424–1429. doi: 10.1073/pnas.0409334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox JE, et al. The platelet cytoskeleton stabilizes the interaction between alphaIIbbeta3 and its ligand and induces selective movements of ligand-occupied integrin. J. Biol. Chem. 1996;271:7004–7011. doi: 10.1074/jbc.271.12.7004. [DOI] [PubMed] [Google Scholar]
- 89.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. J. Cell Biol. 2004;167:1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo BH, Carman CV, Takagi J, Springer TA. Disrupting integrin transmembrane domain heterodimerization increases ligand binding affinity, not valency or clustering. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3679–3684. doi: 10.1073/pnas.0409440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo BH, Springer TA, Takagi J. A specific interface between integrin transmembrane helices and affinity for ligand. PLoS Biol. 2004;2:e153. doi: 10.1371/journal.pbio.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Partridge AW, Liu S, Kim S, Bowie JU, Ginsberg MH. Transmembrane domain helix packing stabilizes integrin alphaIIbbeta3 in the low affinity state. J. Biol. Chem. 2005;280:7294–7300. doi: 10.1074/jbc.M412701200. [DOI] [PubMed] [Google Scholar]
- 93.MacKenzie KR, Prestegard JH, Engelman DM. A transmembrane helix dimer: structure and implications. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- 94.Gottschalk KE, Adams PD, Brunger AT, Kessler H. Transmembrane signal transduction of the alpha(IIb)beta(3) integrin. Protein Sci. 2002;11:1800–1812. doi: 10.1110/ps.4120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gottschalk KE, Kessler H. Evidence for hetero-association of transmembrane helices of integrins. FEBS Lett. 2004;557:253–258. doi: 10.1016/s0014-5793(03)01443-1. [DOI] [PubMed] [Google Scholar]
- 96.Gottschalk KE. A coiled-coil structure of the alphaIIbbeta3 integrin transmembrane and cytoplasmic domains in its resting state. Structure (Camb.). 2005;13:703–712. doi: 10.1016/j.str.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Arnaout, M.A., Mahalingam, B., and Xiong, J.P. 2005. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. doi:10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed]
- 98.Poncz M, et al. Structure of the platelet membrane glycoprotein IIb. J. Biol. Chem. 1987;262:8476–8482. [PubMed] [Google Scholar]
- 99.Zimrin AB, et al. Structure of platelet glycoprotein IIIa. J. Clin. Invest. 1988;81:1470–1475. doi: 10.1172/JCI113478. [DOI] [PMC free article] [PubMed] [Google Scholar]