Abstract
Platelets represent an important linkage between inflammation, thrombosis, and atherogenesis. Inflammation is characterized by interactions among platelets, leukocytes, and ECs. These interactions trigger autocrine and paracrine activation processes that lead to leukocyte recruitment into the vascular wall. Platelet-induced chronic inflammatory processes at the vascular wall result in development of atherosclerotic lesions and atherothrombosis. This Review highlights the molecular machinery and inflammatory pathways used by platelets to initiate and accelerate atherothrombosis.
Platelet interaction with endothelium
At the site of vascular lesions, ECM proteins such as vWF and collagen are exposed to the blood. Platelet adhesion to the exposed matrix is considered to be the initial step in thrombus formation. Platelets adhere to vWF via the membrane adhesion receptor glycoprotein Ib/IX/V (GPIb/IX/V) (1) and to collagen via GPVI (2–4). This results in platelet activation and transformation of the integrin receptors αIIbβ3 (GPIIb/IIIa, fibrinogen receptor) (5, 6) and α2β1 (collagen receptor) (4, 7), which firmly bind to the respective ECM components. Subsequently, platelets spread and form a surface for the recruitment of additional platelets via fibrinogen bridges between 2 αIIbβ3 receptors.
In vitro studies with human ECs.
In recent years, however, it has become increasingly evident that endothelial denudation is not an absolute prerequisite to allow platelet attachment to the arterial wall. The intact, nonactivated endothelium normally prevents platelet adhesion to the ECM. The adhesion receptors involved in platelet attachment to the subendothelial matrix, e.g., following rupture of an atherosclerotic plaque, have been well defined during the past decade; however, the molecular determinants that promote the interaction between platelets and endothelium are incompletely understood. Whereas endothelium normally controls platelet reactivity through inhibitory and modulating mechanisms involving COX-2, PGI2, or prostanoid synthetic systems, inflamed ECs develop properties that render them adhesive for platelets. In vitro studies showed that platelets adhere to the intact but activated human EC monolayer (8–10). Platelet adhesion to human umbilical vein ECs (HUVECs) is mediated by a GPIIb/IIIa–dependent bridging mechanism involving platelet-bound fibrinogen, fibronectin, and vWF (10). In HUVECs infected with herpes virus or stimulated with IL-1, platelet adhesion was effectively inhibited by antibodies to vWF or αIIbβ3 integrin, respectively (9, 11, 12). Furthermore, the involvement of the EC receptors ICAM-1, αvβ3 integrin, and GPIb in the binding of activated platelets to HUVECs has been described in vitro (10).
In vivo studies of mouse models.
Most in vitro studies have evaluated platelet-endothelium adhesion to human ECs under static conditions with limited attention to the dynamic situation in vivo. Studies using intravital microscopy, however, confirmed that platelet-endothelium adhesion occurs even under high shear stress in vivo (13–19). The results of these studies provide evidence that, similar to interaction with extracellular matrix proteins at the site of vascular lesions, adhesion of platelets to the intact endothelium is coordinated in a multistep process that involves platelet tethering, followed by rolling and subsequent firm adhesion to the vascular wall (Figure 1). These processes involve interactions involving at least 2 types of receptors, selectins and integrins, which induce receptor-specific activation signals in both platelets and the respective adhesive cell type.
Figure 1.
Platelet-endothelium adhesion. Activated endothelium surface expresses P-selectin. Platelet surface receptors GPIbα and PSGL-1 interact with endothelial P-selectin and mediate platelet rolling. Subsequent firm adhesion is mediated through β3 integrins.
The initial loose contact between circulating platelets and vascular endothelium (“platelet rolling”) is mediated by selectins, present on both ECs and platelets (20). P-selectin (CD62P) is rapidly expressed on the endothelial surface in response to inflammatory stimuli by translocating from membranes of storage granules (Weibel-Palade bodies) to the plasma membrane within seconds. Endothelial P-selectin has been demonstrated to mediate platelet rolling in both arterioles and venules in acute inflammatory processes (14, 15). E-selectin, which is also expressed on inflamed ECs, allows a loose contact between platelets and endothelium in vivo (15). In line with the concept of endothelial inflammation as a trigger for platelet accumulation, the process of platelet rolling does not require previous platelet activation, since platelets from mice lacking P- and/or E-selectin roll as efficiently as wild-type platelets (17).
So far, few studies have addressed the exact nature of the ligands expressed on platelets that bind to endothelial P-selectin. One candidate that has been identified as a potential counterreceptor for platelet P-selectin is the leucine-rich GPIb/IX/V, also known as the vWF receptor complex. Romo et al. (21) have recently demonstrated that cells expressing P-selectin roll on immobilized GPIbα. Platelets rolling on activated endothelium can be inhibited by antibodies against both P-selectin and GPIbα, indicating that the vWF receptor mediates platelet adhesion to both the subendothelial matrix and the intact endothelium (21). Further, PSGL-1, a glycoprotein that avidly associates with P-selectin, is present on platelets and mediates platelet rolling to the endothelial monolayer under high shear rates (22, 23).
The interaction between P-selectin and PSGL-1 or GPIb/IX/V, however, is rapidly reversible and insufficient for stable adhesion. Rapid conversion to stable adhesion requires additional contacts between the platelet and the endothelium. Integrins are recognized as the major class of surface receptor mediating stable adhesion at high shear in hematopoietic cells (1). Although the role of integrins in mediating firm platelet adhesion to ECM proteins at the lesion site is well established, their role in platelet-endothelium adhesion in vivo is incompletely understood so far. In vitro, both β3 integrins (αIIbβ3 and αvβ3) have been shown to mediate firm platelet-endothelium adhesion under static conditions (9, 10). In vivo, firm platelet adhesion to the endothelium can be inhibited by anti-αIIbβ3 mAb, and platelets defective in αIIbβ3 do not firmly adhere to activated ECs (19). Thus, although we are just starting to understand the molecular requirements of platelet-endothelium adhesion under dynamic conditions, it seems to be a well-controlled multistep process involving interaction of platelet PSGL-1 or GPIbα with endothelial P-selectin (“rolling”) followed by subsequent β3 integrin–mediated firm platelet adhesion. These receptor-dependent platelet-EC interactions allow transcellular communication via soluble mediators and might therefore play an important role in the initiation and progression of vascular inflammation (Figure 2).
Figure 2.
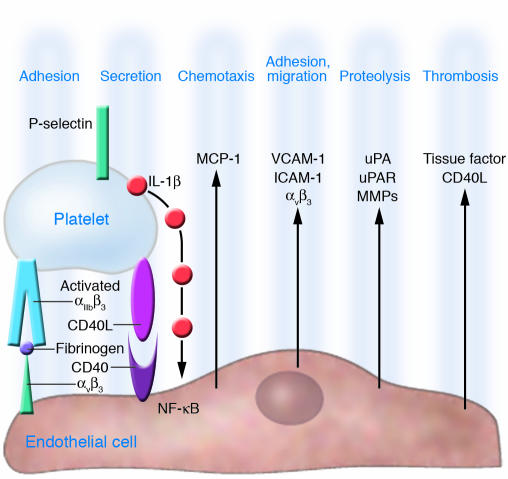
Adherent platelets inflame ECs. Firm platelet adhesion involving αIIbβ3 induces platelet surface exposure of P-selectin (CD62P) and release of CD40L and IL-1β, which stimulate ECs to provide an inflammatory milieu that supports proatherogenic alterations of endothelium.
Platelet-derived mediators stimulate inflammation
During the adhesion process, platelets become activated and release an arsenal of potent inflammatory and mitogenic substances into the local microenvironment, thereby altering chemotactic, adhesive, and proteolytic properties of ECs (24). These platelet-induced alterations of the endothelial phenotype support chemotaxis, adhesion, and transmigration of monocytes to the site of inflammation (Figure 2).
Released from dense granules, α-granules, lysosomes, the canalicular system, or the cytosol, platelets secrete or expose adhesion proteins (e.g., fibrinogen, fibronectin, vWF, thrombospondin, vitronectin, P-selectin, GPIIb/IIIa), growth factors (e.g., PDGF, TGF-β, EGF, bFGF), chemokines (e.g., RANTES, platelet factor 4 [CXC chemokine ligand 4], epithelial neutrophil-activating protein 78 [CXC chemokine ligand 5]), cytokine-like factors (e.g., IL-1β, CD40 ligand, β-thromboglobulin), and coagulation factors (e.g., factor V, factor XI, PAI-1, plasminogen, protein S). These proteins act in a concerted and finely regulated manner to influence widely differing biological functions such as cell adhesion, cell aggregation, chemotaxis, cell survival and proliferation, coagulation, and proteolysis, all of which accelerate inflammatory processes and cell recruitment. For example, IL-1β has been identified as a major mediator of platelet-induced activation of ECs (25, 26). The IL-1β activity expressed by platelets appears to be associated with the platelet surface, and coincubation of ECs with thrombin-activated platelets induces IL-1β–dependent secretion of IL-6 and IL-8 from ECs (26). Furthermore, incubation of cultured ECs with thrombin-stimulated platelets significantly enhances the secretion of endothelial monocyte chemoattractant protein-1 (MCP-1) in an IL-1β–dependent manner (12). MCP-1 belongs to the CC family of chemokines and is thought to play a key role in the regulation of monocyte recruitment to inflamed tissue and in atherosclerosis (27, 28).
However, platelet IL-1β does not only modify endothelial release of chemotactic proteins. IL-1β additionally can increase endothelial expression of adhesion molecules. Surface expression of ICAM-1 and αvβ3 on ECs is significantly enhanced by activated platelets via IL-1β (12). Both enhanced chemokine release and upregulation of endothelial adhesion molecules through platelet-derived IL-1β act in concert and promote neutrophil and monocyte adhesion to the endothelium. IL-1β–dependent expression of early inflammatory genes, such as MCP-1 or ICAM-1, involves the activation of the transcription factor NF-κB. Transient adhesion of platelets to the endothelium initiates degradation of IκB and supports activation of NF-κB in ECs, thereby inducing NF-κB–dependent chemokine gene transcription (29, 30). Likewise, platelet-induced NF-κB activation was largely reduced by IL-1β antagonists, which supports the notion that platelet IL-1β is the molecular determinant of platelet-dependent activation of the transcription factor. Activation of NF-κB involves a cascade of phosphorylation processes. One family of kinases that is involved in NF-κB–dependent gene expression is the MAPKs, such as p38 MAPK. In a manner similar to that of recombinant human IL-1β, activated platelets have the potential to induce phosphorylation of p38 MAPK. Correspondingly, transfection of a dominant-negative p38 mutant significantly reduced platelet-induced MCP-1 secretion in ECs (31).
Once recruited to the vascular wall, platelets may promote inflammation by chemoattraction of leukocytes through mediators such as platelet-activating factor and macrophage inflammatory protein-1α, may stimulate smooth muscle cell proliferation (TGF-β, PDGF, serotonin) (32), and may contribute to matrix degradation by secretion of MMP-2 (33).
A finely regulated functional interaction of platelets with chemokines has also been implicated in atherogenesis (34). Activated platelets can release chemokines and can induce the secretion of chemokines in various cells of the vascular wall; in turn, certain chemokines can enhance platelet aggregation and adhesion in combination with primary agonists and can trigger monocyte recruitment (35). One such candidate for monocyte recruitment is RANTES, which has been shown to trigger monocyte arrest on inflamed and atherosclerotic endothelium (35). Deposition of platelet-derived RANTES induces monocyte recruitment mediated by P-selectin (36, 37). Another platelet-derived chemokine is platelet factor 4 (PF4), the most abundant protein secreted by activated platelets. First, PF4 acts as a chemoattractant for monocytes promoting their differentiation into macrophages (38). Second, PF4 may directly aggravate the atherogenic actions of hypercholesterolemia by promoting the retention of lipoproteins. Sachais and colleagues have recently shown that PF4 can facilitate the retention of LDL on cell surfaces by inhibition of its degradation by the LDL receptor (39). In addition, PF4 markedly enhances the esterification and uptake of oxidized LDL by macrophages (40). The fact that PF4 has been found in human atherosclerotic lesions and was found associated with macrophages in early lesions and with foam cells in more advanced lesions (41) supports the concept that PF4 released from locally activated platelets enters the vessel wall and promotes vascular inflammation and atherogenesis.
Furthermore, release of platelet-derived CD40 ligand (CD40L, CD154) induces inflammatory responses in endothelium. Henn et al. (42) showed that platelets store CD40L in high amounts and release it within seconds after activation in vitro. Ligation of CD40 on ECs by CD40L expressed on the surface of activated platelets increased the release of IL-8 and MCP-1, the principal chemoattractants for neutrophils and monocytes (42). In addition, platelet CD40L enhanced the expression of endothelial adhesion receptors including E-selectin, VCAM-1, and ICAM-1, all molecules that mediate the attachment of neutrophils, monocytes, and lymphocytes to the inflamed vessel wall (42). Moreover, CD40L induces endothelial tissue factor expression (43). Hence, like IL-1β, CD40L expressed on platelets induces ECs to release chemokines and to express adhesion molecules, thereby generating signals for the recruitment of leukocytes in the process of inflammation. CD40 ligation on ECs, smooth muscle cells, and macrophages initiates the expression and release of matrix-degrading enzymes, the MMPs. These enzymes, which degrade ECM proteins, significantly contribute to destruction and remodeling of inflamed tissue. Activated platelets release MMP-2 during aggregation (33, 44). Furthermore, adhesion of activated platelets to ECs results in generation and secretion of MMP-9 and of the protease receptor urokinase-type plasminogen activator receptor (uPAR) on cultured endothelium (45). The endothelial release of MMP-9 is dependent on both the fibrinogen receptor GPIIb/IIIa and CD40L, since inhibition of either mechanism resulted in reduction of platelet-induced matrix degradation activity of ECs. Moreover, GPIIb/IIIa ligation results in substantial release of CD40L in the absence of any further platelet agonist (45, 46) (Figure 2). These results suggest that the release of platelet-derived proinflammatory mediators like CD40L is dependent on GPIIb/IIIa–mediated adhesion. This mechanism may be pathophysiologically important to localize platelet-induced inflammation of the endothelium at a site of firm platelet-endothelium adhesion.
Platelets synthesize biologically active proteins
The platelet “secretome” is derived from intracellular storage granules, eicosanoid and phospholipid synthesis (47), and, as recently recognized, synthesis of proteins from constitutive mRNAs (48–50). It is now clear that platelets synthesize biologically relevant proteins in response to physiological stimuli that are regulated via gene expression programs at the translational level (51). A small amount of constitutive protein synthesis (e.g., GPIIb/IIIa, vWF) occurs in nonstimulated platelets (52). However, synthesis of specific proteins is remarkably enhanced in response to activation. It seems that the pattern of protein synthesis is dependent on distinct agonists and requires ligation and engagement of β3 integrins for maximal production of the protein product. For example, platelet activation through platelet-activating factor or thrombin induces rapid and sustained synthesis of pro–IL-1β and processing into active IL-1β (49). The recent integration of proteomics into biochemical and biological platelet research has proved to be a powerful tool in understanding platelet function. Although platelet proteomics is a young field, remarkable advances have already been accomplished. To date, more than 300 proteins released by human platelets after thrombin activation have been identified (53, 54). Coppinger et al. showed that, while absent in normal vasculature, a variety of newly described platelet-derived proteins that may promote atherogenesis (e.g., secretoganin III, calumenin, cyclophilin A) are present in human atherosclerotic tissue (54). Thus, proteomics and analysis of activation-dependent protein synthesis open a new and promising direction of platelet research and may disclose novel molecular mechanisms of platelet-mediated inflammation and atherothrombosis.
Platelet interactions with leukocytes
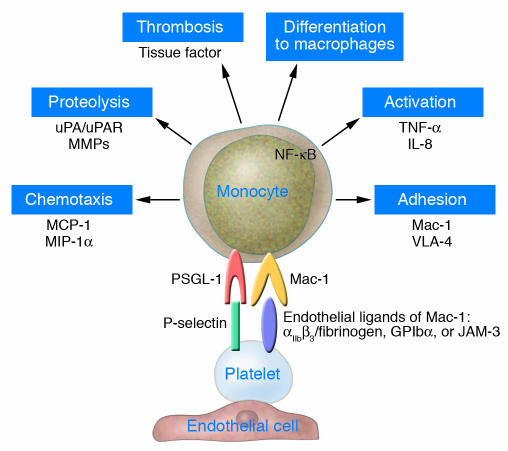
When activated, platelets coaggregate with circulating leukocytes (55). Once adherent to the vascular wall, platelets also provide a sticky surface to recruit leukocytes to the vessel wall. Recruitment of circulating leukocytes to the vascular wall requires multistep adhesive and signaling events that result in the infiltration of inflammatory cells into the blood vessel wall, including selectin-mediated attachment and rolling, leukocyte activation, integrin-mediated firm adhesion, and diapedesis. Leukocytes tether to adherent platelets via PSGL-1–P-selectin interactions (56, 57) and, subsequently, firmly adhere via binding of Mac-1 (CD11b/CD18, αMβ2) to GPIbα (58) and/or other receptors of the platelet membrane, including JAM-3 (59) and ICAM-2 (60), or bridging proteins such as fibrinogen (bound to GPIIb/IIIa) (61, 62) or high–molecular weight kininogen (bound to GPIbα) (63) (Figure 3). However, the exact contribution of each system remains to be elucidated in vivo. During this adhesive process, receptor engagement of PSGL-1 and Mac-1 together with platelet-derived inflammatory compounds induces inflammatory cascades in monocytes (64, 65). In addition, engagement of PSGL-1 by P-selectin also drives translationally regulated expression of proteins such as uPAR, a critical surface protease receptor and regulator of integrin-mediated leukocyte adhesion in vivo (66–68) (Figure 3).
Figure 3.
Adherent platelets recruit and inflame monocytes. Adherent and/or activated platelets mainly interact with monocytic PSGL-1 via P-selectin and with monocytic Mac-1 (αMβ2) via αIIbβ3 (and fibrinogen bridging) or GPIbα. Thereby, platelets initiate monocyte secretion of chemokines, cytokines, and procoagulatory tissue factor, upregulate and activate adhesion receptors and proteases, and induce monocyte differentiation into macrophages. Thus, platelet-monocyte interaction provides an atherogenic milieu at the vascular wall that supports plaque formation.
Platelets in animal models of atherosclerosis
Abundant recent data support the concept of atherosclerosis as a chronic inflammatory disease (69). However, the contribution of platelets to the process of atherosclerosis was unclear for decades. With the help of intravital microscopy and the availability of appropriate atherosclerotic animal models, it has become evident that platelets adhere to the arterial wall in vivo even in the absence of EC denudation (70, 71). Theilmeier and coworkers found in hypercholesteremic rabbits that platelets adhere to predilection sites of atherosclerosis before lesions are detectable (70). Recently, we showed that platelets adhere to the vascular endothelium of the carotid artery in apoE-deficient mice before the development of manifest atherosclerotic lesions (71). Substantial platelet adhesion to the carotid artery early in atherogenesis involved both platelet GPIbα and αIIbβ3. Platelet adhesion to the carotid wall coincided with inflammatory gene expression and preceded the invasion of leukocytes (71). Prolonged antibody blockade of platelet GPIbα profoundly reduced leukocyte accumulation in the arterial intima and attenuated atherosclerotic lesion formation. Moreover, apoE-deficient mice lacking GPIIb exhibit substantially reduced formation of atherosclerotic lesions (72). Further, circulating activated platelets and platelet–leukocyte/monocyte aggregates promote formation of atherosclerotic lesions (73). The importance of P-selectin for atherosclerotic lesion development has also been described (74–76). The importance of platelets in development of atherosclerosis is also documented by Belton et al., who showed that inhibition of COX-1, an enzyme that is exclusively present in platelets, prevented gross lesion formation in apoE–/– mice (77). Interestingly, effective inhibition of downstream activation cascades can also effectively inhibit atherosclerosis in various models. The body of evidence implicating the CD40-CD40L system in atherogenesis is compelling: Disruption of CD40-CD40L in mouse models of atherosclerosis both downregulated early disease events such as initial plaque formation (78, 79) and could halt the progression of established lesions to more advanced unstable lesions (80). Further, IL-1–dependent mechanisms have been shown to promote atherogenesis in mice in vivo (81, 82).
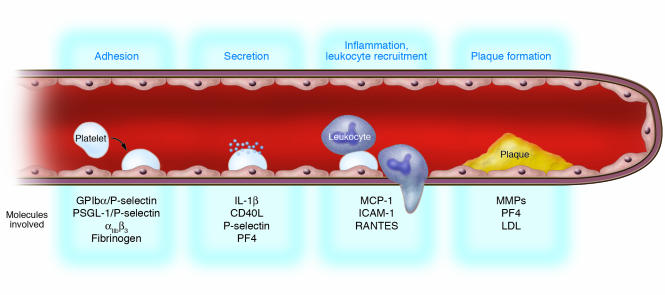
Together, these in vitro and in vivo data overwhelmingly support the hypothesis that platelets trigger early events of atherogenesis and are critical for atherosclerotic lesion formation in mice (Figure 4). This complex process appears to require adhesive mechanisms mediated, basically, by PSGL-1/P-selectin and β3 integrins. Adhesion-induced secretion of proatherogenic proteins (e.g., CD40L, IL-1β) and incorporation of platelet-derived products (e.g., PF4) result in inflammation of the vessel wall and subsequent vascular remodeling.
Figure 4.
Hypothetical model of atherogenesis triggered by platelets. Activated platelets roll along the endothelial monolayer via GPIbα/P-selectin or PSGL-1/P-selectin. Thereafter, platelets firmly adhere to vascular endothelium via β3 integrins, release proinflammatory compounds (IL-1β, CD40L), and induce a proatherogenic phenotype of ECs (chemotaxis, MCP-1; adhesion, ICAM-1). Subsequently, adherent platelets recruit circulating leukocytes, bind them, and inflame them by receptor interactions and paracrine pathways, thereby initiating leukocyte transmigration and foam cell formation. Thus, platelets provide the inflammatory basis for plaque formation before physically occluding the vessel by thrombosis upon plaque rupture.
However, the results from mouse models cannot be uncritically transferred to the human situation, since mouse platelets differ substantially from human platelets in several respects (e.g., higher platelet count, different expression profile of surface receptors). What is the evidence that platelets are also linked to atheroprogression in humans?
Platelets and atherosclerosis in humans
The availability of conclusive data obtained in humans is very limited. Nevertheless, there is some evidence that platelets are involved in atheroprogression in humans. An increase in systemic platelet activation has been described for a variety of atherosclerotic diseases, including coronary artery disease (83), transplant vasculopathy (84), and carotid artery disease (85). Recently, it was found that activation of circulating platelets is associated with enhanced wall thickness of the carotid artery in humans (85, 86). Enhanced systemic platelet activation correlates with progression of intima media thickness of the carotid artery in type 2 diabetes (85). Moreover, PF4 (41) and other platelet-derived chemokines and growth factors (54) are found in human atherosclerotic plaques. Previously, we reported that systemic platelet activation is associated with an accelerated progression of transplant vasculopathy (84). Current antiplatelet drugs (aspirin, clopidogrel) do not seem to have a major impact on atheroprogression in humans. However, most antiplatelet strategies in high-risk patients have been applied for secondary prevention at a rather advanced atherosclerotic disease state. Clinical studies are required that evaluate the efficacy of a long-term antiplatelet strategy for primary prevention in high-risk patients at an early stage of atherosclerotic disease.
Future considerations
It has become clear that, besides their role in hemostasis and thrombosis, platelets regulate a variety of inflammatory responses and are key players in atherothrombosis. Thrombosis and inflammation are therefore linked rather than separate entities. Because atherothrombotic diseases are a major cause of morbidity and mortality in developed countries, understanding the role of platelets in vascular inflammation and atherosclerosis is an important challenge. Major achievements have been made in elucidating the molecular mechanisms of platelet interaction with ECs of the arterial wall. Defining the specific requirements for platelets adhering to endothelium may lead to the development of novel therapeutic strategies. The era of genomics and proteomics has recently been introduced in platelet research and will continue to offer major tools to help understand platelet pathology in the course of atherosclerosis.
Footnotes
Nonstandard abbreviations used: CD40L, CD40 ligand; GP, glycoprotein; HUVEC, human umbilical vein EC; MCP-1, monocyte chemoattractant protein-1; PF4, platelet factor 4; uPAR, urokinase-type plasminogen activator receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nat. Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.Massberg S, et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J. Exp. Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieswandt B, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 5.Arya M, et al. Glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3): effects of receptor clustering and von Willebrand factor adhesion. J. Thromb. Haemost. 2003;1:1150–1157. doi: 10.1046/j.1538-7836.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 6.Kasirer-Friede A, et al. Lateral clustering of platelet GP Ib-IX complexes leads to up-regulation of the adhesive function of integrin alpha IIbbeta 3. J. Biol. Chem. 2002;277:11949–11956. doi: 10.1074/jbc.M108727200. [DOI] [PubMed] [Google Scholar]
- 7.Kahn ML. Platelet-collagen responses: molecular basis and therapeutic promise. Semin. Thromb. Hemost. 2004;30:419–425. doi: 10.1055/s-2004-833477. [DOI] [PubMed] [Google Scholar]
- 8.Gawaz M, Neumann FJ, Ott I, Schiessler A, Schomig A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation. 1996;93:229–237. doi: 10.1161/01.cir.93.2.229. [DOI] [PubMed] [Google Scholar]
- 9.Gawaz M, et al. Vitronectin receptor (alpha(v)beta3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation. 1997;96:1809–1818. doi: 10.1161/01.cir.96.6.1809. [DOI] [PubMed] [Google Scholar]
- 10.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J. Exp. Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etingin OR, Silverstein RL, Hajjar DP. von Willebrand factor mediates platelet adhesion to virally infected endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5153–5156. doi: 10.1073/pnas.90.11.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gawaz M, et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148:75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RC, et al. Blood cell dynamics in P-selectin-deficient mice. Blood. 1995;86:1106–1114. [PubMed] [Google Scholar]
- 14.Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenette PS, et al. Platelet-endothelial interactions in inflamed mesenteric venules. Blood. 1998;91:1318–1324. [PubMed] [Google Scholar]
- 16.Massberg S, et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood. 2004;103:136–142. doi: 10.1182/blood-2002-11-3417. [DOI] [PubMed] [Google Scholar]
- 17.Massberg S, et al. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood. 1998;92:507–515. [PubMed] [Google Scholar]
- 18.Massberg S, et al. Increased adhesion and aggregation of platelets lacking cyclic guanosine 3′,5′-monophosphate kinase I. J. Exp. Med. 1999;189:1255–1264. doi: 10.1084/jem.189.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massberg S, et al. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]
- 20.Subramaniam M, et al. Defects in hemostasis in P-selectin-deficient mice. Blood. 1996;87:1238–1242. [PubMed] [Google Scholar]
- 21.Romo GM, et al. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J. Exp. Med. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenette PS, et al. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J. Exp. Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laszik Z, et al. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. 1996;88:3010–3021. [PubMed] [Google Scholar]
- 24.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J. Exp. Med. 1991;174:785–790. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplanski G, et al. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- 27.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2–/– mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 28.Lu B, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gawaz M, et al. Activated platelets induce monocyte chemotactic protein-1 secretion and surface expression of intercellular adhesion molecule-1 on endothelial cells. Circulation. 1998;98:1164–1171. doi: 10.1161/01.cir.98.12.1164. [DOI] [PubMed] [Google Scholar]
- 30.Gawaz M, et al. Transient platelet interaction induces MCP-1 production by endothelial cells via I kappa B kinase complex activation. Thromb. Haemost. 2002;88:307–314. [PubMed] [Google Scholar]
- 31.Dickfeld T, et al. Transient interaction of activated platelets with endothelial cells induces expression of monocyte-chemoattractant protein-1 via a p38 mitogen-activated protein kinase mediated pathway. Implications for atherogenesis. Cardiovasc. Res. 2001;49:189–199. doi: 10.1016/s0008-6363(00)00220-0. [DOI] [PubMed] [Google Scholar]
- 32.Ross R, Bowen-Pope DF, Raines EW. Platelets, macrophages, endothelium, and growth factors. Their effects upon cells and their possible roles in atherogenesis. Ann. N. Y. Acad. Sci. 1985;454:254–260. doi: 10.1111/j.1749-6632.1985.tb11865.x. [DOI] [PubMed] [Google Scholar]
- 33.Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW. Release of gelatinase A during platelet activation mediates aggregation. Nature. 1997;386:616–619. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- 34.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ. Res. 2005;96:612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 35.von Hundelshausen P, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 36.Schober A, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 37.von Hundelshausen P, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood. 2005;105:924–930. doi: 10.1182/blood-2004-06-2475. [DOI] [PubMed] [Google Scholar]
- 38.Scheuerer B, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 39.Sachais BS, et al. Platelet factor 4 binds to low-density lipoprotein receptors and disrupts the endocytic machinery, resulting in retention of low-density lipoprotein on the cell surface. Blood. 2002;99:3613–3622. doi: 10.1182/blood.v99.10.3613. [DOI] [PubMed] [Google Scholar]
- 40.Nassar T, et al. Platelet factor 4 enhances the binding of oxidized low-density lipoprotein to vascular wall cells. J. Biol. Chem. 2003;278:6187–6193. doi: 10.1074/jbc.M208894200. [DOI] [PubMed] [Google Scholar]
- 41.Pitsilos S, et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb. Haemost. 2003;90:1112–1120. doi: 10.1160/TH03-02-0069. [DOI] [PubMed] [Google Scholar]
- 42.Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 43.Slupsky JR, et al. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb. Haemost. 1998;80:1008–1014. [PubMed] [Google Scholar]
- 44.Fernandez-Patron C, et al. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb. Haemost. 1999;82:1730–1735. [PubMed] [Google Scholar]
- 45.May AE, et al. Engagement of glycoprotein IIb/IIIa (alpha(IIb)beta3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation. 2002;106:2111–2117. doi: 10.1161/01.cir.0000033597.45947.0f. [DOI] [PubMed] [Google Scholar]
- 46.Nannizzi-Alaimo L, Alves VL, Phillips DR. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 2003;107:1123–1128. doi: 10.1161/01.cir.0000053559.46158.ad. [DOI] [PubMed] [Google Scholar]
- 47.Reed GL. Platelet secretory mechanisms. Semin. Thromb. Hemost. 2004;30:441–450. doi: 10.1055/s-2004-833479. [DOI] [PubMed] [Google Scholar]
- 48.Lindemann S, et al. Integrins regulate the intracellular distribution of eukaryotic initiation factor 4E in platelets. A checkpoint for translational control. J. Biol. Chem. 2001;276:33947–33951. doi: 10.1074/jbc.M104281200. [DOI] [PubMed] [Google Scholar]
- 49.Lindemann S, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindemann SW, Weyrich AS, Zimmerman GA. Signaling to translational control pathways: diversity in gene regulation in inflammatory and vascular cells. Trends Cardiovasc. Med. 2005;15:9–17. doi: 10.1016/j.tcm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Weyrich AS, et al. Change in protein phenotype without a nucleus: translational control in platelets. Semin. Thromb. Hemost. 2004;30:491–498. doi: 10.1055/s-2004-833484. [DOI] [PubMed] [Google Scholar]
- 52.Kieffer N, Guichard J, Farcet JP, Vainchenker W, Breton-Gorius J. Biosynthesis of major platelet proteins in human blood platelets. Eur. J. Biochem. 1987;164:189–195. doi: 10.1111/j.1432-1033.1987.tb11010.x. [DOI] [PubMed] [Google Scholar]
- 53.McRedmond JP, et al. Integration of proteomics and genomics in platelets: a profile of platelet proteins and platelet-specific genes. Mol. Cell. Proteomics. 2004;3:133–144. doi: 10.1074/mcp.M300063-MCP200. [DOI] [PubMed] [Google Scholar]
- 54.Coppinger JA, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 55.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb. Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 56.Evangelista V, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- 57.Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb. Haemost. 1999;81:1–7. [PubMed] [Google Scholar]
- 58.Simon DI, et al. Platelet glycoprotein ibalpha is a c ounterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J. Exp. Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santoso S, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J. Clin. Invest. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright SD, et al. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altieri DC, Bader R, Mannucci PM, Edgington TS. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J. Cell Biol. 1988;107:1893–1900. doi: 10.1083/jcb.107.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavakis T, et al. High molecular weight kininogen regulates platelet-leukocyte interactions by bridging Mac-1 and glycoprotein Ib. J. Biol. Chem. 2003;278:45375–45381. doi: 10.1074/jbc.M304344200. [DOI] [PubMed] [Google Scholar]
- 64.Weyrich AS, et al. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neumann FJ, et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997;95:2387–2394. doi: 10.1161/01.cir.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 66.May AE, et al. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J. Exp. Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preissner KT, Kanse SM, May AE. Urokinase receptor: a molecular organizer in cellular communication. Curr. Opin. Cell Biol. 2000;12:621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 68.May AE, et al. Urokinase receptor surface expression regulates monocyte adhesion in acute myocardial infarction. Blood. 2002;100:3611–3617. doi: 10.1182/blood-2002-03-0778. [DOI] [PubMed] [Google Scholar]
- 69.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theilmeier G, et al. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–4493. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 71.Massberg S, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massberg S, et al. Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb. Circulation. 2005;112:1180–1188. doi: 10.1161/CIRCULATIONAHA.105.539221. [DOI] [PubMed] [Google Scholar]
- 73.Huo Y, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 74.Dong ZM, et al. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 76.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 77.Belton OA, Duffy A, Toomey S, Fitzgerald DJ. Cyclooxygenase isoforms and platelet vessel wall interactions in the apolipoprotein E knockout mouse model of atherosclerosis. Circulation. 2003;108:3017–3023. doi: 10.1161/01.CIR.0000104565.78013.AD. [DOI] [PubMed] [Google Scholar]
- 78.Lutgens E, et al. Requirement for CD154 in the progression of atherosclerosis. Nat. Med. 1999;5:1313–1316. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 79.Schonbeck U, Sukhova GK, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 81.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 82.Kirii H, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 83.Willoughby S, Holmes A, Loscalzo J. Platelets and cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2002;1:273–288. doi: 10.1016/s1474-5151(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 84.Fateh-Moghadam S, et al. Changes in surface expression of platelet membrane glycoproteins and progression of heart transplant vasculopathy. Circulation. 2000;102:890–897. doi: 10.1161/01.cir.102.8.890. [DOI] [PubMed] [Google Scholar]
- 85.Fateh-Moghadam S, et al. Platelet degranulation is associated with progression of intima-media thickness of the common carotid artery in patients with diabetes mellitus type 2. Arterioscler. Thromb. Vasc. Biol. 2005;25:1299–1303. doi: 10.1161/01.ATV.0000165699.41301.c5. [DOI] [PubMed] [Google Scholar]
- 86.Koyama H, et al. Platelet P-selectin expression is associated with atherosclerotic wall thickness in carotid artery in humans. Circulation. 2003;108:524–529. doi: 10.1161/01.CIR.0000081765.88440.51. [DOI] [PubMed] [Google Scholar]