Abstract
A previously uncharacterized gene, DBC2 (deleted in breast cancer), was cloned from a homozygously deleted region at human chromosome 8p21. DBC2 contains a highly conserved RAS domain and two putative protein interacting domains. Our analyses indicate that DBC2 is the best candidate tumor suppressor gene from this region. It lies within the epicenter of the deletions and is homozygously deleted in 3.5% (7/200) of breast tumors. Mutation analysis of DBC2 led to discovery of two instances of somatic missense mutations in breast tumor specimens, whereas no missense mutations were found in other candidates from the region. Unlike other genes in the region, expression of DBC2 is often extinguished in breast cancer cells or tissues. Moreover, our functional analysis revealed that DBC2 expression in breast cancer cells lacking DBC2 transcripts causes growth inhibition. By contrast, expression of a somatic mutant discovered in a breast cancer specimen does not suppress the growth of breast cancer cells.
In the course of carcinogenesis, cancer cells acquire a number of genetic changes, many of which may have no effect on cellular function. There are, however, critical genetic alterations that affect two classes of genes: oncogenes that are activated or altered in function and tumor suppressor genes that are down-regulated or ablated in cancer cells. Discovery and analysis of these genetic changes have contributed to a better understanding of the molecular basis of cancer development. Studies on BRCA1 and -2 tumor suppressor genes have provided useful information for understanding familial breast cancer (1). Other tumor suppressor genes are involved in sporadic breast cancer, including PTEN and p53 (2, 3). However, it is likely that many, if not most, tumor suppressor genes responsible for sporadic breast cancer remain to be discovered. Representational difference analysis (RDA) is one tool that has been shown to be effective in the hunt for tumor suppressor genes (3, 4). We have analyzed a few breast cancer biopsies by RDA and isolated a number of deletion probes. An RDA probe detecting homozygous deletion was mapped to human chromosome 8p21, and we find this region to be deleted in other breast cancer specimens as well. We have mapped this region, cloned new genes residing therein, and have found somatic mutations in cancer cells for one of the genes from this locus.
Materials and Methods
Materials.
Breast tumor specimens were procured from a variety of sources. Aneuploid and diploid nuclei from breast tumor specimens were prepared by fluorescence-activated sorting by using an Elite EPS (Beckman Coulter). DNA from the sorted nuclei was immortalized by making DpnII and BfaI representations, by using methods described elsewhere (5). Cell lines were purchased from American Type Culture Collection or are from our permanent collection inherited from Jorgen Fogh (Memorial Sloan–Kettering Cancer Center). Chemicals and buffers were obtained from Sigma unless otherwise noted. Enzymes were obtained from New England Biolabs except TaqDNA Polymerase (AmpliTaq), from Perkin–Elmer. All enzymatic reactions were performed with buffers recommended by the supplier.
Deletion Analysis.
Deletion mapping was performed by quantitative PCR (Q-PCR) with the ABI Prism 7700 Sequence Detector (6). The Q-PCR was carried out in three stages: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 sec and 60°C for 1 min. DNA markers from 8p21 and other chromosomes were used for Q-PCR. DNA markers were derived from the original RDA probe WD31; from sequence tag sites WI-14743, WI-15206, and WI-16696; from genes we discovered [deleted in breast cancer 1 (DBC1) (GenBank accession no. AF293335) and deleted in breast cancer 2 (DBC2) (accession no. AY009093)]; and from known genes from this region, including EGR3 (accession no. NM004430), tumor necrosis factor receptor super family 10B (TNFRSF10A) (accession no. AF014794), TNFRSF10B (accession no. AC007868), and KIAA0273 (accession no. NM014759). Additionally, DNA probe ER48 was a subcloned fragment from a genomic clone from human chromosome 3p14, and WB23 was a cloned fragment from human chromosome 21p.
The protocols for RDA are described elsewhere (4). All oligonucleotides were synthesized by Operon Technologies (Alameda, CA), except that Taqman probes for Q-PCR were synthesized by Perkin–Elmer with 6-carboxyfluorescein at the 5′ end and 6-carboxy-tetramethyl-rhodamine at the 3′ end.
Gene Cloning.
Gene discovery methods included exon trapping (7), rapid isolation of cDNA by hybridization (8), cDNA library screening, and sequence determination of genomic clones followed by database searches. RNA ligase-mediated amplification of cDNA ends (RLM-RACE) was performed by using the First Choice RLM-RACE kit (Ambion, Austin, TX) (9). Computational analyses of the fragments were performed by basic local alignment search tool (blast), gcg Wisconsin Package, and a pfam hmm search (10, 11).
Mutation Analysis.
Mutational analyses were performed by a protein truncation test (PTT), denatured HPLC (DHPLC), and single-strand conformation polymorphism (SSCP). For PTT, the entire ORF of TNFRSF10B was amplified with primers, TAATACGACTCACTATAGGGAGCCACCATGGAACAACGGGGACAG and AATTGTGGCACTTTCCTACTGACT. The PTT procedure is described elsewhere (12). WAVE Nucleic Acid Fragment Analysis System (Transgenomic, Omaha, NE) was used for DHPLC (13). The target fragments were amplified by PCR with the GeneAmp PCR System 9600 (Perkin–Elmer). Control fragments were generated by PCR from normal human DNA. The samples and control fragments were mixed, denatured, reannealed to generate heteroduplexes, and then analyzed by DHPLC. The samples with different peak patterns were sequenced. Protocols for SSCP are described elsewhere (14). The primer sequences for Q-PCR, DHPLC, and SSCP used in this study will be provided on request.
Expression Analysis.
Expression analyses were mainly done by RT-PCR. Cytoplasmic RNA was extracted from cultured cells, and total RNA was extracted from tissue samples with guanidinium thiocyanate followed by centrifugation in cesium chloride solutions. The protocols are described elsewhere (15). RNA of normal tissues was either purchased from BD Biosciences (Palo Alto, CA) or extracted from surgical specimens. Avian myeloblastosis virus reverse transcriptase purchased from Boehringer Mannheim was used to synthesize cDNA with oligo-dT primers. PCR was then performed with AmpliTaq. The 50-μl PCR reaction mixture contains 1 μl of the cDNA, 200 μM of each dNTP, 1 μM of each primer, 2 units of AmpliTaq, and 1× AmpliTaq buffer. After initial denaturation at 94°C for 10 min, 30 cycles of 94°C for 30 sec, 60°C for 30 sec, and 72°C for 3 min were performed. The primer sequences used in this analysis were: AACATGGAGGCCCTGTGCCAGCAGAA and ACTCGCAGCTCCTGCATCTCT (DBC1); ACCATGTGGTACCCAGAAATC and TTAGACAACAGCAGCAGGGTCAGA (DBC2); AGTTTGACCAGAGATGC and TGACCAAGGCTGAATAAATCCC (TNFRSF10A); CTTGATTGTGGCTGTGTTTGTT and GCCACCTTTATCTCATTGTCCA (TNFR10B); AGCATGGAGCTGCTGTCCAC and TTAGCTCCCTGAACCTGGGCTACC (KIAA0273); and CCATGGAGGAGCCGCAGTCAGA and TTAGTCTGAGTCAGGCCCTTCTGT (p53).
Mutagenesis.
Mutation was introduced by using the Exsite Mutagenesis kit (Stratagene). Sequences of primers used for mutagenesis were ATGAACCTGAGTGAGGGGGAGCT and CAGCTCCCCCTCACTCAGGTT. The generated mutation was confirmed by sequencing of both strands.
Gene Expression.
HeLa and T-47D cells were grown in DMEM supplemented with 15% FCS. For constitutive gene expression, a mammalian expression vector pcDNA3.1/His (Invitrogen) was used. For inducible gene expression, an ecdysone gene expression system was used. Stable host cell lines carrying ecdysone receptors were established by using retroviral vectors (16). The genes of interest were cloned into pIND (Invitrogen) with an Xpress tag at the 5′ end and transfected into the host cell lines. All constructs were verified by sequencing. The cells were transformed with 15 μg of plasmid by electroporation (field strength = 0.25 kV/cm, capacitor = 950 μF) using Gene Pulser II (Bio-Rad) and selected with G418 (400 μg/ml) and Zeocin (Invitrogen) (150 μg/ml). Muristerone A (Invitrogen) (5 μM) was administered to induce the genes in pIND. Cell growth was monitored by using the CyQuant Cell Proliferation Assay kit (Molecular Probes).
Sequencing.
DNA sequencing was performed with the ABI Thermal Cycler Sequencing kit and ABI Prism 377 DNA Sequencer (Perkin–Elmer). Both strands were sequenced unless otherwise specified.
Results
Homozygous Deletion.
RDA of breast cancer samples generated a number of deletion probes, including WD31 from a tumor called BBr67. Homozygous deletion (HD) of WD31 in BBr67 was confirmed both by Southern blotting of the DNA representations made from the aneuploid nuclei of BBr67 and by the semiquantitative PCR analysis of genomic DNA made from these nuclei. WD31 was mapped to 8p21 by radiation hybrid panels and genomic library screening, and the locus was scrutinized further. We constructed a contig of genomic clones and applied positional cloning methods, described in Materials and Methods. We discovered two previously uncharacterized genes and identified four known genes extending outward from WD31: DBC1, DBC2, TNFRSF10A, TNFRSF10B, EGR3, and KIAA0273. These genes were mapped by us, and our mapping order was confirmed by comparison to the human genome sequence (www.ncbi.nlm.nih.gov/) (Fig. 1).
Figure 1.
Deletion map of 8p21. The vertical lines indicate the localization of sequence tag sites and genes used for deletion mapping, all of which were tested against 200 tumor samples. Genes are represented by a horizontal line with an arrowhead. The STSs are listed above the line, and the lines with a square indicate probes derived from the genes. The arrow with double heads indicates approximately 300 kb. Horizontal lines in the lower half represent deletions found in the tumor samples (named, Left). The DBC2 probe and WI-16696 demonstrated the highest deletion frequency. Both DBC2 and TNFRSF10B genes are overlapping with different orientations and deleted in all tumor specimens.
Deletion Analysis.
These genes, as well as nearby sequence tag sites, were then used for deletion analysis. Aneuploid and diploid nuclei from breast cancer were sorted and analyzed to determine deletion frequency and the deletion epicenter. Although aneuploid nuclei will be enriched for tumor cells, and diploid nuclei will be enriched for normal stroma, each fraction will be contaminated to varying extents with each other. Real-time PCR or Q-PCR was used to analyze genetic loss for markers in the region. Even in the presence of low levels of contamination, we can detect a homozygous deletion as a cycle difference between aneuploid and diploid samples (Fig. 2). We used multiple control markers for normalization, from chromosome 8 and other chromosomes. For example, we used ER48 and WB23 (from other chromosomes) to standardize the DNA amount and markers on the short arm of chromosome 8 to control for chromosome 8 copy number. In all cases tested, Q-PCR results were confirmed by Southern blotting of representations made from diploid and aneuploid nuclei (Fig. 2). The deletion mapping using 200 breast tumors revealed that the deletion frequency was 3.5%, and the deletion epicenter lay around a DNA marker WI-16696 that was surrounded by two genes. WI-16696 is contained in the intron 7 of DBC2 and located within 1 kb of the 3′ end of TNFRSF10B. On the basis of the transcription and deletion mapping (see Fig. 1), these two genes resided at the deletion epicenter, and hence we studied them further.
Figure 2.
Deletion analysis of tumor samples. (A) Real-time PCR analysis of a breast tumor sample CHTN41 is demonstrated. The abscissa is cycle number of PCR and the ordinate is quantity of PCR products. The control primer set in this figure is WB23 from chromosome 22. “D” and “A” represent DNA from diploid and aneuploid fractions, respectively. The amplification curve from aneuloid DNA with WD31 primer set has a several cycle difference from the others, indicating deletion of WD31 in this tumor. (B) Southern blot analysis of DpnII representation with WD31 probe is shown. Each lane contains 5 μg of DpnII restriction fragments. Lanes 1 and 2 are DNA from a tumor and a matched normal (CHTN40), respectively, showing no deletion. Lanes 3 and 4 contain DNA from a tumor and a matched normal pair (CHTN41), showing deletion. ER48 is a control probe derived from chromosome 3. Lane 3 has a very faint band for DBC2, whereas the control probe exhibits the same intensity for tumor and normal samples, representing homozygous deletion. Contamination of normal stromal cells is considered to contribute the faint band.
Mutation Analysis.
We began our study with TNFRSF10B, which encodes the Trail2 receptor for TNF, because mutations in the death domain of TNFRSF10B in nonsmall cell lung cancer had been previously reported (17). Mutation analysis of the entire ORF of TNFRSF10B was performed on 60 cancer cell lines by both protein truncation test and DHPLC followed by sequencing of variants, but no frameshift, missense, or nonsense mutations were found (see Table 1).
Table 1.
Frequency of codon-altering point mutations found in three genes in the deleted region
| Gene | Materials | Protein truncation test | SSCP | DHPLC |
|---|---|---|---|---|
| TNFRSF10B | Cell lines | 0/60 | NA | 0/60 |
| Primary tumor | NA | 0/90 | NA | |
| DBC2 | Cell lines | 0/60 | 2/60 | 2/65 |
| Primary tumor | NA | 2/65 | NA | |
| DBC1 | Cell lines | 0/60 | NA | 0/60 |
| Primary tumor | NA | NA | NA |
NA, not applicable.
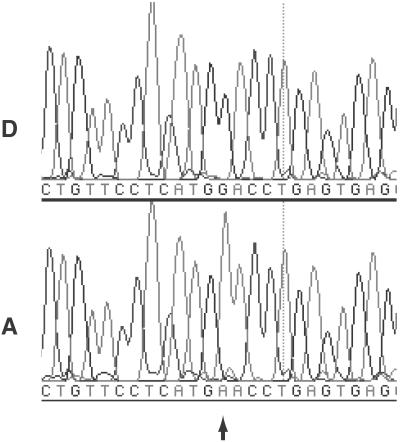
The coding region of DBC2 was also screened for mutations by DHPLC and SSCP, and variants further studied by sequencing, revealing two missense mutations in the BTB/POZ (broad-complex/tramtrack/bric-a-brac/poxvirus/zinc finger) domain, found in lung and breast cancer cell lines. Ninety-five primary tumors and matched normals were then screened by either DHPLC or SSCP for mutations in either of these two genes (see Table 1). Samples showing variants in retention time (DHPLC) or mobility shifts (SSCP) were sequenced, and tumors were compared with matched normals (Fig. 3). No frameshift, missense, or nonsense mutations were found in TNFRSF10B. However, two missense mutations within DBC2 were again discovered within tumors but not in matched normals, indicating that somatic mutations had occurred. One of these mutations, causing Asp-299→Asn, was in the BTB/POZ domain and was confirmed by an independent PCR amplification and sequencing reaction. This mutation was used for functional studies described below. In total, from cell lines and tumors, four missense mutations in the coding region of DBC2 were found (see Fig. 4), but no significant mutations were found in TNFRSF10B.
Figure 3.
Electrophoretogram of sequencing analysis. “D” and “A” designate sequencing results of DNA from diploid and aneuploid nuclei of tumor sample CHTN56, respectively. Arrow indicates position of somatic mutation.
Figure 4.
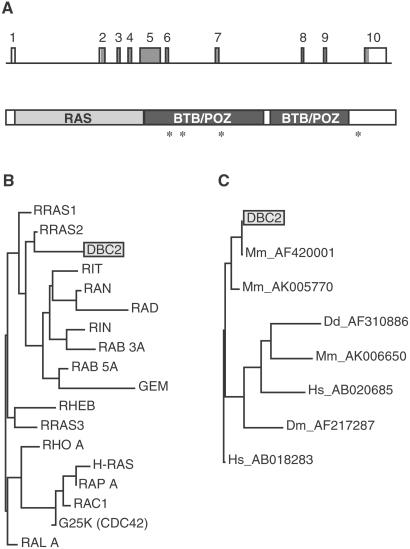
Structure of DBC2. (A) The intron–exon structure of DBC2 (GenBank accession no. AF315385) and predicted functional domains of DBC2 are shown. The rectangles with a number represent exons. The shaded area indicates coding sequences. Based on deduced amino acid sequence, molecular mass of the predicted DBC2 product is 83 kDa. The asterisks denote missense mutations we have discovered. There were three in exon 5: T to G (7762), causing Tyr-284→Asp in a lung cancer cell line (SK-Mes-1), G to A (7807), causing Asp-299→Asn in a primary breast tumor (CHTN56), and A to C (8015), causing Asp-368→Ala in a breast cancer cell line (BT483); and one in exon 9: C to A (16389), causing Phe-647→Thr in a primary breast tumor (P-1). (B) Phylogenic tree demonstrating overall homology between DBC2 and human RAS family members is shown. A table of the pairwise distances between the genes was prepared by GCG Wisconsin Package, and a phylogram was then created from the table by using the neighbor-joining method. The length of a horizontal line is proportional to estimated divergence along each branch. Accession numbers of the genes used for this analysis are: RRAS1 (P10301); RRAS2 (P17082); RIT (AAB42213); RAN (P17080); RAD (P55042); RIN (AAB42214); RAB3A (P20336); GEM (P55040); RHEB (Q15382); RRAS2 (O14807); RHO A (P06749); H-RAS (P01112); RAP A (P10113); RAC1 (P15154); G25K (P25763); and RAL A (P11233). (C) RAS-BTB proteins from different organisms are compared. Accession numbers of the genes are listed after the designation of the organism: Hs, Homo sapiens; Mm, M. musculus; Dm, D. melanogaster; and Dd, Dictyostelium discoideum.
Computational Analysis.
To the best of our knowledge, DBC2 is a previously unreported gene. The cDNA for DBC2 was obtained by cDNA library screening and completeness confirmed by using RNA ligase-mediated amplification of cDNA ends. pfam analysis of our sequence revealed DBC2 contained a RAS domain at its 5′ end and two BTB/POZ domains. The RAS domain of DBC2 has high homology to that of small GTP-binding proteins (G proteins), including the sites for binding to, and hydrolysis of, GTP (Fig. 4). However, DBC2 is distinct from the typical RAS family members. First, DBC2 has additional functional domains. Second, DBC2 lacks the C-terminal lipid-binding sites that anchor the typical family member to membranes. blast analysis revealed two human genes with a similar RAS-BTB/POZ structure: KIAA0740 and KIAA0878 (accession nos. AB018283 and AB020685, respectively), the first one with high homology, and several genes in other organisms including Mus musculus (accession nos. AF420001, AK005770, and AK006650), Drosophila melanogaster (accession no. AF217287), and Dictyostelium discoideum (accession no. AF310886) (Fig. 4).
The BTB/POZ domain functions as a specific protein–protein interaction motif in known proteins (18). It is evolutionarily conserved and found primarily at the N terminus of zinc-finger transcription factors. Many BTB/POZ proteins are transcriptional regulators that mediate gene expression through the control of chromatin conformation; examples are BACH1 and -2, PATZ, and PLZF, all of which interact with other proteins through their BTB/POZ domains. Unlike these proteins, DBC2 has two BTB/POZ domains located at the C terminus.
Expression Analysis.
To gain more support for the hypothesis that loss of DBC2 may play a role in breast cancer, and possibly other cancers, we examined its expression pattern in a variety of neoplastic and normal cells and tissues. We used RT-PCR to monitor the presence of DBC2 mRNA, as well as two other genes from its locus, DBC1 and TNFRSF10B. In a subset of cases, we tested our results by RNA blotting, and in all those cases, our RT-PCR results were confirmed. In Fig. 5, we show some representative data, illustrating the lack of expression of DBC2 in T-47D, a breast cancer cell line. Our data are summarized in Table 2. For neoplastic cells and tissues, we drew on our collection of cultured tumor cell lines and frozen tissue biopsies from various sources, and for normal, we used multiple normal tissue samples, including breast and placenta (Materials and Methods). We find that all three genes are expressed ubiquitously in all normal sources. However, DBC2 expression is extinguished in about half of breast and lung cancer specimens, but not in colon or other cancers we examined. In contrast, neither TNFRSF10B nor DBC1 expression is substantially extinguished in cancers from any source.
Figure 5.
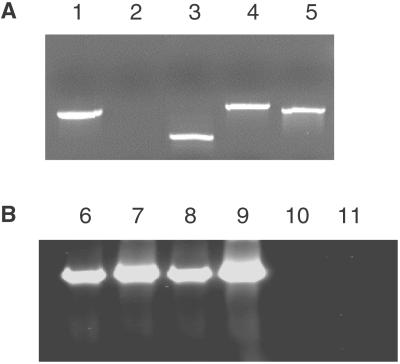
RT-PCR analysis of tumor specimens. (A) RT-PCR products from mRNA of T-47D with five primer sets. Lanes 1–5 contain RT-PCR products with primers of DBC1, DBC2, KIAA0273, TNFRSF10A, and p53, respectively. Transcripts of all genes but DBC2 were detected in T-47D. (B) RT-PCR products with the DBC2 primer pair. Lanes 6–11 contain RT-PCR products from NCI-H82, Saos-2, SK-BR-3, SW 982, T-47D, and T84, respectively. DBC2 expression was observed in NCI-H82, Saos-2, SK-BR-3, and SW 982 but not in T-47D or T84.
Table 2.
RT-PCR analysis of DBC2, TNFRSF10B, and DBC1
| Tissue of origin | DBC2 | TNFRSF10B | DBC1 | |
|---|---|---|---|---|
| Neoplasm | Breast | 42% (8/19) | 89% (17/19) | 84% (16/19) |
| Lung | 50% (7/14) | 93% (13/14) | 86% (12/14) | |
| Colon | 83% (10/12) | 100% (12/12) | 92% (11/12) | |
| Other | 91% (10/11) | 82% (9/11) | 100% (11/11) | |
| Normal | Breast | 100% (13/13) | 100% (8/8) | 100% (8/8) |
| Lung | 100% (1/1) | 100% (1/1) | 100% (1/1) | |
| Placenta | 100% (2/2) | 100% (2/2) | 100% (2/2) | |
| Brain | 100% (3/3) | 100% (3/3) | 100% (3/3) | |
| Total | 100% (19/19) | 100% (14/14) | 100% (14/14) |
The percentage of the specimens with detectable expression of the indicated gene is listed. The denominator in parentheses indicates the number of specimens tested and the numerator, the number of positives. Neoplasm specimens are cell lines; normal specimens are tissue.
Functional Analysis of DBC2.
To study the function of DBC2, we expressed wild-type and mutated DBC2 in cancer cell lines. We generated the G-to-A mutation at nucleotide position 7807 (Asp-299→Asn), which was discovered in breast tumor specimen CHTN56.
First, wild-type DBC2 and the mutant were cloned into pcDNA3.1/His for constitutive expression. Use of this vector results in expression of an epitope-tagged product. Wild-type and mutant DBC2 vectors were introduced into HeLa cells that express endogenous DBC2. Expression of exogenous genes was verified by Western blot analysis, and neither affected the growth of the HeLa cells. Next we tested T-47D, the breast cancer cell line described above and in Fig. 5. T-47D cells transformed with wild-type DBC2 were difficult to obtain, and even when transformants were cloned, expression of DBC2 could not be detected.
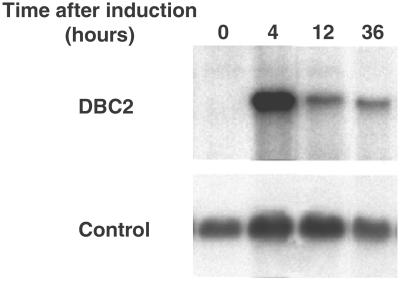
We therefore used an ecdysone inducible gene expression system to analyze effects of DBC2 expression in T-47D, using pIND. T-47D stopped proliferating 12 h after induction of wild-type DBC2. Growth suppression lasted for about 36 h. In contrast, induced expression of mutant DBC2 (Asp-299→Asn) did not suppress growth (Fig. 6). Induced expression was confirmed by RNA blot analysis (see for example Fig. 7).
Figure 6.
Effects of gene induction on the growth of T47D. T-47D cells with various constructs were treated with 5 μM Muristerone A. Percent increase of the cell count from 12 h after induction is shown. Expression of wild-type DBC2 inhibits cell growth of T47D between 12 and 48 h after gene induction, whereas mutant DBC2 and the empty vector have no influence on T47D proliferation.
Figure 7.
RNA blot analysis of T47D with inducible DBC2. T-47D cells with inducible DBC2 were treated with 5 μM of Muristerone A. DBC2 expression was examined at various time points after induction. The DBC2 transcript was detected only after induction. The control is β actin.
Discussion
HDs have been a good starting point for the discovery of tumor suppressor genes. Many researchers have tried to detect these HDs by comparing tumors with corresponding normal tissues (19). Arbitrarily primed PCR (APPCR) has been successfully used in some cases, but it can examine only a small portion of genome, because certain combinations of primer will amplify limited numbers of fragments. Furthermore, cloning the differences detected by APPCR is often difficult. Comparative genomic hybridization can screen the entire genome and is a powerful tool for detecting loss of heterozygosity (LOH) and amplification, but its relatively low resolution neither allows HD detection nor provides much help in localizing regions. DNA microarray technology may be sufficiently sensitive to detect HD (20), but the true utility of this technique for identifying tumor suppressor genes remains to be established. RDA is currently an effective method to clone HD probes (3, 4), although it too has certain limitations. These limitations include the need for further analysis to distinguish HD from LOH, and that HD probes found by RDA are generally from large deletions. Hence, detailed deletion mapping is required to define the targeted genetic regions. The first problem is not insurmountable. RDA favors detection of HD over LOH, and RDA probes can be further analyzed to distinguish between these events. The second problem is likely to ease as the transcription map of humans becomes better defined.
The initial discovery of an RDA HD probe has resulted in the definition of a region on chromosome 8p that is frequently deleted in breast cancer. The HD region at 8p21, as illustrated here, contains at least six genes. In this report, we have focused on the analysis of two genes, TNFRSF10B and DBC2, because they reside in the deletion epicenter. There are four other genes that map to this region and are deleted in at least one tumor, but they are less frequently deleted. One of these, DBC1, was discovered by us; although it has a long ORF, it shows no homology to any known genes. It is expressed ubiquitously, but we found no point mutations within its coding domains (see Table 1). KIAA0273, found on the border of the region, corresponds to a spliced EST with no known function, and we do not find it expressed in normal breast tissue (data not presented). It was not studied further. EGR3 is a transcription factor implicated in the development of neuromuscular junctions. Knock-out mice are not reported to show increased tumorigenicity, and it was not studied further (21). TNFRSF10A encodes a decoy receptor for TNF, and we would therefore not suspect it as a tumor suppressor.
In contrast to EGR3, DBC1, KIAA0273, and TNFRSF10A, TNFRSF10B looked like a reasonable tumor suppressor candidate. TNFRSF10B encodes the Trail2 receptor for TNF, and mutations in the death domain of TNFRSF10B in nonsmall cell lung cancer had been previously reported. Overexpression of TNFRSF10B induces apoptosis of tumor cells in a p53-dependent manner (22). However, we did not detect mutations in TNFRSF10B in breast cancers, implying either that TNFRSF10B is not the tumor suppressor gene involved in breast cancer, or that its inactivation mechanism is not frequently caused by mutations in coding regions. We believe the latter to be unlikely, but TNFRSF10B cannot be completely ruled out as a candidate tumor suppressor for breast cancer.
The discovery of missense mutations in the coding region of DBC2, residing at the epicenter of deletion, makes DBC2 the lead candidate for a tumor suppressor gene within the deletion locus at 8p21. Three of four mutations we discovered are located in the BTB/POZ domain and create radical amino acid substitutions likely to change protein function. In fact, functional analysis revealed that DBC2 suppresses growth of a breast cancer cell line T-47D, but a mutant (Asp-299→Asn) does not. If we sum all deletions and point mutations, we find genetic alteration of DBC2 in nearly 10% of breast cancer samples. Furthermore, our work indicates that, whereas DBC2, TNFRSF10B, and DBC1 are all expressed in normal breast tissue, only expression of DBC2 is frequently extinguished in breast cancers (11/19).
All these data suggest the involvement of DBC2 in breast cancer, but its involvement in other cancers is not excluded. In fact, mutation in DBC2 was found in one lung cancer cell line, and DBC2 expression was found extinguished in lung cancers.
DBC2 expression is not suppressed in colon cancer or other types of tumors we have tested. These findings imply that DBC2 may function as a tumor suppressor in a tissue specific manner. A possible explanation is that DBC2 tumor suppressor function may be circumvented by alternative biological pathways in certain tissues. The existence of altered response to DBC2 is supported by the observation that nearly half of breast cancer cells express DBC2. This hypothesis will also explain resistance of HeLa cells to growth inhibition caused by DBC2 expression.
The structure of DBC2 suggests that it encodes a signal transduction protein. It encodes a protein with significant RAS homology and contains known protein–protein interaction domains. Its novel combination of structural domains, lack of a membrane anchor sequence at the C terminus, and divergence from RAS proteins with known functions suggest that its function may be distinct from other members of the superfamily. Curiously, although DBC2 is an evolutionarily conserved protein, with homologs M. muculus and D. melanogaster, homologs are not found in all eukaryotes and are conspicuously absent in Caenorhabditis elegans (23) and Saccharomyces cerevisiae (http://genome-www.stanford.edu/Saccharomyces/). Further work is clearly warranted to illuminate the role of DBC2 in normal and pathophysiology.
Acknowledgments
This research was supported by grants to M.H. from the V Foundation, GCCARES (Glen Cove CARES, Inc.), and the Department of Defense Breast Cancer Research Program, which is managed by the U.S. Army Medical Research and Materiel Command (BC991222). This work was supported by a grant to M.C.K. from the National Institutes of Health (R01 CA27632). This work was also supported by grants to M.W. from the National Institutes of Health and National Cancer Institute (OIG-CA39829; 5R01-CA78544; 1R21-CA81674; 5P50-CA68425-05/SPORE); Tularik Incorporated; 1 in 9: The Long Island Breast Cancer Action Coalition; Lillian Goldman; and the Breast Cancer Research Foundation. M.W. is an American Cancer Society Research Professor.
Abbreviations
- DHPLC
denatured HPLC
- HD
homozygous deletion
- Q-PCR
quantitative PCR
- SSCP
single-strand conformation polymorphisms
- RDA
representational difference analysis
Footnotes
References
- 1.Bishop D T. Ann Oncol. 1999;10:113–119. [PubMed] [Google Scholar]
- 2.Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 4.Lisitsyn N A, Lisitsina N M, Dalbagni G, Barker P, Sanchez C A, Gnarra J, Linehan W M, Reid B J, Wigler M H. Proc Natl Acad Sci USA. 1995;92:151–155. doi: 10.1073/pnas.92.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucito R, Nakimura M, West J A, Han Y, Chin K, Jensen K, McCombie R, Gray J W, Wigler M. Proc Natl Acad Sci USA. 1998;95:4487–4492. doi: 10.1073/pnas.95.8.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieche I, Olivi M, Champeme M H, Vidaud D, Lidereau R, Vidaud M. Int J Cancer. 1998;78:661–666. doi: 10.1002/(sici)1097-0215(19981123)78:5<661::aid-ijc22>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Hamaguchi M, Sakamoto H, Tsuruta H, Sasaki H, Muto T, Sugimura T, Terada M. Proc Natl Acad Sci USA. 1992;89:9779–9783. doi: 10.1073/pnas.89.20.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamaguchi M, O'Connor E A, Chen T, Parnell L, McCombie R W, Wigler M H. Proc Natl Acad Sci USA. 1998;95:3764–3769. doi: 10.1073/pnas.95.7.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Gorovsky M A. Nucleic Acids Res. 1993;21:4954–4960. doi: 10.1093/nar/21.21.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman A, Birney E, Durbin R, Eddy S R, Howe K L, Sonnhammer E L. Nucleic Acids Res. 2000;28:263–266. doi: 10.1093/nar/28.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den Dunnen J, Van Ommen G. Hum Mutat. 1999;14:95–102. doi: 10.1002/(SICI)1098-1004(1999)14:2<95::AID-HUMU1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Wagner T, Stoppa-Lyonnet D, Fleischmann E, Muhr D, Pages S, Sandberg T, Caux V, Moeslinger R, Langbauer G, Borg A, Oefner P. Genomics. 1999;62:369–376. doi: 10.1006/geno.1999.6026. [DOI] [PubMed] [Google Scholar]
- 14.Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Proc Natl Acad Sci USA. 1989;86:2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. In: Molecular Cloning: A Laboratory Manual. Ford N, Nolan C, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Stolarov S, Chang K, Reiner A, Wigler M, Mittal V. Proc Natl Acad Sci USA. 2001;98:13043–13048. doi: 10.1073/pnas.221450598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S H, Shin M S, Kim H S, Lee H K, Park W S, Kim S Y, Lee J H, Han S Y, Park J Y, Oh R R, et al. Cancer Res. 1999;59:5683–5686. [PubMed] [Google Scholar]
- 18.Bardwell V J, Treisman R. Genes Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- 19.Gray J W, Collins C. Carcinogenesis. 2000;21:443–452. doi: 10.1093/carcin/21.3.443. [DOI] [PubMed] [Google Scholar]
- 20.Pollack J R, Perou C M, Alizadeh A A, Eisen M B, Pergamenschikov A, Williams C F, Jeffrey S S, Botstein D, Brown P O. Nat Genet. 1999;23:41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 21.Tourtellotte W G, Milbrandt J. Nat Genet. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- 22.Takimoto R, El-Deiry W S. Oncogene. 2000;19:1735–1743. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- 23.The C. elegans Sequencing Consortium. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]