Abstract
In mammals, some fungi, and plants, DNA methylation plays a central role in the epigenetic control of gene transcription. Recently, however, a subclass of Arabidopsis mutants revealed that the release of transcriptional gene silencing (TGS) does not necessarily require DNA demethylation. Here, we address the fundamental question of whether these mutants delineate a previously uncharacterized, methylation-independent level of epigenetic regulation, or whether they just act downstream of DNA methylation signals. Two mutants described earlier, ddm1 and mom1, reactivate previously silent loci: ddm1 impairs TGS by reducing chromosomal DNA methylation, and mom1 releases TGS without affecting DNA methylation. We examined the epistatic relationship between ddm1 and mom1 by constructing double mutant strains. The synergistic release of TGS revealed by gene expression patterns from silent loci, drastic developmental abnormalities, and characteristic changes in nuclear architecture in these double mutants implies that DDM1 and MOM are likely to operate at independent levels in TGS control. Our results indicate that the methylation-independent silencing mechanism reinforces the methylation-based system and prevents extremely rapid epigenetic deregulation in plants with DNA methylation deficiencies.
The functional relationship, epistatic interaction, and hierarchy of genetic elements can be addressed by combining multiple genetic defects. Combined mutants provide substantial information, even where the number of gene products in a pathway or their modes of action are not well defined (1). We applied this approach to the analysis of transcriptional gene silencing (TGS) in plants. Methylation-dependent and -independent release of TGS is represented by the two recently isolated genes DDM1 (for decreased DNA methylation 1; ref. 2) and MOM (for Morpheus' molecule), respectively (3). Sequence comparison identified DDM1 as a member of the SWI2/SNF2 family of chromatin remodeling factors (2). MOM defines a new group of proteins with only limited homology to the SWI2/SNF2 family (3). The properties of these proteins and their modes of action in gene silencing are not yet known. Although mutations in each gene release silencing from a range of common target loci (4), the ddm1 and mom1 mutations have different consequences for plant morphology. Homozygous ddm1 mutants show rapid, progressive loss of vigor on inbreeding (5), whereas mom1 plants are morphologically normal even after many generations of selfing (3). After outcrossing of ddm1 to the wild type, the heterozygous hybrids show no or very slow resilencing and remethylation of the activated targets (6–8), whereas resilencing in mom1 heterozygotes is fast (3). These differences are likely related to the fact that ddm1 affects TGS by reducing DNA methylation levels, whereas mom1 restores transcriptional activity despite maintaining the templates in a hypermethylated state. Thus, mom1 challenges the concept that DNA methylation is an obligate component of the epigenetic switch. However, MOM could act by “translating” the DNA methylation signal into a functional suppression of transcription. In this case, MOM would act downstream of DNA methylation and very likely downstream of DDM1. Alternatively, MOM could be part of a regulatory mechanism that acts independently of DNA methylation, defining a different level of epigenetic control in an organism that utilizes DNA methylation for gene regulation.
We set up a genetic test of these two alternatives by generating strains combining the ddm1 and mom1 mutations. If the two mutations contribute to the same mechanism linked directly or indirectly to the DNA methylation, the phenotype and effect on TGS of the double mutant would be expected to resemble that of the single mutants, in this case that of the more severe ddm1. In contrast, if the mutations were part of different or only partially convergent mechanisms, then the double mutant could display a stronger phenotype than either single mutant because of an additive or accelerated loss of silencing.
Materials and Methods
The single mutants mom1 and ddm1–5 (som8) have been described (2, 3); the essentials are summarized in Results. Plants were grown in phytotrons at 70% humidity and daily cycles of 12 h light at 21°C and 12 h darkness at 16°C. Genomic DNA was prepared with Nucleon Phytopure (Amersham Pharmacia Life Science) and RNA was prepared with TRIzol reagent (GIBCO/BRL) according to the suppliers' recommendations. Procedures for crossing and for Southern and Northern blot analysis have been described (9). Hybridization was performed with 32P-labeled DNA fragments in 0.5 M sodium phosphate buffer (pH 7.2), 1 mM EDTA, and 7% SDS. Genotyping of the segregating F2 and F3 plants was performed by PCR with 30 cycles (94°C/50°C/72°C), using 100 ng of genomic DNA as template. Primers A (5′-CGCTCTCGAAATCGCTCGCTGTTC) and B (5′-AAAGGACCCATTTACAGAACAC) (designed by Eric Richards, Washington University, St. Louis) for the DDM1 sequence amplify a fragment of 332 or 414 bp in case of genotype DD or dd; primer C (5′-CATGACTCCCCCAGCCAGTAG) is in the genomic MOM sequence deleted in the mom1 mutant; primer D (5′-CATCTACGGCAATGTACCAGC) binds within the T-DNA in mom1; and primer E (5′-CACTTTCCGATTTCGATTCTCG) is located downstream of the MOM gene in wild type and mutant. The combinations amplify fragments of 260 bp (C/E in the case of genotype MM or Mm) and/or 447 bp (D/E in the case of Mm or mm).
For cytological analysis, young rosette leaves of about 1–2 cm in size were fixed in ethanol-acetic acid (3:1) and stored at −20°C. Fluorescent in situ hybridization (FISH) and DAPI (4′,6-diamidino-2-phenylindole) staining were carried out according to ref. 10, with minor modifications. In brief, the probe specific to 180-bp centromeric repeats (6) was prepared by PCR with 0.1 mM dATP, dCTP, and dGTP, 0.065 mM dTTP, and 0.035 mM Biotin-dUTP (Roche Molecular Biochemicals). One microliter of the reaction was added to 20 μl of hybridization mix. DNA of the probe and the specimen were denatured for 2 min at 80°C. After hybridization for ≈15 h, slides were either washed as described (10) or for 5 min in 2× SSC, 5 min in 0.1× SSC, 3 min in 2× SSC at 42°C, and 5 min in 2× SSC with 0.1% Tween 20 at room temperature (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7). Slides were analyzed using a Leitz DMR fluorescence microscope, and images were taken with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI), using the spot advanced software.
Results and Discussion
The mom1 mutation was generated by insertion of a T-DNA with a marker gene encoding PPT herbicide resistance (11) into a transgenic line possessing a transcriptionally silent multicopy insert of the hygromycin phosphotransferase gene (HPT) (8). T-DNA integration produced a 2-kb deletion spanning the entire C-terminal part of the MOM gene (3). For the ddm1 mutation, we chose a fast-neutron-generated allele with an insertion of 82 bp in the 5′ region of the DDM1 gene [ddm1–5 (2)], formerly som8 (8). The insertion forms two stop codons in the DDM1 reading frame, terminating translation after 30 or 53 aa, respectively. A possible reinitiation of translation at the first downstream start codon would result in a frameshift. However, although detectable in wild-type plants, we could not find any DDM1 mRNA in ddm1–5, suggesting its decreased stability (data not shown). Therefore, ddm1–5 can be considered as a null allele. The presence of each mutant allele could be determined by PCR. Because the mutations are in the same genetic background (3, 8), differences caused by factors other than these particular mutations or their epigenetic effects were rather unlikely. To avoid the secondary epigenetic effects seen in later inbred generations of ddm1 (5), plants homozygous for ddm1–5 were recovered from segregating heterozygotes before the generation of the double mutant. These MMdd plants were crossed to plants homozygous for the mom1 allele (mmDD). The two genes are on different chromosomes and therefore expected to segregate independently. Several F2 populations were derived from F1 plants heterozygous for both mutations (MmDd). F2 plants were grown in soil and genotyped in a PCR assay. In all cases presented here, genotypes were determined by PCR analysis of mutant or wild-type alleles of both genes and by segregation of markers in the F3 (data not shown).
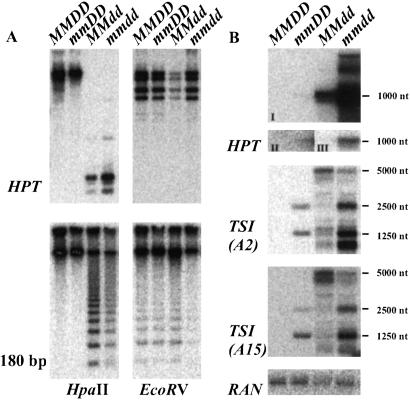
A double-mutant line (F3) was analyzed and compared with the single mutants with respect to the degree of DNA methylation at CpG sites (HpaII) within the HPT transgene, as well as at pericentromeric repeats (6). Both sequences substantially lost methylation in ddm1, but were still hypermethylated in mom1. Methylation in the double mutant was reduced, but only to the level found in ddm1 (Fig. 1A). This was also true for the single copy gene FWA that is methylated in wild-type plants (12), where the degree of demethylation at CpG (assayed by CfoI) and CpNpG (HaeIII) remained at the level of ddm1 (data not shown). Therefore, combining both mutations does not cause additional demethylation of DNA. Surprising was an elevated level of the HPT transcript, already higher in ddm1 than in mom1, in a more than additive fashion (Fig. 1B). This finding suggests a synergistic action of the mutations on release of silencing from the transgenic target locus. The single mutations release silencing also from specific endogenous pericentromeric repeats termed TSI (transcriptionally silent information) at different levels (13). In the double mutant, TSI expression patterns were additive, and individual transcripts appeared even more abundant than additive (Fig. 1B Middle). Interestingly, amounts of the longest TSI transcripts (5,000 nt) that are abundant in ddm1 were reduced in double mutants. It is likely that the 5,000-nt transcript is a precursor for the shorter TSI transcripts (13); thus, the combination of both mutations possibly also accelerates not only transcriptional activation of TSI, but the processing of TSI RNAs as well. In summary, loss of both proteins in the double mutant leads to superimposed or even synergistic transcriptional reactivation of the studied templates, suggesting that DDM1 and MOM have a concerted action in silencing.
Figure 1.
Analysis of DNA methylation and transcript levels in the double mutant. (A) Southern blot of genomic DNA of line A (MMDD), mom1 (mmDD), ddm1–5 (MMdd), and double mutant (mmdd) restricted with methylation-sensitive HpaII (Left) and methylation-insensitive EcoRV (Right), hybridized with the HPT gene (Upper) or the pericentromeric 180-bp repeat (Lower) (6). (B) Northern blot of line A (MMDD), mom1 (mmDD), ddm1–5 (MMdd) and double mutant (mmdd), hybridized with the HPT gene (Top; I, equal exposure of all slots; II, long exposure of slot 1 and 2; III, short exposure of slot 3 and 4), two clones representing the two main groups of the pericentromeric TSI sequences (Middle) (13), and a loading control (Bottom).
MOM deficiency in a wild-type background does not have detectable effects on plant growth and development (3), which suggests that MOM is a component of a minor TGS pathway, or that its role is only essential when alternative silencing pathway(s) are compromised. Examination of phenotypes in segregating F2 and F3 populations strongly supports the second possibility. We have genotyped and scored the phenotypes of 124 plants from five segregating populations. These consisted of 51 F2 plants derived from three F1 hybrids, 38 F3 plants from a parent homozygous for mom1 and heterozygous for ddm1, and 35 F3 plants from a parent homozygous for ddm1 and heterozygous for mom1. In total, 26 plants (5 F2 plants, progeny from plants heterozygous for both mutations, 10 and 11 F3 plants from F2 parents homozygous for mom1 or ddm1 and segregating for the other mutation) showed characteristic abnormalities in leaf morphology—namely, upward curling of the younger leaves parallel to the midrib, and growth retardation (Fig. 2). All these plants had double mutant genotype (mmdd). Among those, one plant developed the characteristic morphology only later in development. The double mutants were observed with approximately the expected frequency (6.25% in F2 and 25% in F3). The specific morphology was never seen in any stage of development among the 97 segregants from the same populations (siblings) identified as wild type, heterozygous, or homozygous for any single mutation. Therefore, each gene seems to be haplosufficient, even in the absence of the other. However, in the double mutant, the loss of MOM that on its own provokes no phenotypic deviation, causes immediate and severe developmental abnormalities.
Figure 2.
Phenotype of mom1 (Left), ddm1 (Right), and double mutant plants (Center).
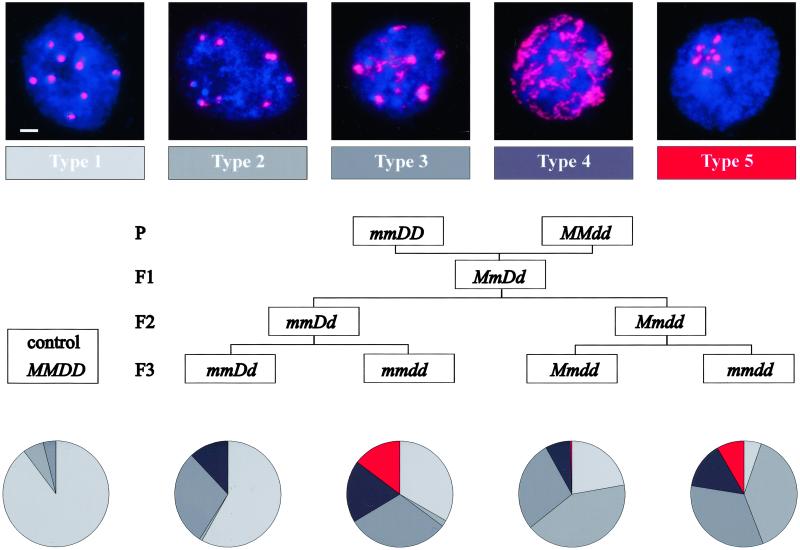
We further examined the effects of single and combined mutations on heterochromatin organization in interphase nuclei by using fluorescence in situ hybridization (FISH). The probe specific for centromeric repetitive DNA (6) revealed differences in heterochromatin distribution, which we assigned to five different types (Fig. 3). In wild-type nuclei, the probe hybridizes to discrete, bright DAPI (4′,6-diamidino-2-phenylindole)-stained chromocenters (Fig. 3, type 1), sometimes a little less definite (Fig. 3, type 2). Nuclei of mom1 plants are indistinguishable from the wild type. In contrast, most nuclei of ddm1–5 mutants are either of type 2 or show an even stronger disintegration of chromocenters (Fig. 3, types 3 and 4), indicating decondensation of the centromeric heterochromatin. Intriguingly, nuclei of mmdd double mutants exhibit structural peculiarities never observed in the single mutants. Not only do the chromocenters disintegrate, but they also have the tendency to aggregate to a superheterochromatic structure (Fig. 3, type 5). We compared the frequency of the different types of nuclei in double mutants and heterozygous controls segregating from F2 parents that were already homozygous for either mom1 or ddm1 (Fig. 3). The presence of type 3 and 4 nuclei even in F3 of heterozygous ddm1–5 is likely to be a heritage of the original parental ddm1–5 plant. Type 5 nuclei occur only in double mutant plants, regardless which single mutation was homozygous already in the previous generation (Fig. 3). The frequency of the heterochromatin aggregation characteristic for the double mutant seems to be correlated with the severity of developmental abnormalities of individual mmdd plants (Fig. 4). Therefore, MOM1 and DDM1 may have complementary functions that are essential for maintenance of nuclear organization required for epigenetic inheritance and normal development.
Figure 3.
Chromatin organization in interphase nuclei of single and double mutants. (Top) Classification based on distribution of the pericentromeric heterochromatin (red, 180-bp repeats revealed by fluorescence in situ hybridization analysis; blue, DNA stained with 4′,6-diamidino-2-phenylindole). (Scale bar, 2 μm.) (Middle) Genetic pedigree of the plant material. (Bottom) Representation of the five types of nuclei in different F3 genotypes. Results from 6–12 independent experiments are combined. Number of nuclei evaluated: MMDD, 829; mmDD, 1,002; mmdd, 2,257; Mmdd, 778; mmdd, 2,280.
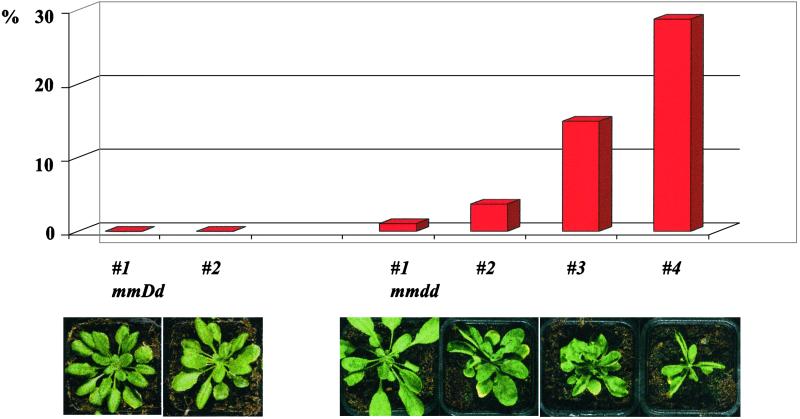
Figure 4.
Correlation of frequency of type 5 nuclei with double mutant phenotypes, progressive from #1 with a weak and delayed phenotype appearance, to #4 with an early and strong phenotype. A minimum of 300 nuclei was scored for each of six plants with a phenotype as illustrated below.
Although an indirect, epistatic interaction cannot be completely excluded, considering secondary epigenetic effects of both mutations, a hypomorphic interaction in the double mutant is rather unlikely because ddm1–5 is expected to be a null allele. Therefore, the data rather support the concept of a nonlinear relationship between DDM1 and MOM, and the existence of two independent mechanisms of transcriptional silencing, with DDM1 or MOM as essential components.
Although DNA methylation is widespread among eukaryotes and believed to play an important role in epigenetic regulation and genome evolution, there is little conservation of the phenomenon even between close relatives (14). The occurrence of methylation does not necessarily exclude other epigenetic mechanisms, such as those well documented for organisms with no or very low levels of DNA methylation (15–17). Thus, previously unknown components of methylation-independent epigenetic regulation, such as MOM, could be part of previously uncharacterized regulatory networks controlling heritable patterns of gene expression. Moreover, a methylation-independent mode of silencing might explain the exceptional cases of gene regulation in mC-containing organisms, where gene repression and hypermethylation are not linked (18, 19).
Acknowledgments
We thank Ueli Grossniklaus, Barbara Hohn, Patrick King, and Frederick Meins for valuable comments on the manuscript, and Paul Fransz for his help with the fluorescent in situ hybridization technique. This work was supported by European Union Grant EU QLRT-2000-00078 and Swiss Federal Office for Education and Sciences Grant BBW 00.0224-2 (both to O.M.S.).
Abbreviations
- TGS
transcriptional gene silencing
- TSI
transcriptionally silent information
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Avery L, Wasserman S. Trends Genet. 1992;8:312–316. doi: 10.1016/0168-9525(92)90263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeddeloh J A, Stokes T L, Richards E J. Nat Genet. 1999;22:94–97. doi: 10.1038/8803. [DOI] [PubMed] [Google Scholar]
- 3.Amedeo P, Habu Y, Afsar K, Mittelsten Scheid O, Paszkowski J. Nature. 2000;405:203–206. doi: 10.1038/35012108. [DOI] [PubMed] [Google Scholar]
- 4.Vaucheret H, Fagard M. Trends Genet. 2001;17:29–35. doi: 10.1016/s0168-9525(00)02166-1. [DOI] [PubMed] [Google Scholar]
- 5.Kakutani T, Jeddeloh J A, Flowers S K, Munakata K, Richards E J. Proc Natl Acad Sci USA. 1996;93:12406–12411. doi: 10.1073/pnas.93.22.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vongs A, Kakutani T, Martienssen R A, Richards E J. Science. 1993;260:1926–1928. doi: 10.1126/science.8316832. [DOI] [PubMed] [Google Scholar]
- 7.Kakutani T, Munakata K, Richards E J, Hirochika H. Genetics. 1999;151:831–838. doi: 10.1093/genetics/151.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittelsten Scheid O, Afsar K, Paszkowski J. Proc Natl Acad Sci USA. 1998;95:632–637. doi: 10.1073/pnas.95.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittelsten Scheid O, Paszkowski J, Potrykus I. Mol Gen Genet. 1991;228:104–112. doi: 10.1007/BF00282454. [DOI] [PubMed] [Google Scholar]
- 10.Fransz P, Armstrong S, Alonso-Blanco C, Fischer T C, Torres-Ruiz R A, Jones G. Plant J. 1998;13:867–876. doi: 10.1046/j.1365-313x.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- 11.Mengiste T, Amedeo P, Paszkowski J. Plant J. 1997;12:945–948. doi: 10.1046/j.1365-313x.1997.12040945.x. [DOI] [PubMed] [Google Scholar]
- 12.Soppe W J J, Jacobsen S E, Alonso-Blanco C, Jackson J P, Kakutani T, Koornneef M, Peeters A J M. Mol Cell. 2000;6:791–802. doi: 10.1016/s1097-2765(05)00090-0. [DOI] [PubMed] [Google Scholar]
- 13.Steimer A, Amedeo P, Afsar K, Fransz P, Mittelsten Scheid O, Paszkowski J. Plant Cell. 2000;12:1165–1178. doi: 10.1105/tpc.12.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colot V, Rossignol J L. BioEssays. 1999;21:402–411. doi: 10.1002/(SICI)1521-1878(199905)21:5<402::AID-BIES7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Lustig A J. Curr Opin Genet Dev. 1998;8:233–239. doi: 10.1016/s0959-437x(98)80146-9. [DOI] [PubMed] [Google Scholar]
- 16.Pirrotta V. Cell. 1998;93:333–336. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- 17.Kelly W G, Fire A. Development (Cambridge, UK) 1998;125:2451–2456. doi: 10.1242/dev.125.13.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wölfl S, Schräder M, Wittig B. Proc Natl Acad Sci USA. 1991;88:271–275. doi: 10.1073/pnas.88.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmen M W, Leitgeb S, Charlton J, Jones S J M, Harris B R, Clark V H, Bird A. Science. 1999;283:1164–1167. doi: 10.1126/science.283.5405.1164. [DOI] [PubMed] [Google Scholar]