Abstract
The cytokine macrophage migration inhibitory factor (MIF) exerts a multitude of biological functions. Notably, it induces inflammation at the interface between the immune system and the hypothalamus–pituitary–adrenal stress axis. The role of MIF in infectious diseases is not understood completely. Here, we show that MIF-deficient (MIF−/−) knockout mice fail to control an infection with wild-type Salmonella typhimurium. Increased susceptibility was accompanied by a reduced Th1 response, demonstrated by decreased levels of IL-12, IFNγ, and tumor necrosis factor α. In Salmonella-infected MIF−/− mice, levels of IL-1β were markedly increased. Additionally, infected MIF−/− mice showed elevated serum levels of nitric oxide and corticosterone as compared with control mice. Our results point to MIF as a key mediator in the host response to S. typhimurium. MIF not only promotes development of a protective Th1 response but ameliorates disease by altering levels of reactive nitrogen intermediates and corticosteroid hormones, which both exert immunosuppressive functions.
Experimental infection of mice with Salmonella enterica serovar Typhimurium provides a useful model of human typhoid fever caused by S. enterica serovar Typhi (1). Salmonella typhi and Salmonella typhimurium are facultative intracellular pathogens that infect, replicate, and persist in macrophages. After oral uptake, S. typhimurium reaches the small intestine, where it traverses the M cells of the Peyer's patches to reach the underlying tissue. From there, it spreads via the circulation to become systemic. This results either in fatal bacteremia, when unrestricted, or in the development of neutrophil- and mononuclear-cell-rich microabscesses in spleen and liver, which serve as the main target organs of Salmonella. These microabscesses restrict bacterial replication and limit further spreading of the pathogen (2). The first line of defense is mediated by IFNγ and tumor necrosis factor α (TNFα), which are produced primarily by natural killer cells and macrophages. The bactericidal capacities of macrophages include production of reactive oxygen and nitrogen intermediates (3). Ultimately, T cell-mediated immunity is critical for effective clearance of S. typhimurium (4). Although the central role of cytokine-producing CD4+ T cells of the Th1 type is beyond doubt, studies have shown that CD8+ T cells also participate in acquired immunity to S. typhimurium (5, 6). The acquired immune response to S. typhimurium takes weeks to fully develop, and it has been shown that Salmonella can suppress the host response (7). Hence, the initial action of the innate immune system critically influences the ultimate outcome of infection.
Recently, macrophage migration inhibitory factor (MIF) has been characterized as a proinflammatory cytokine (8, 9). MIF first was described in 1966 as a factor produced by T lymphocytes that was associated with migration of macrophages during delayed-type hypersensitivity responses (10, 11). More recent findings have identified the mononuclear phagocyte system as well as the anterior pituitary gland as major sources of MIF. In the pituitary, the cytokine is produced in direct vicinity of the adrenocorticotropic hormone, which is a central hormone within the hypothalamus–pituitary–adrenal (HPA) stress axis. In response to proinflammatory cytokines, including IL-1, IL-6, and TNFα, the hypothalamus releases the corticotropin-releasing hormone, which induces secretion of adrenocorticotropic hormone from the pituitary. Adrenocorticotropic hormone, in turn, induces the release of glucocorticoids (like corticosterone) from the adrenal gland. Generally, glucocorticoids inhibit circulation of leukocytes and impair activation of macrophages and Th1 cells (12, 13). Thus, glucocorticoids play a key role in down-regulation of excessive, and potentially harmful, inflammatory responses. The crosstalk between MIF and the HPA axis has direct functional consequences. Although MIF is induced by minute amounts of glucocorticoids, it can override their immunosuppressive functions. The immunomodulatory role of MIF has been studied in various models (14, 15). In a murine septic shock model, MIF represented a major mediator released by the pituitary gland in response to lipopolysaccharide (LPS), which amplifies the fatal action of TNFα, causing lethal endotoxemia (16). Blockade of MIF with neutralizing anti-MIF antibodies delays the onset and lowers the frequency of arthritis (17, 18), and it inhibited up-regulation of TNFα, iNOS, and intercellular adhesion molecule-1 in gastric inflammation in rats (19). This is associated with reduced macrophage and neutrophil accumulation, thus attenuating ulceration. Additionally, treatment with neutralizing anti-MIF antibodies results in increased serum levels of corticosterone in crescentic glomerulonephritis (20). These data suggest that MIF suppresses the endogenous antiinflammatory glucocorticoid response, thus exerting detrimental inflammatory effects. Even though both MIF and glucocorticoids counteract each other, they communicate with one another at the interface between the endocrine and the immune system. Hence, both beneficial and detrimental sequelae of this crosstalk can be envisaged.
Because the role of MIF in bacterial infections has received little attention, we analyzed its role in S. typhimurium infection by using MIF-deficient (MIF−/−) knockout mice. Our results identify MIF as a key factor in immunity to typhoid. MIF not only promoted protective Th1 responses but also participated in the reduction of immunosuppressive stress responses.
Methods
Mice.
MIF gene-disrupted mutant mice (21) and control (C57BL/6 × Sv129) F1 mice were housed under specific-pathogen-free conditions at our facilities at the Federal Institute for Health Protection of Consumers and Veterinary Medicine in Berlin. Male mice (8–10 weeks old) were used. For measurements of corticosterone levels, all mice involved in the investigation were handled individually daily before and during the experiments. To mimic the stress situation of i.v. infections, control mice were mock-infected with the same volume of PBS. Mice were typed for their Nramp1 genotype by PCR as described by Weintraub et al. (22).
Bacteria and Infection.
SL1344 is a wild-type strain (rspL, hisG) of S. typhimurium. It was kindly provided by B. A. D. Stocker, Department of Medical Microbiology, Stanford University, Stanford, CA. Salmonella were grown overnight in LB medium, washed twice in PBS, aliquoted, and stored at −80°C. For determination of bacterial burdens in organs, mice were killed at the time points indicated. Livers and spleens were homogenized in PBS, serial dilutions of homogenates were plated on LB agar, and colonies were counted after overnight incubation at 37°C.
Treatment of Mice with Recombinant Murine IFNγ (r-IFNγ).
r-IFNγ (Strathmann, Hamburg, Germany) was given daily at a concentration of 105 units per mouse i.p. starting 1 day before Salmonella infection.
Determination of Cytokines.
Single-cell suspensions of S. typhimurium (SL1344)-infected spleens were prepared, and erythrocytes were lysed. Spleen cells (2 × 105 per well) were cultured in 96-well, round-bottomed plates in RPMI medium 1640 supplemented with 2 mM l-glutamine/1 mM Na-pyruvate/100 μg/ml penicillin/100 μg/ml streptomycin (all Biochrom, Berlin), 50 μM 2-mercaptoethanol (GIBCO/BRL), and 10% FCS (Sigma). Spleen cells were restimulated with heat-killed Salmonella (HKS) (107 per ml) or 5 μg/ml plate-bound anti-CD3 mAb (clone 145 2C11). Culture supernatants were screened for cytokines after culture for 3 days. Cytokines were measured by a standard sandwich ELISA as described (23). IL-1β and IL-12 were determined from culture supernatants by using kits (BioTrend), following the manufacturer's instructions. The TNFα bioassay was performed by using the L929 mouse fibroblast cell line as described (24).
Enzyme-linked immunospot assays were performed as described (23, 25). In all experiments, 2 × 105 spleen cells were assayed for cytokine release after 20 h of incubation.
Determination of Nitrite.
The reactive nitrogen intermediates (RNI) were determined by measuring nitrite. Sera were diluted with normal mouse serum. A standard curve was prepared by using NaNO2. NADPH and nitrate reductase were added. Samples were incubated for 20 min at room temperature. Griess reagent (26) and 10% trichloroacetic acid were added. After centrifugation, supernatants were analyzed at 540 nm photometrically.
Blockade of NO Production.
NO production was blocked with NG-methyl-l-arginine (NMA), a competitive inhibitor of inducible NO synthase. NMA was applied in a concentration of 12 μg/ml in drinking water during Salmonella infection (27).
Determination of Corticosterone.
Serum levels of corticosterone were measured by the 125I double-antibody radio immunoassay kit (ICN). Measurements were carried out by following the manufacturer's instructions.
Statistics.
Significant differences between data were analyzed by nonparametric Mann–Whitney test, Student's unpaired t test, or log-rank test for survival assays by using the prism computer program (GraphPad, San Diego). P < 0.05 was considered statistically significant.
Results
MIF−/− Mice Fail to Control S. typhimurium Infection.
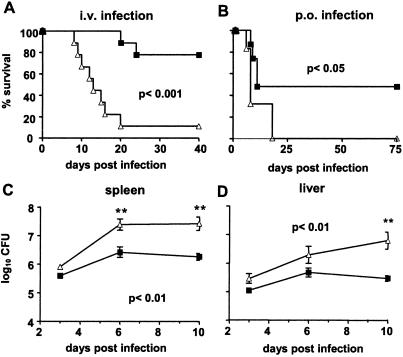
The MIF−/− mice used in our experiments were on a mixed C57BL/6 (Nramp1s) and Sv129 (Nramp1r) genetic background. Phenotypic characterization by PCR revealed that all mice were Nramp1r (data not shown). Hence, we used (C57BL/6 × Sv129) F1 animals with the dominant Nramp1r phenotype as control mice. Survival of Nramp1r MIF−/− and control mice after i.v. infection with 103 S. typhimurium was monitored over 40 days (Fig. 1A). The vast majority of MIF−/− mice died within 20 days of infection, whereas 80% of control mice survived. Because the natural route of Salmonella infection is via the gastrointestinal tract, we also infected mice per os with a dose of 2 × 1010 S. typhimurium. Within 25 days, all MIF−/− mice succumbed to the infection, whereas 50% of control mice survived for more than 75 days (Fig. 1B). After i.v. infection, both mouse strains showed an initial increase in bacterial load in livers and spleens. Bacterial titers reached a plateau at day 6 postinfection (p.i.), and bacterial counts never exceeded 107 bacteria per organ in control mice. In contrast, bacterial growth in livers and spleens of MIF−/− mice was increased, and bacterial numbers reached levels >107 colony-forming units (Fig. 1 C and D). Histologically, livers and spleens from control and MIF−/− mice did not differ over the first 10 days (data not shown).
Figure 1.
Survival and bacterial replication in livers and spleens of S. typhimurium-infected MIF−/− and control mice. Mice were infected i.v. with 103 (A) or infected per os with 2 × 1010 (B) S. typhimurium. ■, Control mice; ▵, MIF−/− mice. Survival was monitored over 40 days (A) or 75 days (B) (10 animals per group). Bacterial loads were determined in spleens (C) and livers (D) after 3, 6, and 10 days of i.v. infection (five animals per group). Experiments were repeated twice with similar results.
MIF−/− Mice Display Impaired IL-12 (p40) and IFNγ Responses to S. typhimurium.
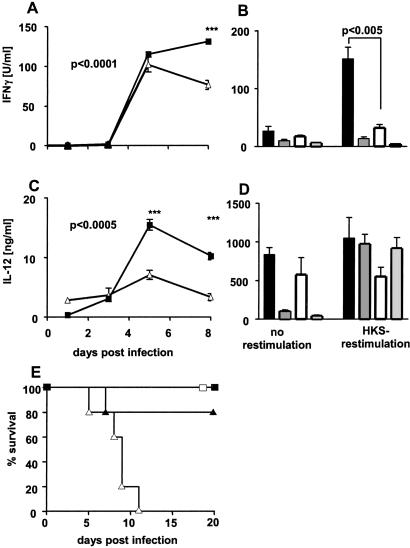
IFNγ plays a critical role in the control of S. typhimurium infection (4, 28, 29). Besides natural killer cells, T cells are a major source of IFNγ, and the development of a Th1 response is essential for successful defense (30). To determine whether increased susceptibility of MIF−/− mice correlates with an insufficient IFNγ response, we determined IFNγ concentrations in sera of infected animals. After an initial increase in both control and MIF−/− mice, IFNγ levels decreased in MIF−/− mice from day 5 p.i., whereas IFNγ levels remained high in control mice (Fig. 2A). Consistent with these in vivo data, spleen cells from infected control mice produced more IFNγ after restimulation with HKS in vitro as compared with splenocytes from MIF−/− mice (Fig. 2B).
Figure 2.
Determination of IL-12 (p40) and IFNγ in MIF−/− and control mice infected with S. typhimurium. Mice were infected i.v. with 103 S. typhimurium. Blood was collected at days 1, 3, 5, and 8 p.i., and IFNγ (A) and IL-12 (C) levels were determined in pooled sera of seven mice. Cytokine levels in sera of noninfected mice were below the detection level. Results represent mean ± SD of triplicate samples of pooled sera. ■, Infected control mice; ▵, infected MIT−/− mice. After 9 days of infection, spleen cells were harvested and cultured with medium alone or were restimulated with 2 × 107 per ml HKS. After 3 days, IFNγ (B) and IL-12 (D) in supernatants were determined by ELISA. Data are represented as mean ± SD of triplicate cultures of five individually analyzed mice. Solid bars, infected control mice; open bars, infected MIF−/− mice; darkly shaded bars, naive control mice; lightly shaded bars, naive MIF−/− mice. Mice were treated with r-IFNγ starting 1 day before infection with 103 S. typhimurium, and survival was monitored for 20 days (E). □ and ▵, Control/MIF−/− mice without r-IFNγ; ■ and ▴, control/MIF−/− mice treated with r-IFNγ. IFNγ-treated MIF−/− mice showed prolonged survival compared with untreated MIF−/− mice (P < 0.05; five mice per group).
The macrophage and dendritic cell product IL-12 promotes Th1 cell development. To determine whether IL-12 production in response to S. typhimurium infection was impaired, we measured serum levels of IL-12 in infected animals. Control mice showed increased serum levels of IL-12, reaching a maximum 5 days p.i. IL-12 in the sera of infected MIF−/− mice was significantly lower at days 5 and 8 p.i. (Fig. 2C). Consistent with these results, in response to HKS stimulation, spleen cells from infected control mice showed a trend to produce more IL-12 than spleen cells from infected MIF−/− mice (Fig. 2D). In contrast, production of IL-4 and the frequency of IL-4-producing spleen cells in Salmonella-infected control and MIF−/− mice were comparable (data not shown). The detrimental consequences of impaired IFNγ responses to Salmonella in MIF−/− mice could be reversed by administration of exogenous r-IFNγ. As shown in Fig. 2E, MIF−/− mice treated with r-IFNγ during Salmonella infection could be rescued. These results reveal a critical role of MIF in the development of protective Th1 responses to Salmonella infection. The impaired Th1 response in MIF−/− mutants is at least partly responsible for the susceptibility of these mice against S. typhimurium.
Proinflammatory Cytokine Production Is Disturbed in MIF−/− Mice After S. typhimurium Infection.
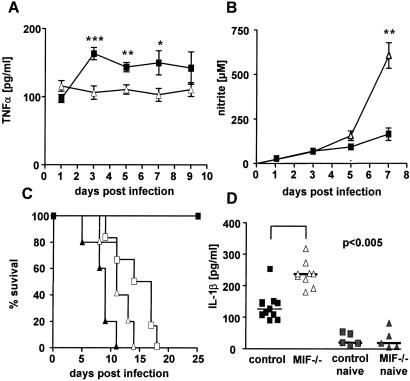
Macrophages produce proinflammatory cytokines including TNFα and IL-1β, which participate in macrophage activation. Macrophage TNFα secretion is up-regulated by MIF (16). To determine whether TNFα is involved in macrophage activation during S. typhimurium infection, we measured TNFα levels in sera of infected mice. The TNFα serum levels were increased significantly in control mice as compared with MIF−/− mice (Fig. 3A). TNFα levels in spleen cell supernatants also were elevated in control compared with MIF−/− mice during the course of Salmonella infection (data not shown). IFNγ and TNFα synergistically stimulate NO production by macrophages. On the one hand, NO induces intracellular killing of Salmonella (3, 31); on the other hand, NO causes immunosuppression during Salmonella infection (32). Although IFNγ and TNFα were decreased in MIF−/− mice, NO production was elevated during the course of infection (Fig. 3B). To determine whether the increased NO production was involved in enhanced susceptibility of MIF−/− mice, we blocked NO production in vivo with NMA, a competitive inhibitor of iNOS (27). Treatment of mice with NMA completely blocked NO production in infected control and MIF−/− mice (data not shown). In MIF−/− mice, blocking of NO synthesis prolonged survival for a few days (Fig. 3C). In contrast, NMA treatment of control mice drastically enhanced susceptibility to infection, an observation reported previously by Schwacha and Eisenstein (33). Together, these results suggest that although NO production is necessary for the control of S. typhimurium, the unbalanced NO production observed in MIF−/− mice appears to be a major cause of higher susceptibility of these mice.
Figure 3.
Determination of TNFα, nitrite, and survival after NMA blockade and IL-1β in MIF−/− and control mice infected with S. typhimurium. Mice were infected i.v. with 103 S. typhimurium. TNFα was determined during the course of infection (9–10 mice per group) (A). Nitrite levels were determined from pooled sera during the course of infection (B). ■, Control mice; ▵, MIF−/− mice (***, P < 0.0005; **, P < 0.005; *, P < 0.05). NO production was blocked by NMA, and survival of mice was monitored (five mice per group) (C). ■, Controls (no NMA); □, controls treated with NMA; ▴, MIF−/− mice (no NMA); ▵, MIF−/− mice treated with NMA. This experiment was repeated once, with similar results. IL-1β was measured after 9 days p.i. in culture supernatants of spleen cells incubated for 3 days with HKS (D).
Because IL-1β was not detectable in sera of animals, we measured IL-1β in supernatants of spleen cells from infected control and MIF−/− mice after HKS restimulation in vitro. The amount of IL-1β produced by spleen cells did not differ during the first days of infection (data not shown), but at later time points, when the bacterial load in MIF−/− mice became fatal, spleen cells of infected MIF−/− mice produced significantly more IL-1β as compared with control mice (Fig. 3D).
Increased Corticosterone Levels in Sera of S. typhimurium-Infected MIF−/− Mice.
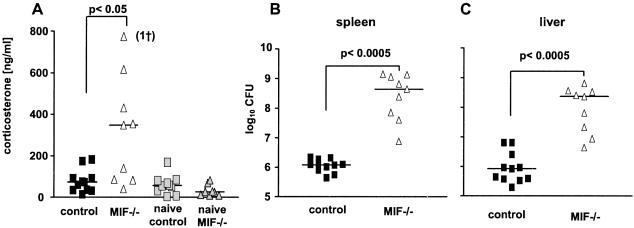
Stress caused by exogenous insults including infection and inflammation is characterized by the activation of the HPA-stress axis, with increased corticosterone production as a major hallmark (34). On the one hand, glucocorticoids down-regulate the immune response, resulting in impaired immune defense, with a potentially fatal outcome (13). On the other hand, glucocorticoid hormones also play an important role in maintaining homeostasis and in protecting the body against an overshoot of potentially damaging immune activation (35). Reciprocally, glucocorticoids are counterregulated by the immune system, with MIF playing an important role (18, 36). Therefore, serum corticosterone was measured in control and MIF−/− mice. Salmonella-infected control mice, as well as mock-infected MIF−/− mice, showed low serum levels of corticosterone. In contrast, corticosterone levels in sera of Salmonella-infected MIF−/− mice were elevated after day 5 p.i., reaching a peak at day 9 p.i. (Fig. 4A and data not shown). At the same time, bacterial loads in spleens and livers of MIF−/− mice were highly increased as compared with controls (Fig. 4 B and C).
Figure 4.
Salmonella infection raises corticosterone levels in MIF−/− mice. Mice were infected i.v. with 103 S. typhimurium. Nine days p.i., mice were killed and bled immediately. Corticosterone was measured in sera of individual mice (A). Colony-forming units were determined in spleens (B) and livers (C). Experiments were repeated twice, with similar results.
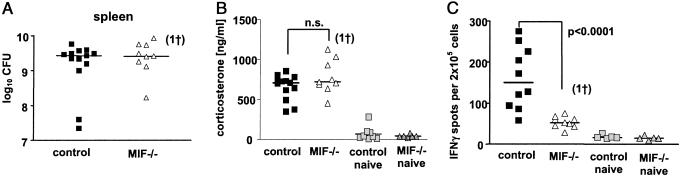
When mice were infected with a lethal dose of S. typhimurium (3 × 106 colony-forming units per mouse), bacterial loads in spleens (Fig. 5A) were similar in control and MIF−/− mice at day 3 p.i. Under these conditions, serum corticosterone levels in control and MIF−/− mice were comparable (Fig. 5B). In contrast, frequencies of IFNγ-producing spleen cells (Fig. 5C) and serum levels of TNFα (data not shown) were reduced markedly in MIF−/− mice as compared with control mice. These data indicate that the fatal bacterial burden in infected mice induced the high levels of corticosterone.
Figure 5.
Determination of bacterial load, corticosterone, and IFNγ in MIF−/− and control mice after high-dose infection with S. typhimurium. Mice were infected with 3 × 106 S. typhimurium. Three days p.i., mice were killed and bled immediately. Bacterial numbers were determined in spleens (A). Corticosterone was measured in serum of individual mice (B). Frequencies of IFNγ-producing spleen cells of individual mice were determined ex vivo by enzyme-linked immunospot assay (C).
Discussion
Our experiments demonstrate a crucial role of MIF in protection against S. typhimurium infection by promoting protective Th1 responses. Moreover, the lack of MIF results in an elevated production of NO and corticosteroids during infection. We assume that the unrestrained function of both immunosuppressive factors further influences the fatal outcome of infection. MIF has been shown to activate macrophages and to promote NO production, which are required for killing of the protozoal pathogen Leishmania major (37, 38). Activated macrophages are critical effectors in the defense against S. typhimurium, and IL-12 and IFNγ are principal cytokines in the protective Th1 response that results in activated macrophages. IL-12 and IFNγ production were decreased in MIF−/− mice as compared with control mice both in vitro and in vivo, suggesting insufficient Th1 cell activation and, consequently, impaired macrophage activation in the absence of MIF. Treatment of MIF−/− mice with r-INFγ could reconstitute the insufficient immune response of these mice to Salmonella. These data demonstrate that MIF−/− mice suffer from a Th1 immune defect. Decreased IFNγ production is at least partly responsible for the increased susceptibility of MIF−/− mice.
Further cytokines involved in macrophage activation for antimicrobial effector functions include TNFα and IL-1. TNFα and MIF synergize with each other, thus amplifying their inflammatory responses (16). Consistent with these findings, serum levels of TNFα in control mice were elevated significantly as compared with the low levels of TNFα detected in MIF−/− mice. We assume that MIF promotes TNFα secretion, which is crucial in protection against S. typhimurium (28). Reduced TNFα levels in MIF−/− mice could be a further reason for impaired protection against S. typhimurium. The proinflammatory cytokine IL-1 can simultaneously activate the HPA axis and the immune system. IL-1β promotes macrophage activation (39) and acts on the hypothalamic thermoregulatory center to induce fever (40). Moreover, IL-1β modulates cerebral functions during systemic and localized inflammation via the HPA axis. Our data suggest down-regulation of IL-1β production by MIF. Spleen cells from infected MIF−/− mice produced twice as much IL-1β than splenocytes of control mice.
The role of RNI during S. typhimurium infection is complex. RNI produced by activated macrophages contribute to the killing of the pathogen (31, 41). At the same time, NO suppresses antibacterial immunity in S. typhimurium-infected mice (33). With progressive infection, NO concentrations gradually increased in MIF−/− mice, resulting in high serum levels at day 9 p.i. as compared with control mice. These results contrast observations in the Leishmania system made by Satoskar et al. and Juttner et al. (37, 38). These reports describe inhibition of NO production by macrophages as a consequence of phenotypic or genetic MIF blockade. Differential effects in NO production between Salmonella and Leishmania infection could result from different biology of the two infectious agents. Moreover, we measured NO in sera of infected animals whereas Satoskar et al. (37) determined NO produced by infected macrophages in vitro. Interestingly, NO is high in Salmonella-infected and low in Leishmania-infected MIF−/− mice, yet these mice are more susceptible to both microbes. Elevated levels of NO in MIF−/− mice during S. typhimurium infection correlated with higher bacterial burden in these mice. That MIF−/− mice showed low IFNγ but high NO levels after Salmonella infection is striking. Immunohistochemical staining of Salmonella-infected spleens and livers for iNOS did not reveal differential expression of this key enzyme in RNI synthesis (data not shown). However, differences in iNOS expression in infected control and MIF−/− organs could be minute and not visible by histological examinations. It is possible that during Salmonella infection mechanisms other than IFNγ trigger NO production in MIF−/− mice, thereby inducing NO production by cells that normally do not synthesize this intermediate via this pathway such as cardiac myocytes or vascular smooth muscle cells (42). MIF−/− mice also may lack counterregulation of iNOS, resulting in enhanced NO production. Accordingly, blocking of NO production by NMA, a competitive inhibitor of iNOS, prolonged survival of MIF−/− mice compared with nontreated mice for a few days. We assume that the high RNI production in S. typhimurium-infected MIF−/− mice counteracts the development of protective Th1 responses.
In general, glucocorticoids exert immunosuppressive effects on peripheral T cells, in that they inhibit expression of a wide variety of activation-induced gene products. Glucocorticoids exert their antiinflammatory action by inducing the synthesis of a phospholipase A2 inhibitor, which, in turn, prevents release of inflammatory mediators and adhesion molecules (43, 44). Moreover, glucocorticoids block translocation of NFκB into the nucleus, thus suppressing transcription of inflammatory genes (45). Our data reveal markedly increased levels of corticosterone in MIF−/− mice during infection as compared with controls. This effect could be due to deficient counterregulation of glucocorticoids by MIF in knockout animals (46). High levels of corticosterone could directly impair killing of S. typhimurium (47). After infection with a lethal dose of S. typhimurium, both MIF−/− and control mice showed similarly high levels of corticosterone. Yet, regardless of the high serum levels of glucocorticoids, control mice still produced TNFα and IFNγ whereas MIF−/− mice failed to do so. We assume that MIF regulates different mechanisms of suppression, which are both operative in S. typhimurium infection and mediated, in part, by RNI or glucocorticoids, respectively.
MIF has been described as a critical mediator of LPS-induced shock, suggesting its detrimental role in septic shock by Gram-negative bacteria (15). Recent findings demonstrate that MIF modulates Toll-like receptor 4 (TLR4) (48). The TLR4 senses LPS, the abundant cell-wall component of Salmonella and other Gram-negative bacteria. In MIF−/− mice, TLR4 expression of macrophages is impaired, resulting in a defective innate immune response to LPS as shown by profound reduction of TNFα levels. This could mean that the reduced levels of TNFα, IL-12, and INFγ in MIF−/− mice during S. typhimurium infection at least partly are caused by impaired sensing of S. typhimurium because of reduced TLR4 expression in these mice.
Our results point to a protective role of MIF against the Gram-negative pathogen S. typhimurium. Experiments using cell-wall components such as LPS fail to mimic the complexity underlying bacterial infections. A harmful role of MIF in defense against the facultative pathogens Pseudomonas aeruginosa and Escherichia coli has been claimed (16, 21), emphasizing that results obtained from different infectious disease models as well as models of dysregulated immune responses cannot be generalized (49, 50). Accordingly, novel therapeutic measures based on beneficial effects of anti-MIF treatment in autoimmune disorders must take into account detrimental consequences on the course of infection.
Acknowledgments
We thank Anne Koehler and Karin Bordarsch for technical assistance and Manuela Staeber for purification and preparation of antibodies. We thank Dr. Uwe Klemm and Janette Scherff for maintenance of animals. We are grateful to Dr. Helen Collins for critically reading the manuscript.
Abbreviations
- MIF
migration inhibitory factor
- HPA
hypothalamus–pituitary–adrenal
- TNF
tumor necrosis factor
- RNI
reactive nitrogen intermediate(s)
- NMA
NG-methyl-l-arginine
- p.i.
postinfection
- LPS
lipopolysaccharide
- HKS
heat-killed Salmonella
References
- 1.Raupach B, Kaufmann S H. Curr Opin Immunol. 2001;13:417–428. doi: 10.1016/s0952-7915(00)00236-3. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann S H, Raupach B, Finlay B B. Microbes Infect. 2001;3:1177–1181. doi: 10.1016/s1286-4579(01)01498-8. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang F C. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess J, Ladel C, Miko D, Kaufmann S H. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 5.Lo W F, Ong H, Metcalf E S, Soloski M J. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 6.Gautreaux M D, Deitch E A, Berg R D. Infect Immunol. 1994;62:2874–2884. doi: 10.1128/iai.62.7.2874-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwacha M G, Meissler J J, Jr, Eisenstein T K. Infect Immunol. 1998;66:5862–5866. doi: 10.1128/iai.66.12.5862-5866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucala R. FASEB J. 1996;10:1607–1613. doi: 10.1096/fasebj.10.14.9002552. [DOI] [PubMed] [Google Scholar]
- 9.Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David J R. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom B R, Bennett B. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 12.Sapolsky R M, Romero L M, Munck A U. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 13.Ashwell J D, Lu F W, Vacchio M S. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 14.Metz C N, Bucala R. Adv Immunol. 1997;66:197–223. doi: 10.1016/s0065-2776(08)60598-2. [DOI] [PubMed] [Google Scholar]
- 15.Bernhagen J, Calandra T, Bucala R. J Mol Med. 1998;76:151–161. doi: 10.1007/s001090050204. [DOI] [PubMed] [Google Scholar]
- 16.Calandra T, Echtenacher B, Roy D L, Pugin J, Metz C N, Hultner L, Heumann D, Mannel D, Bucala R, Glauser M P. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 17.Mikulowska A, Metz C N, Bucala R, Holmdahl R. J Immunol. 1997;158:5514–5517. [PubMed] [Google Scholar]
- 18.Santos L, Hall P, Metz C, Bucala R, Morand E F. Clin Exp Immunol. 2001;123:309–314. doi: 10.1046/j.1365-2249.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X R, Chun Hui C W, Chen Y X, Chun B, Wong Y, Fung P C, Metz C, Cho C H, Hui W M, Bucala R, et al. Gastroenterology. 2001;121:619–630. doi: 10.1053/gast.2001.27205. [DOI] [PubMed] [Google Scholar]
- 20.Yang N, Nikolic-Paterson D J, Ng Y Y, Mu W, Metz C, Bacher M, Meinhardt A, Bucala R, Atkins R C, Lan H Y. Mol Med. 1998;4:413–424. [PMC free article] [PubMed] [Google Scholar]
- 21.Bozza M, Satoskar A R, Lin G, Lu B, Humbles A A, Gerard C, David J R. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weintraub B C, Eckmann L, Okamoto S, Hense M, Hedrick S M, Fierer J. Infect Immunol. 1997;65:2306–2312. doi: 10.1128/iai.65.6.2306-2312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittrücker H W, Kohler A, Mak T W, Kaufmann S H. J Immunol. 1999;163:6769–6776. [PubMed] [Google Scholar]
- 24.Formica S, Roach T I, Blackwell J M. Immunology. 1994;82:42–50. [PMC free article] [PubMed] [Google Scholar]
- 25.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 26.Rockett K A, Awburn M M, Aggarwal B B, Cowden W B, Clark I A. Infect Immunol. 1992;60:3725–3730. doi: 10.1128/iai.60.9.3725-3730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolph M S, Ramshaw I A, Rockett K A, Ruby J, Cowden W B. Virology. 1996;217:470–477. doi: 10.1006/viro.1996.0141. [DOI] [PubMed] [Google Scholar]
- 28.Nauciel C, Espinasse-Maes F. Infect Immunol. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everest P, Wain J, Roberts M, Rook G, Dougan G. Trends Microbiol. 2001;9:316–320. doi: 10.1016/s0966-842x(01)02067-4. [DOI] [PubMed] [Google Scholar]
- 30.Pie S, Truffa-Bachi P, Pla M, Nauciel C. Infect Immunol. 1997;65:4509–4514. doi: 10.1128/iai.65.11.4509-4514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb J L, Harvey M W, Holden D W, Evans T J. Infect Immunol. 2001;69:6391–6400. doi: 10.1128/IAI.69.10.6391-6400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacFarlane A S, Schwacha M G, Eisenstein T K. Infect Immunol. 1999;67:891–898. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwacha M G, Eisenstein T K. Infect Immunol. 1997;65:4897–4903. doi: 10.1128/iai.65.12.4897-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson I M, Lorentzen J C, Ericsson-Dahlstrand A. J Neuroendocrinol. 2000;12:1096–1104. doi: 10.1046/j.1365-2826.2000.00565.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller A H. Psychiatr Clin North Am. 1998;21:443–463. doi: 10.1016/s0193-953x(05)70015-0. [DOI] [PubMed] [Google Scholar]
- 36.Bucala R. Ann NY Acad Sci. 1998;840:74–82. doi: 10.1111/j.1749-6632.1998.tb09551.x. [DOI] [PubMed] [Google Scholar]
- 37.Satoskar A R, Bozza M, Rodriguez S M, Lin G, David J R. Infect Immunol. 2001;69:906–911. doi: 10.1128/IAI.69.2.906-911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juttner S, Bernhagen J, Metz C N, Rollinghoff M, Bucala R, Gessner A. J Immunol. 1998;161:2383–2390. [PubMed] [Google Scholar]
- 39.Rivest S. Psychoneuroendocrinology. 2001;26:761–788. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- 40.Dinarello C A. Ann NY Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan C. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 42.Parratt J R. J Antimicrob Chemother. 1998;41, Suppl. A:31–39. doi: 10.1093/jac/41.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 43.Aljada A, Ghanim H, Mohanty P, Hofmeyer D, Tripathy D, Dandona P. J Clin Endocrinol Metab. 2001;86:5988–5991. doi: 10.1210/jcem.86.12.8212. [DOI] [PubMed] [Google Scholar]
- 44.Cronstein B N, Kimmel S C, Levin R I, Martiniuk F, Weissmann G. Proc Natl Acad Sci USA. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S., Jr Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 46.Calandra T, Bucala R. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 47.Zwet T L, Thompson J, Furth R. Infect Immunol. 1975;12:699–705. doi: 10.1128/iai.12.4.699-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roger T, David J R, Glauser M, Calandra T. Nature. 2001;414:920–923. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 49.Froidevaux C, Roger T, Martin C, Glauser M P, Calandra T. Crit Care Med. 2001;29:S13–S15. doi: 10.1097/00003246-200107001-00006. [DOI] [PubMed] [Google Scholar]
- 50.de Jong Y P, Abadia-Molina A C, Satoskar A R, Clarke K, Rietdijk S T, Faubion W A, Mizoguchi E, Metz C N, Sahli M A, ten Hove T, et al. Nat Immunol. 2001;2:1061–1066. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]