Abstract
There are numerous studies to indicate that irradiation induces reactive oxygen species (ROS), which play an important causative role in radiation damage of the cell. We evaluated the effects of ginsan, a polysaccharide fraction extracted from Panax ginseng, on the γ-radiation induced alterations of some antioxidant systems in the spleen of Balb/c mice. On the 5th day after sublethal whole-body irradiation, homogenized spleen tissues of the irradiated mice expressed only marginally increased mRNA levels of Mn-SOD (superoxide dimutase) in contrast to Cu/Zn-SOD, however, catalase mRNA was decreased by ∼50% of the control. In vivo treatment of non-irradiated mice with ginsan (100 mg kg−1, intraperitoneal administration) had no significant effect, except for glutathione peroxidase (GPx) mRNA, which increased to 144% from the control. However, the combination of irradiation with ginsan effectively increased the SODs and GPx transcription as well as their protein expressions and enzyme activities. In addition, the expression of heme oxygenase-1 and non-protein thiol induced by irradiation was normalized by the treatment of ginsan. Evidence indicated that transforming growth factor-β and other important cytokines such as IL-1, TNF and IFN-γ might be involved in evoking the antioxidant enzymes. Therefore, we propose that the modulation of antioxidant enzymes by ginsan was partly responsible for protecting the animal from radiation, and could be applied as a therapeutic remedy for various ROS-related diseases.
Keywords: antioxidant enzyme, cytokine, ginsan, radiation
Introduction
In a variety of cells, ionizing radiation has been shown to enhance the production of reactive oxygen species (ROS) that can induce oxidative damage to vital cellular molecules including DNA, proteins and lipids (1–5). ROS are tightly controlled by antioxidant defense systems, including non-enzymatic radical scavengers and enzymes that can either directly detoxify ROS or indirectly regulate their levels. The major enzymatic antioxidants are superoxide dimutase (SOD), which degrades superoxide anion radical, catalase and the glutathione (GSH) redox system, which inactivates H2O2 and organic hydroperoxides (6–9). There are three forms of SOD in eukaryotic cells: manganese-SOD (Mn-SOD), which is located in mitochondria; a copper- and zinc-containing isoform (Cu/Zn-SOD), which resides in cytoplasm; and extracellular SOD, which lines blood vessels. Catalase is localized in peroxisomes and cytoplasm, while glutathione peroxidase (GPx) is located in many subcellular compartments and comprises four distinct forms of which classical cytosolic GPx (cGPx) is the most common form among them (10). In addition to the above enzymes, microsomal enzyme heme oxygenase (HO), especially its isoform HO-1, plays an essential role in regulating the ROS level indirectly through the production of other antioxidants (bilirubin and ferritin) and removal of prooxidants (free-heme). HO oxidatively breaks down free heme to biliverdin, which is quickly converted to bilirubin (11,12). Vitamin E, β-carotene, uric acid, flavonoids and bilirubin are some of the compounds that may function as non-enzymatic antioxidants (13,14). GSH is a main intracellular non-protein thiol (NP-SH), which is present in high concentrations and possesses not only direct radical scavenging ability but also is an essential cofactor for various enzymes that decrease oxidative stress (15).
In order to overcome the potential harmful effect of free radicals and to reduce the damage by oxidants, a variety of pharmacological antioxidants such as GSH, ceruloplasmin and transferrin have been examined (16). In addition, many botanical drugs, particularly those containing phenolic components, have been tried as an antioxidant. Panax ginseng is one of the most popular natural tonics used in oriental countries. It exhibits a wide range of pharmacological actions such as antiaging, immunoenhancement, antistress and antitumor (17,18). Recently, most effects of P. ginseng have been attributed to its antioxidant action (19). Ginseng extracts exhibit protective effects against peroxidation of unsaturated fatty acid caused by iron and hydrogen peroxide (20), ginsenosides protect pulmonary vascular endothelium from free radical-induced injury (21) and panaxadiol fractions inhibit aging and mutation via induction of Cu/Zn-SOD expression at the transcriptional level (22).
Ginsan, a polysaccharide fraction extracted from P. ginseng, has been shown to be strongly radioprotective through its ability to stimulate hematopoietic stem cells (colony-forming unit-spleen) and produce a battery of cytokines such as IL-1, IL-6, IL-12 and TNF-α (23). Since several cytokines such as GM-CSF, TNF and IL-1 are produced in various cells after irradiation and they have been demonstrated to have radioprotective effect (24) as well as induce Mn-SOD mRNA in vitro and in vivo (25), it seemed to be of a great interest to evaluate whether ginsan might exert its radioprotective effect on irradiated mice through activation of antioxidant defense systems. Therefore, in the present study, we investigated the effects of ginsan on the activities of antioxidant entities such as SOD, catalase, GPx, HO and the level of NP-SH in the spleen of mice after irradiation.
Materials and Methods
Mice
Six- to seven-week-old BALB/c female mice were purchased from Korea Laboratory Animal Co. (Daejeon, Korea) and were kept for 7 days for acclimatization in our animal quarters. Sterile standard mouse chow (NIH-7 open formula) and water were given ad libitum, and they were housed randomly at 60% humidity and 22 ± 2°C on a 12 h light/12 h dark cycle. Each experimental group consisted of at least 5–8 animals. Studies were conducted under guidelines for the use and care of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences.
Materials
Ginsan polysaccharide was purified from the ethanol-insoluble fraction of P. ginseng water extract, as described previously (23). The endotoxin level in the purified ginsan preparation was <0.03 EU mg−1 as measured by limulus amebocyte lysate assay (Endosafe®, Charles River Laboratories, USA) according to the manufacturer's instruction. Moreover, the treatment of polymyxin B did not inhibit the proliferation of lymphocytes, thus excluding possible endotoxin contamination in ginsan. Ginsan was dissolved in phosphate-buffered saline (PBS, pH 7.4) and filtered through 0.25 μm millipore membranes. Mice were administered 100 mg kg−1 of ginsan or PBS intraperitoneally 24 h before irradiation. Other chemicals and solvents used were of the highest analytical grade available.
Irradiation
The mice were randomized, placed in ventilated Plexiglas containers and exposed to γ-radiation from the 60Co Theratron-780 (Atomic Energy of Canada Ltd, Canada) at a dose of 4.5 Gy (0.5 Gy min−1).
Scavenging Activity on 1,1-Diphenyl-2-picrylhydrazyl Radicals
The scavenging activity of ginsan on 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radicals was measured according to the method of Saiga et al. (26). Ginsan was dissolved in 1 ml of distilled water and mixed with 0.25 ml of 0.02% DPPH–ethanol solution. After incubation for 60 min at room temperature, absorbance of each sample was measured at 512 nm.
Tissue Preparation and Assay of Antioxidant Enzyme Activity
Spleens were removed from the mice and, after washing with saline, the tissues were homogenized with Tissue-Tearor with 7 mm probe (BioSpec Products, Inc., USA) on ice in 9 volumes of sucrose/EDTA (0.25 M/1 mM) buffer. The homogenates were centrifuged at 12 000 g for 15 min at 4°C, and the resulting supernatants were analyzed for SOD, catalase and GPx activity, using standard spectrophotometric assays. Protein content in an aliquot of tissue homogenate was determined according to Bradford method (27) using bovine serum albumin as a standard protein.
SOD activity was measured according to the procedure of McCord et al. (28) by monitoring the inhibition of formazan formation from nitroblue tetrazolium (NBT) (Sigma) in superoxide radical generating xanthine–xanthine oxidase system. One unit of SOD activity was defined as the amount of protein that inhibits the rate of reduction of NBT by 50%. The catalase activity was determined by the reduction velocity of 10 mM H2O2 in 50 mM phosphate buffer at 240 nm for 3 min, as previously described (29). One unit activity was defined as the amount of enzyme to degrade mmoles of H2O2 per min per mg protein. GPx activity was measured by the method of Flohe et al. (30), in which GPx activity was coupled to the oxidation of NADPH (Sigma) by glutathione reductase (Sigma). The oxidation of NADPH was followed spectrophotometrically at 340 nm at 37°C for 20 min. One unit of activity was equal to oxidize mmoles of NADPH per min per mg of protein. HO activity was assayed by the method of Ryter et al. (31). Briefly, the reaction mixture in a final volume of 500 μl contained spleen supernatant in MgCl2 buffer (3-6 mg of protein per ml), liver 105 000 g supernatant (1 mg of protein per ml) as a source of biliverdin reductase, 2 mM glucose-6-phosphate (ICN, USA), 1 U of glucose-6-phosphate dehydrogenase (ICN), 25 μM hemin (ICN) and 1 mM NADPH. Reaction was initiated by the addition of NADPH, and the mixture was incubated at 37°C in the dark for 10 min in a water bath, and 500 μl of chloroform was added to terminate the reaction. The lower chloroform layer was preserved, and difference in OD between 464 and 530 nm (ΔOD464–530) was measured using UV/Visible spectrophotometer (Ultrospec 3100 pro, Biochrom, England). Bilirubin concentrations were calculated, using an extinction coefficient (ɛ464–530) of 40 mM−1 cm−1, and HO activity was expressed in picomoles of bilirubin produced per milligram of protein per hour.
NP-SH Content Assay
NP-SH content in spleen tissues was determined with 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) (Sigma) by the method of Sedlak and Lindsay (32). In brief, spleen supernatants homogenized in 10% TCA and 0.02 M EDTA-Na2 was mixed with 0.4 M Tris–HCl buffer (pH 8.9), and 0.01 M DTNB in methanol was then added. Optical density of yellow color developed in 5 min was measured at 412 nm. NP-SH contents were calculated assuming an extinction coefficient (ɛ412) of 13.1 mM−1 cm−1 and expressed in micromoles per gram of wet tissue weight.
Semiquantitative RT–PCR
Total RNA was isolated from the spleen of mice (five mice per group) using TRI reagent (MRC, Cincinnati, OH, USA). One microgram of intact total RNA was reversibly transcribed into first strand cDNA, which was then amplified using PCR. The final volume of 20 μl of reverse transcriptase (RT) reaction mixture contained; 50 mM Tris–HCl (pH 8.3), 3 mM MgCl2, 75 mM KCl, 2.5 μg ml−1 pd(N)6 primer, 0.5 mM each of dNTP and 10 U of AMV-RT (Amersham, USA). The reaction mixture for PCR contained 10 μl of cDNA template from RT reaction, 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.5 mM each of dNTP mixture, 1.0 μM each of primer and 0.5 U Taq DNA polymerase (Takara, Japan). PCR was performed with GeneAmpR PCR system 9700 (Applied Biosystems, USA) at 94°C for 1 min, at 55–60°C for 1 min and at 72°C for 1 min per cycle. The numbers of amplification cycles were determined according to individual primer set in order to maintain exponential rate of product amplification (28–35 cycles). The amplified products were visualized by electrophoresis on a 1% agarose gel in the presence of 0.5 μg ml−1 ethidium bromide, and the density of bands was quantitated by Image analyzer (Fluor-S™ multiImager, Bio-Rad, USA).
Western blotting
To analyze the expression of antioxidant enzymes, cell lysates were prepared by extracting proteins with lysis buffer [40 mM Tris-HCl (pH 8.0), 120 mM NaCl and 0.1% Nonidet-40 (NP-40)] supplemented with protease inhibitors. Proteins (30–50 μg per well) were separated by SDS–PAGE and transferred to nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline and then incubated for 1 h at room temperature with primary antibodies against Mn-SOD, Cu/Zn–SOD, catalase and GPx. Blots were developed by peroxidase-conjugated secondary antibody, and proteins were visualized by enhanced chemiluminescence procedures (NEN), according to the manufacturer's recommendation.
Statistical Analysis
All analyses were performed at least in triplicate. Results are presented as means ± SEM. Data were analyzed with one-way ANOVA for significant differences.
Results
Scavenging Activity of Ginsan on DPPH Radical
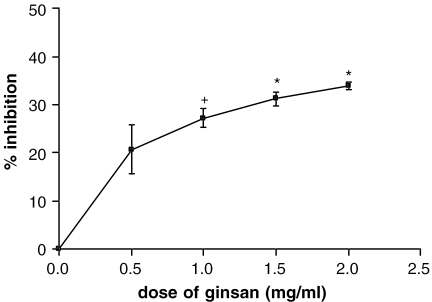
The method of scavenging DPPH free radicals was used to evaluate the antioxidant activity of specific compounds or extracts in a short time. As shown in Fig. 1, ginsan was found to scavenge the stable DPPH radical in a dose-dependent manner, and the highest scavenging activity (34% inhibition) was detected at 2.0 mg ml−1 of ginsan. Since ginsan naturally has a slightly yellowish color, the solution of ginsan at high concentration (>5.0 mg ml−1) interferes with the colorimetric quantification of DPPH radical.
Figure 1.
DPPH radical scavenging activity of ginsan at different concentrations. Data are shown as means ± SEM of triplicate measurements. +P < 0.05 and ★P < 0.005 with respect to normal control values.
Transcriptional Modulation of SODs, Catalase and GPx in Irradiated Mice
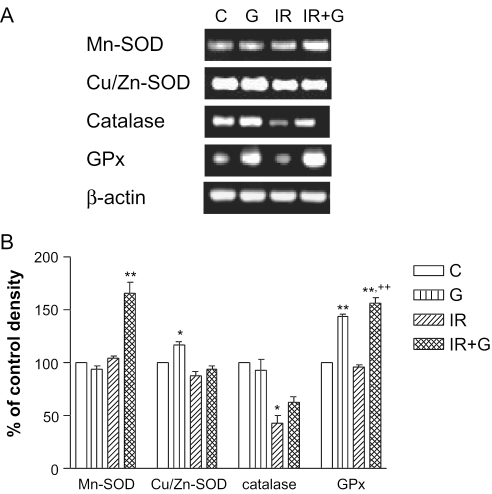
Based on our previous study which exhibited optimal radioprotection (23), 100 mg kg−1 ginsan was administered to mice intraperitoneally. On the 5th day after irradiation, ginsan-induced transcriptional modulation of several redox proteins in spleens was evaluated by semiquantitative RT–PCR using the specific primers described in Table 1. As shown in Fig. 2, the level of Cu/Zn-SOD mRNA was slightly decreased after irradiation, while that of Mn-SOD was not changed. This could be due to different localization of each enzyme: Cu/Zn-SOD is predominantly a cytosolic enzyme, whereas Mn-SOD is rich in mitochodria. Ginsan itself had no effect on the mRNA of Mn-SOD, however, 1.7-fold increase of its expression was detected in ginsan-treated mice before irradiation. By contrast, ginsan itself increased Cu/Zn-SOD mRNA ∼117% of the control (P < 0.05), but Cu/Zn-SOD mRNA of irradiated mice was not different from that of control as well as ginsan-treated mice. However, the mRNA of catalase was decreased by more than half, compared with the non-irradiated group. Although ginsan appeared to enhance the catalase mRNA of irradiated mice, the level did not reach to the control level. The expression of GPx mRNA level was not changed by radiation, however, it was 1.4- and 1.6-fold increased by treatment of ginsan alone or together with radiation, respectively, compared with non-irradiated control mice (P < 0.001).
Table 1.
Pairs of synthetic primers used in RT–PCR
| Oligonuleotides | Sequence | Expected size |
|---|---|---|
| IL-12 | 5′-primer 5′-ACCTCAGTTTGGCCAGGGTC-3′ | 500 |
| 3′-primer 5′-GTCACGACGCGGGTGGTGAAG-3′ | ||
| IFN-γ | 5′-primer 5′-TACTGCCACGGCACAGTCATTGAA-3′ | 405 |
| 3′-primer 5′-GCAGCGACTCCTTTTCCGCTTCCT-3′ | ||
| IL-1β | 5′-primer 5′-TGAAGGGCTGCTTCCAAACCTTTGACC-3′ | 361 |
| 3′-primer 5′-TGTCCATTGAGGTGGAGAGCTTTCAGC-3′ | ||
| TNF-α | 5′-primer 5′-GCGACGTGGAACTGGCAGAAG-3′ | 340 |
| 3′-primer 5′-TCCATGCCGTTGGCCAGGAGG-3′ | ||
| Mn-SOD | 5′-primer 5′-GCACATTAACGCGCAGATCA-3′ | 241 |
| 3′-primer 5′-AGCCTCCAGCAACTCTCCTT-3′ | ||
| Cu/Zn-SOD | 5′-primer 5′-AAGGCCGTGTGCGTGCTGAA-3′ | 246 |
| 3′-primer 5′-CAGGTCTCCAACATGCCTCT-3′ | ||
| Catalase | 5′-primer 5′-GCAGATACCTGTGAACTGTC-3′ | 229 |
| 3′-primer 5′-GTAGAATGTCCGCACCTGAG-3′ | ||
| GPx | 5′-primer 5′-CCTCAAGTACGTCCGACCTG-3′ | 197 |
| 3′-primer 5′-GTAGAATGTCCGCACCTGAG-3′ | ||
| HO-1 | 5′-primer 5′-AACAAGCAGAACCCAGTC-3′ | 375 |
| 3′-primer 5′-TGTCATCTCCAGAGTGTTC-3′ | ||
| TGF-β1 | 5′-primer 5′-GCTCACTGCTCTTGTGACAGCAAAG-3′ | 360 |
| 3′-primer 5′-CAAGGACCTTGCTGTACTGTGTGTC-3′ | ||
| β-actin | 5′-primer 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ | 349 |
| 3′-primer 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ |
Figure 2.
Transcriptional modulation of important redox enzymes by ginsan. (A) Representative RT–PCR-amplified mRNA transcripts of Mn-SOD, Cu/Zn-SOD, catalase and GPx in spleen on the 5th day after 4.5 Gy irradiation. Total tissue RNA was extracted, reverse transcribed and subjected to PCR. The products were electrophoresed on 1% agarose gel with ethidium bromide. (B) Data are represented as the percentage of normal control levels and represent mean ± SEM of values obtained from three experiments. C, control group; G, 100 mg kg−1 of ginsan-treated group; IR, irradiated froup; IR + G, group treated with 100mg kg−1 of ginsan and radiation. ★P < 0.05 and ★★P < 0.001 with respect to normal control values. ++P < 0.001 compared with irradiated controls.
Protein Levels of SODs, Catalase and GPx
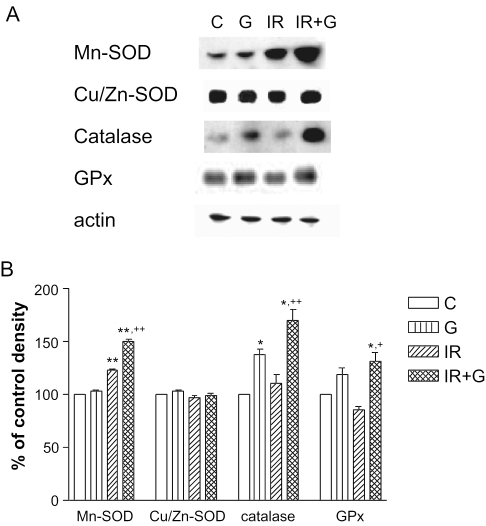
On the basis of the results of RT–PCR, we then examined the protein levels of SODs, catalase and GPx in the spleen of irradiated mice on the 5th day after irradiation. As shown in Fig. 3, the Mn-SOD level of irradiated mice was significantly higher than that of control. In addition, ginsan increased this level by ∼1.45-fold. However, Cu/Zn-SOD expression was not altered by irradiation and/or ginsan treatment. There were significant increases of catalase (P < 0.001) and GPx expression (P < 0.01) in irradiated mice with ginsan treatment, but not in irradiated mice without ginsan treatment.
Figure 3.
Western blot analysis of redox proteins in spleen treated with or without ginsan. (A) Samples containing 30 mg of proteins of spleen lysates from indicated experimental group were assessed by western blot analysis for Mn-SOD, Cu/Zn-SOD, catalase and GPx as described in Materials and Methods. (B) Data are represented as the percentage of normal control levels and represent mean ± SEM of values obtained from three experiments. See legend to Fig. 2. ★P < 0.05 and ★★P < 0.001 with respect to normal control values. +P < 0.01, ++P < 0.001 compared with irradiated controls.
Effect of Ginsan on Antioxidant Enzyme Activity
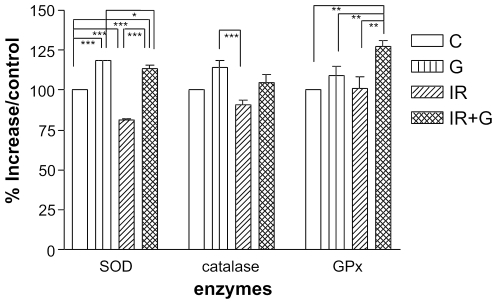
As seen in Fig. 4, the activity of SOD, which scavenges superoxide anion, was reduced in the irradiation group as compared with the non-irradiated group (P < 0.05). However, the activity of SOD was significantly increased in the ginsan-treated group with or without irradiation (P < 0.001). No significant change in catalase activity was observed. Interestingly, GPx activity was not altered in the irradiated group, whereas it was increased by administration of ginsan before irradiation, similar to the results of mRNA and protein expression (P < 0.01).
Figure 4.
Induction of ROS scavenging enzyme activities by ginsan in irradiated mice. Activities of SOD, catalase and GPx were determined on lysates of spleen (five mice per group) as described in Materials and Methods. Results are expressed as means ± SEM of three independent experiments. See legend to Fig. 2. ★P < 0.05 and ★★P < 0.001 with respect to normal control values. +P < 0.001 compared with irradiated controls.
Effect of Ginsan on HO Activity
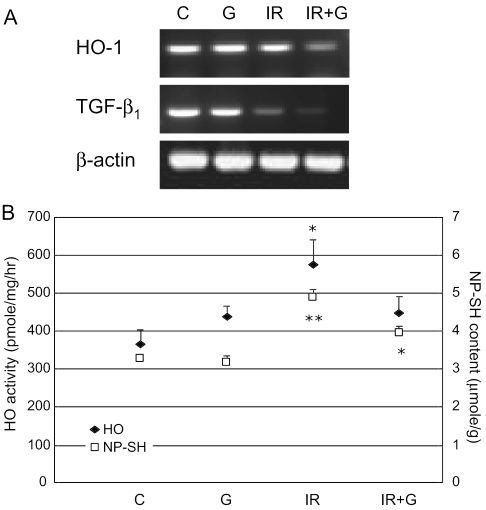
HO has recently been shown to be the major 32 KDa stress protein inducible by oxidative stress. Thus, we next examined the expression of HO-1 mRNA and the enzyme activity. Figure 5A shows that mRNA level of HO-1 was not changed by irradiation or ginsan alone, however, markedly decreased by combined treatment of both ginsan and radiation. Unexpectedly, radiation was found to significantly increase the HO activity by 1.6-fold in spleen, compared with control, and it was downregulated by ginsan pretreatment (Fig. 5B). It is well known that reduced GSH in vivo is an important protective antioxidant, and GSH depletion plays a major role in the induction of HO (33–35), so that it can be expected that the increase of HO results from decreased GSH contents. In the present study, however, NP-SH concentration increased roughly by 50% in the irradiated mice, compared with that of control animals (3.29 ± 0.03 mol g−1 spleen). The ginsan pretreatment of the irradiated mice rather decreased the NP-SH concentration, this tendency was similar to the results of HO. Therefore, we investigated another candidate, transforming growth factor-β1 (TGF-β1), because it has been demonstrated that TGF-β1 is a potent HO-1 inducer in pulmonary and renal epithelial cells (36,37). Unfortunately, however, we did not find any decisive clue, because the radiation per se decreased the expression of TGF-β1 mRNA and more decrease was observed in the ginsan pretreated irradiated mice (Fig. 5A).
Figure 5.
The modulating effect of ginsan on HO activity and NPSH content partially through downregulation of TGF-β. (A) HO-1 and TGF-β mRNA expressions are shown in each spleen indicated on 5th day after 4.5 Gy irradiation. (B) HO-1 activity and NP-SH concentration were determined as described in Materials and Methods. Five mice were used in each group. Results are represented as means ± SEM of three replicate experiments. See legend to Fig. 2. *P < 0.01, **P < 0.001 compared with controls.
Discussion
It is well known that the cytokines such as TNF-α and IL-1β are produced in various cells after irradiation (38) and they induce Mn-SOD mRNA in vitro and in vivo (25). We previously reported that ginsan increased the production of these cytokines in irradiated mice and all ginsan-treated mice survived after 30 days of lethal irradiation (9 Gy) (23). However, ginsan could not completely recover the number of immune cells and peripheral blood cells to the normal value in the irradiated mice, suggesting that other mechanisms may exist by which ginsan defends the animals from radiation. Thus, we attempted in this study to evaluate whether the administration of ginsan affects the activities of antioxidant enzymes such as SODs, catalase and GPx after irradiation.
Although radiation-induced free radicals activate antioxidant defense systems, the most prominent changes were found in catalase transcription and SOD activity. More than 2-fold decrease of catalase mRNA expression was observed in irradiated mice, whereas the protein level and the activity of catalase were not altered by irradiation. Similarly, we also found discrepancies between mRNAs, protein level and the activity of SODs. The mRNAs of Mn-SOD and Cu/Zn-SOD were not affected by irradiation, whereas the protein level of Mn-SOD was increased. Ginsan treatment changed only Cu/Zn-SOD mRNA, however, both transciptional and translational expressions of Mn-SOD were significantly induced when combined with radiation. Mn-SOD and Cu/Zn-SOD differ in their primary structure, biological function and intracellular localization (39). Cu/Zn-SOD is mostly located in the cytosol, whereas Mn-SOD resides in the mitochondrial matrix. Takahashi et al. (40) reported that overexpression of Cu/Zn-SOD significantly prevents the ultraviolet B-induced apoptosis in SV40-transformed human keratinocytes, while Mn-SOD shows no protective effect. In support of the above observation, there are many studies to report that certain types of SODs are selectively induced by cytokines (IL-1, TNF, IFN-γ) (25,41–43), LPS (44), herbicide paraquat (45) and anticancer agent (46). Although the precise mechanism of these different responses of various SODs to ginsan and irradiation remains to be determined, it seems to be partly due to different localization of each enzyme. The other H2O2 scavenger, GPx, was significantly induced in ginsan-treated mice, whereas not affected by irradiation. Recently, it has been shown that IFN-α increased the protein level of GPx and the enzyme activity of Mn-SOD and Cu/Zn-SOD in a dose-dependent manner (47), and treatment with recombinant human IL-1α increased GPx level in hypothalamus that had been decreased by radiation exposure (48). This finding appears to be slightly conflicting with our present data. We speculated that this conflicting phenomenon might have arisen from different experimental conditions such as times, organs and animal species to evaluate the antioxidant enzymes after oxidative stimuli.
In addition to GPx, we also determined NP-SH concentration in irradiated mice. The well-known NP-SHs are reduced GSH, cysteine and coenzyme A. Among these, the most abundant NP-SH is reduced GSH (tripeptide of l-γ-glutamyl-l-cysteinylglycine), comprising ∼75–90% of total intracellular NP-SH (49–51). GSH plays extremely potent role in antioxidant defense, because it possesses not only direct radical-scavenging ability but also is an essential component of GPx system, which eliminates different hydroperoxides without producing free radicals (52). In our experiment, the NP-SH level increased ∼1.5-fold in the irradiated mice, compared with that of control animals, however, pretreatment of the irradiated mice with ginsan rather decreased it. It has been reported that the depletion of GSH can promote the expression of HO-1 either through increasing ROS accumulation or directly influencing signal transduction pathways (53,54). However, our present results indicate that HO-1 induction seemed to act together with NP-SH concentration in organs. It is, therefore, quite possible that the decrease of HO activity in ginsan-treated and irradiated mice might be due to unnecessary HO, because radiation-induced oxidative processes were already downregulated by other preceding antioxidant enzymes that were activated by ginsan. However, it might be related with decreased level of TGF-β1. TGF-β is a multifunctional cytokine exerting biological activities such as regulation of cell proliferation and differentiation, suppression of immune response and regulation of deposition of extracellular matrix component. TGF-β is induced by ROS stimuli and may signal certain events through the generation of ROS (37). In good agreement with the results of Chang et al. (55), TGF-β in irradiated mice spleens was not induced in this study, however, ginsan treatment before irradiation rather decreased TGF-β expression, thus diminishing of HO activity. Furthermore, it has recently been reported that SOD reduces TGF-β expression in myofibroblast, and IFN-γ can prevent the cellular response to TGF-β through the induction of Smad 7 protein (56). In consistent with the above observation, we showed that ginsan was able to restore broken cytokine balance (shifted T helper type-2) by irradiation through increasing T helper type-1 cytokines (IL-12, IFN-γ). Therefore, it is quite possible that ginsan increased IFN-γ production, and IFN-γ in turn inhibited TGF-β and HO in irradiated mice. The action of HO-1 is very complex, because of its pleiotropic effects of the end products resulting from heme catabolism, therefore, it is not easy to predict whether HO-1 protects cells or worsen the injury in a given situation.
Our observations showed that ginsan itself did not alter antioxidant systems in vivo. However, under stressful circumstances such as exposure to radiation which cause redox-state disturbance, ginsan may modulate the oxidant and antioxidant status by not only increasing SODs and GPx activity but also decreasing HO activity and NP-SH contents. Polysaccharides, widely distributed in animals, plants and fungi, have been suggested to play an important role as a dietary radical scavenger to prevent oxidative damage in living systems (57,58), and ginsan also exhibited weak scavenging activity against DPPH free radicals. Nevertheless, the antioxidant effect of ginsan dose not seem to be due to direct scavenging of free radicals, since the antioxidant enzyme activities were induced on the 5th day after irradiation.
In conclusion, the radioprotective action of ginsan in irradiated mice is partly due to rapid regeneration of hematopoietic cells and also due to induction of antioxidant enzymes. The modulatory effect of ginsan on cellular and molecular redox systems, together with the possible close relationship of ROS with aging, carcinogenesis and pathogenesis of a variety of inflammatory disorders, are expected to provide antioxidant therapeutic strategies for the treatment of various detrimental conditions.
Acknowledgments
We are grateful to Mr Shin Keun Kang for expert technical assistance. We thank professor Woon Ki Paik at Hanyang University for critical review of the manuscript. This work was supported by grant from Korea Institute of Science and Technology Evaluation and Planning (KISTEP) and Ministry of Science and Technology (MOST), Korean Government, through its National Nuclear Technology Program.
References
- 1.Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–93. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Mechanisms involved in the generation of free radical. Pathol Biol. 1996;44:6–13. [PubMed] [Google Scholar]
- 3.Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, et al. Chemopreventive effect of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81–5. doi: 10.1093/carcin/19.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Inoue M, Watanabe N, Morino Y, Tanaka Y, Amachi T, Sasaki J. Inhibition of oxygen toxicity by targeting superoxide dismutase to endothelial cell surface. FEBS Lett. 1990;269:89–92. doi: 10.1016/0014-5793(90)81126-9. [DOI] [PubMed] [Google Scholar]
- 5.Sano M, Ernersto C, Thomas RG. A controlled study trial of selegiline, alpha-tocopherol, or both as treatment of Alzheimer's disease. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese FJ, Canada AT. Catalase: its role in xenobiotic detoxification. Pharmacol Ther. 1989;44:297–307. doi: 10.1016/0163-7258(89)90069-7. [DOI] [PubMed] [Google Scholar]
- 7.Canada AT, Calabrese EJ. Superoxide dismutase: its role in xenobiotic detoxification. Pharmacol Ther. 1989;44:285–95. doi: 10.1016/0163-7258(89)90068-5. [DOI] [PubMed] [Google Scholar]
- 8.Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol. 1992;44:1905–15. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- 9.Meister A. Biosynthesis and functions of glutathione, an essential biofactor. J Nutr Sci Vitaminol (Tokyo) Spec. 1992:1–6. doi: 10.3177/jnsv.38.special_1. [DOI] [PubMed] [Google Scholar]
- 10.Muse KE, Oberley TD, Sempf JM, Oberley LW. Immunolocalization of antioxidant enzymes in adult hamster kidney. Histochem J. 1994;26:734–53. doi: 10.1007/BF00158205. [DOI] [PubMed] [Google Scholar]
- 11.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–68. [PubMed] [Google Scholar]
- 12.Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–69. [Google Scholar]
- 13.Heffner JA, Repine JE. State of the art: pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989;140:531–54. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–509. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 15.Bast A, Haenen GR, Doelman CJ. Oxidants, antioxidants: state of arts. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 16.Gutteridge JM. Antioxidant properties of the proteins ceruloplasmin, albumin and transferring. A study of their activity in serum and synovial fluid from patients rheumatoid arthritis. Biochem Biophys Acta. 1986;869:119–27. doi: 10.1016/0167-4838(86)90286-4. [DOI] [PubMed] [Google Scholar]
- 17.Banerjce U, Izquierdo JA. Antistress, antifatigue properties of panax ginseng; Comparison with piracetan. Acta Physiol Lat Am. 1982;32:277–85. [PubMed] [Google Scholar]
- 18.Deng HL, Zhang JT. Anti-lipid peroxidative effect of ginsenoside Rbl and Rgl. Chin Med J (Engl) 1991;104:395–8. [PubMed] [Google Scholar]
- 19.Sohn JO, Lim HB, Lee YC, Lee DW, Kim YT. Effect of subchronic administration of antioxdants against cigarette smoke exposure in rats. Arch Toxicol. 1993;67:667–73. doi: 10.1007/BF01973689. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata T, Ma Y, et al. Ginseng extract scavenges hydroxyl radical, protects unsaturated fatty acid from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996;20:145–50. doi: 10.1016/0891-5849(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Chen X, Gillis N. Ginsenosides protect pulmonary vascular endothelium against free radical-induced injury. Biochem Biophys Res Commun. 1992;189:670–6. doi: 10.1016/0006-291x(92)92253-t. [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Park KH, Rho HM. Transcriptional activation of the Cu, Zn-superoxide dismutase gene through the AP2 site by ginsenoside Rb2 extracted from a medical plant, Panax ginseng. J Biol Chem. 1996;271:24539–43. doi: 10.1074/jbc.271.40.24539. [DOI] [PubMed] [Google Scholar]
- 23.Song JY, Han SK, Bae KG, Lim DS, Son SJ, Jung IS, et al. Radioprotective effects of ginsan, an immunomodulator. Radiat Res. 2003;159:768–74. doi: 10.1667/0033-7587(2003)159[0768:reogai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Aksahi M, Hachiya M, Paquette RL, Osawa YS, Shimizu SG. Irradiation increases manganese superoxide dismutase mRNA levels in human fibroblasts. J Biol Chem. 1995;270:15864–9. doi: 10.1074/jbc.270.26.15864. [DOI] [PubMed] [Google Scholar]
- 25.Wong GH, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–4. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 26.Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51:3661–7. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reaction. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–63. [PubMed] [Google Scholar]
- 29.Abei HE, Bergmyer H, Bergmyer J, Gral M. Methods of Enzymatic Analysis. 3rd edition. Vol. 3. Canada: John Wiley & Sons; 1983. Verlag Chemie, 273. [Google Scholar]
- 30.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 31.Ryter SW, Kvam E, Tyrrell RM. Heme oxygenase activity: current methods, applications. Methods Mol Biol. 2000;99:369–91. doi: 10.1385/1-59259-054-3:369. [DOI] [PubMed] [Google Scholar]
- 32.Sedlack J, Lindsay RH. Estimation of total, protein-bound, nonprotein sulfhydryl groups in tissues with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 33.Ewing JE, Maines MD. Glutathione depletion induced heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993;60:1512–19. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- 34.Maines MD, Kappas A. Regulation of heme pathway enzymes and cellular glutathione content by metals that do not chelate with tetrapyrroles: blockade of metals effects by thiol. Proc Natl Acad Sci USA. 1977;74:1875–8. doi: 10.1073/pnas.74.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maines MD, Sinclair P. Cobalt regulation of heme synthesis and degradation in avian embryo liver cell culture. J Biol Chem. 1977;252:219–23. [PubMed] [Google Scholar]
- 36.Ning W, Song R, Li C, Park E, Mohsenin A, Choi AM, et al. TGF-β1 stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1094–L1102. doi: 10.1152/ajplung.00151.2002. [DOI] [PubMed] [Google Scholar]
- 37.Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwall A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-(in human renal epithelial cells. J Biol Chem. 2002;275:40904–9. doi: 10.1074/jbc.M006621200. [DOI] [PubMed] [Google Scholar]
- 38.Aksahi M, Hachiya M, Paquette RL, Osawa YS, Shimizu SG. Irradiation increases manganese superoxide dismutase mRNA levels in human fibroblasts. J Biol Chem. 1995;270:15864–9. doi: 10.1074/jbc.270.26.15864. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Saito N, Takemori N, Iizuka S, Suzuki K, Taniguchi N, Iizuka H. Ultrastructural localization of superoxide dismutase in human skin. Acta Derm Venereol (Stockholm) 1993;73:41–5. doi: 10.2340/00015555734145. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi H, Hashimoto Y, Aoki N, Kinouchi M, Ishida YA, Iizuka H. Copper, zinc-superoxide dismutase protects from ultraviolet B-induced apoptosis of SV40-transformed human keratinocytes: the protection is associated with the increased levels of antioxidant enzymes. J Dermatol Sci. 2000;23:12–21. doi: 10.1016/s0923-1811(99)00060-2. [DOI] [PubMed] [Google Scholar]
- 41.Masuda A, Longo DL, Kobayashi Y, Appelia E, Oppenheim JJ, Matsushima K. Induction of mitochondrial manganese superoxide dismutase by interleukin 1. FASEB J. 1988;2:3087–91. doi: 10.1096/fasebj.2.15.3263930. [DOI] [PubMed] [Google Scholar]
- 42.Matsubara N, Fuchimoto S, Iwagaki H, Nonaka Y, Kimura T, Kashino H, et al. The possible involvement of free radical scavenging properties in the actions of cytokines. Res Commun Chem Pathol Pharmacol. 1991;71:239–42. [PubMed] [Google Scholar]
- 43.Harris CA, Derbin KS, Hunte-McDonough B, Krauss MR, Chen KT, Smith DN, et al. Manganese superoxide dismutase is induced by IFN-γ in multiple cell types: Synergistic induction by IFN-γ and tumor necrosis factor or IL-1. J Immunol. 1991;147:149–54. [PubMed] [Google Scholar]
- 44.Visner GA, Dougall WC, Wilson JM, Burr IA, Nick HS. Regulation of manganese superoxide dismutase by lipopolysaccharidem interleukin-1, and tumor necrosis factor. J Biol Chem. 1990;265:2856–64. [PubMed] [Google Scholar]
- 45.Niwa Y, Ishimoto K, Kanoh T. Induction of superoxide dismutase in leukocytes by paraquat: correlation with age, possible predictor of longevity. Blood. 1990;76:835–41. [PubMed] [Google Scholar]
- 46.Sugiura Y, Suzuki T. Nucleotide sequence specificity of DNA cleavage by iron-bleomycin: alteration on ethidium bromide-, actinomycin-, distamycin-intercalated. J Biol Chem. 1982;257:10544–6. [PubMed] [Google Scholar]
- 47.Lu G, Shimizu I, Cui X, Itonaga M, Tamaki K, Fukuno H, et al. Interferon-alpha enhances biological defense activities against oxidative stress in cultured rat hepatocytes and hepatic stellate cells. J Med Invest. 2002;49:172–81. [PubMed] [Google Scholar]
- 48.Kandasamy SB, Kumar JS, Harris AH. Involvement of superoxide dismutase and glutathione peroxidase in attenuation of radiation-induced hyperthermia by interleukin-1 alpha in rats. Brain Res. 1993;606:106–10. doi: 10.1016/0006-8993(93)91576-e. [DOI] [PubMed] [Google Scholar]
- 49.Moran LK, Gutteridge JMC, Quinlan GJ. Thiols in cellular redox signaling and control. Curr Med Chem. 2001;8:763–72. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- 50.Sedlack J, L'Hanus Changes of glutathione, protein bound SH-groups concentrations in rat adrenals under acute and repeated stress. Endocrinol Exp. 1982;16:103–9. [PubMed] [Google Scholar]
- 51.Sedlack J. Long-term effect of hypophysectomy on various fractions of sulfhydryl groups in thyroid, adrenal and some other organs in rats. Endocrinol Exp. 1985;19:186–92. [PubMed] [Google Scholar]
- 52.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–26. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 53.Rahman I, MacNee W. Regulation of redox glutathione levels, gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000;28:1405–20. doi: 10.1016/s0891-5849(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 54.Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive, UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992;13:227–32. doi: 10.1093/carcin/13.2.227. [DOI] [PubMed] [Google Scholar]
- 55.Chang CM, Limanni A, Bakjer WH, Dobson ME, Kalinich JF, Jackson W, et al. Bone marrow and splenic granulocyte-macrophage colony-stimulating factor and transforming growth factor-β mRNA levels in irradiated mice. Blood. 1995;86:2130–6. [PubMed] [Google Scholar]
- 56.Martin M, Lefaix JL, Delanian S. TGF-β1 and radiation fibrosis: a master switch, a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–90. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 57.Jun L, Mei Z, Yuan C. Reversal of inhibition of reactive oxygen species on respiratory burst of macrophages by polysaccharide from Coriolus versicolor. Int J Immunopharmacol. 1993;15:429–33. doi: 10.1016/0192-0561(93)90055-4. [DOI] [PubMed] [Google Scholar]
- 58.Pang ZJ, Chen Y, Zhou M. The effect of polysaccharide krestin on GPx gene expression in macrophages. Acta Biochim Biophys Sinica. 1999;31:284–8. [PubMed] [Google Scholar]