Abstract
In response to increased popularity and greater demand for medicinal plants, a number of conservation groups are recommending that wild medicinal plants be brought into cultivation systems. We collected four medicinal herbs Cichorium pumilum, Eryngium creticum, Pistacia palaestina and Teucrium polium used in traditional Arab medicine for greenhouse cultivation to assess the effects of different fertilization regimes on their growth and antioxidant activity. Wild seedlings were collected and fertilized with either 100% Hoagland solution, 50% Hoagland solution, 20% Hoagland solution or irrigated with tap water. Plant height was measured and the number of green leaves and branches counted weekly. Thereafter, the aboveground parts of plants were harvested for preparing a water-soluble powder extracts of which antioxidant activity was measured by their ability to suppress the oxidation of β-carotene. Of the fertilization regimes, we found either 20 or 50% Hoagland solution produced the most consistent response of the plant growth parameters. All powders prepared from the four wild growing plants inhibited oxidation of β-carotene. Increasing the amount of fertilizer caused a significant concentration-dependent increase in antioxidant activity of the cultivated T. polium compared with the wild type. In contrast, increasing the amount of fertilizer caused a significant concentration-dependent reduction in the antioxidant activity of powders prepared from the cultivated E. creticum when compared with wild plants. Our results showed that cultivation success should not rely solely on parameters of growth but should incorporate assessment related to indices of therapeutic potential.
Keywords: antioxidant activity, medicinal plants, plant conservation, plant cultivation
Introduction
In recent years, the use of herbal therapies has gained in popularity (1–4). This has lead to an increased demand for medicinal plants and there is now increasing alarm about the potential extinction of some medicinal plants (5). In response, a number of conservation groups are recommending that wild medicinal plants be brought into cultivation systems (6). Numerous ethnopharmacological surveys have attested to the use of indigenous plants among practitioners of traditional Arab medicine in Israel, the Palestinian territories and Jordan (7–11). Against this background, we decided to collect several indigenous medicinal plants for cultivation under greenhouse conditions and to assess the effects of different fertilization regimes on their growth.
Plants have an almost limitless ability to synthesize aromatic substances that have been evaluated for their therapeutic potential. These include alkaloids, coumarins, saponins and flavonoids (12,13). Flavonoids are probably the best known of these substances due to their antioxidant properties (14,15). Accordingly, we decided to use antioxidant activity as a surrogate index of their therapeutic potential to compare the antioxidant potential of extracts prepared from wild growing medicinal plants with those cultivated in the greenhouse.
Materials and Methods
Plant Species
The four plants were Cichorium pumilum Jacq. (RDC 1029), Eryngium creticum Lam. (RDC 1046), Pistacia palaestina L. (RDC 1130) and Teucrium polium L. (RDC 1117). Following their field identification by botanists, seedlings were collected for transplanting and wild specimens for preparation of plant powders (Table 1).
Table 1.
Plant species studied, time of seedling collection for replanting, time of harvest of cultivated or wild plant specimens, plant parts and the extraction yield of wild species
| Plant species | Time of seedling collection | Harvest time | Plant parts used for extraction | % Extraction yield (w/w) |
|---|---|---|---|---|
| Cichorium pumilum | February | April–May | Leaves and stems | 15.4 |
| Eryngium creticum | January | March–April | Leaves and stems | 13.2 |
| Pistacia palaestina | March | July–August | Leaves | 14.8 |
| Fruits | 6.5 | |||
| Teucrium polium | March | May–July | Leaves | 8.0 |
| Stems | 8.1 |
Collection of Wild Plants for Extraction
Leaves, stems and fruits of the four plants were collected separately from the hills of the Galilee region of Israel during the spring and summer (Table 1). After collection, the plants were dried for 7–10 days at room temperature. The dried plant parts were then ground. The ground product was stored in cloth bags at 5°C until their transfer to the laboratory for preparation of the plant powder.
Plant Cultivation
Wild seedlings were cultivated in a greenhouse (relative humidity 50–70%; temperature 25–35°C) during the spring and the summer. Each seedling was grown in a pot (25 cm height and 20 cm diameter) filled with sandy-loamy soil. Treatments consisted of four different fertilization regimes: (i) full (100%) Hoagland solution (16); (ii) 50% Hoagland solution; (iii) 20% Hoagland solution; and (iv) control (irrigation with tap water). For each treatment, 10 uniform seedlings were grown for 9–11 weeks before being harvested. Each week, plant height was measured and the number of green leaves and branches counted. At the end of the period, aboveground parts of the plants were harvested and weighed. The plants were then dried and ground for preparing the plant powder, as previously described.
Preparation of Plant Powders
The method for preparing water-soluble plant powders has been previously described (17). Briefly, dried plant material (31.5 g) was stirred in 350 ml of distilled water for 15 min at 100°C, followed by rapid filtration through a crude cellulose filter and then by a more delicate filtration through Whatman filter paper no. 1. The resulting filtrate was frozen and freeze-dried. The powder was stored at −18°C in a desiccant until required.
β-Carotene Oxidation Levels of Plant Powders
The antioxidant potential of different aqueous plant extracts was evaluated by measuring their ability to suppress the oxidation of β-carotene as described by Vaya et al. (18), with two minor modifications: the plant powder was dissolved in water instead of alcohol to a final concentration of 100 µg ml−1 and the spectrophotometric readings were taken at 454 nm. The assay was performed at 50°C. The OD454 readings of the rate of β-carotene bleaching were recorded at 20 min intervals for 2 h against the water blank. This method allowed evaluation of the antioxidative activity of the preparations along a time scale. The antioxidant butylated hydroxyanisole (BHA) (100 µg ml−1) (Sigma Chemical Co., St Louis, MO, USA) served as a positive control. The assay was repeated three times for each powder.
Statistical Analysis
The data were analyzed by either a student's t-test or a repeated measures of the one-way analysis of variance (ANOVA) with Dunnett's post-test using Instat™ version 3 (GraphPad Software Inc., San Diego, CA, USA). All data are presented as mean ± standard deviation (SD). Significance was set at 5%.
Results
Fertilization Regimes Affect Parameters of Plant Growth under Greanhouse Conditions
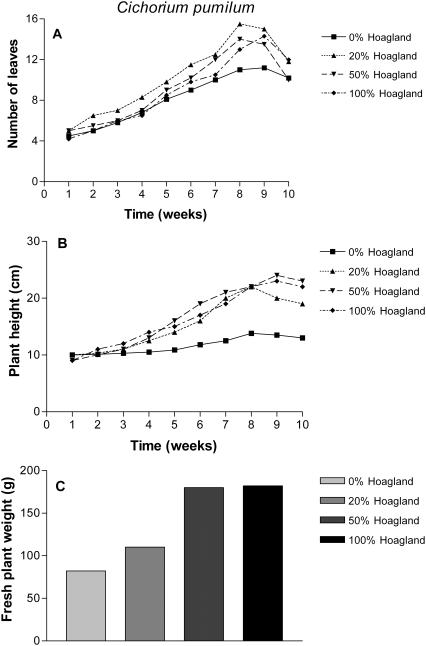
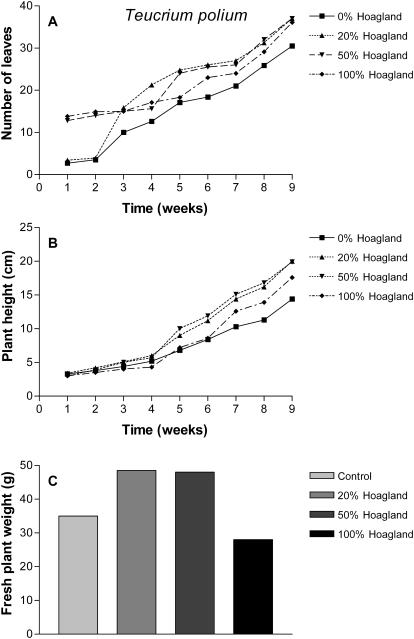
In the greenhouse experiment, we succeeded in cultivating the four plant species. In general, all plants responded positively to the different fertilization regimes (Figs 1–4). Although we found individual differences among the plants, the overall picture indicated that fertilization with either 20 or 50% Hoagland solution produced the most consistent response when measuring the number of leaves, plant height and fresh weight at harvest as indices of growth. Full (100%) Hoagland solution caused some reduction in the fresh weights of P. palaestina and T. polium (Figs 3C and 4C). We did not observe any effect of the different fertilization regimes on numbers of branches of the plants (data not shown).
Figure 1.
Effect of different fertilization regimes on the average number of green leaves (A), plant height (B) and fresh weight (C) of C. pumilum cultivated for 10 weeks under greenhouse conditions. Values presented are mean of 10 plants.
Figure 4.
Effect of different fertilization regimes on the average number of green leaves (A), plant height (B) and fresh weight (C) of T. polium cultivated for 9 weeks under greenhouse conditions. Values presented are mean of 10 plants.
Figure 3.
Effect of different fertilization regimes on the average number of green leaves (A), plant height (B) and fresh weight (C) of P. palaestina cultivated for 11 weeks under greenhouse conditions. Values presented are mean of 10 plants.
Antioxidant Activity of Powders Prepared from Wild and Cultivated Plants
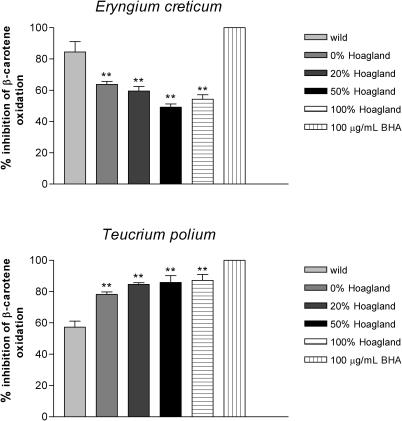
All powders prepared from the aerial parts of the four wild growing plants inhibited oxidation of β-carotene (Fig. 5). The extent of inhibition was dependent on the species and the plant parts. The extent of inhibition caused by powders prepared from C. pumilum, E. creticum and P. palaestina was greater than 84%, whereas the powders prepared from either the leaves or fruits of T. polium were less effective (60–65%) (Fig. 5). Fig. 6 describes the effect of different fertilization regimes on antioxidant potential of powders prepared from E. creticum and T. polium. Increasing the amount of fertilizer caused a significant concentration-dependent reduction in antioxidant activity of powders prepared from the cultivated E. creticum when compared with the wild plant (Fig. 6, top). In contrast, we observed a significant concentration-dependent increase in antioxidant activity of the cultivated T. polium compared with the wild type (Fig. 6, bottom).
Figure 5.
The effect of powders (100 µg ml−1) prepared from various aerial parts of C. pumilum (top left), E. creticum (bottom left), P. palaestina (top and bottom middle) and T. polium (top and bottom right) on the inhibition of β-carotene oxidation. The P-values represent the significance of the difference from the extent of inhibition obtained by plant powders (100 µg ml−1) and 100 µg ml−1 of BHA control. Values presented are mean of three replicates ± SD.
Figure 6.
The effect of different fertilization regimes on powders (100 µg ml−1) prepared from cultivated E. creticum (top) and T. polium (bottom) on the inhibition of β-carotene oxidation. *P < 0.05 and represent the significance of the difference from the extent of inhibition obtained by powders (100 µg ml−1) prepared from the wild growing species and 100 µg ml−1 BHA control. Values presented are mean of three replicates ± SD.
Discussion
The increased demand for medicinal plants has created an ecological crisis for medicinal herbs growing in the wild raising alarm about their rate of extinction (5,19,20). Accordingly, conservation agencies are now recommending that wild species be brought into cultivation systems (6). The four plant species analyzed in this study are well-known medicinal herbs used to treat various diseases including liver, diabetes, rheumatism and microbial inflammation in traditional Arab medicine (8,11,21–23). Against this background, the first aim was to try to cultivate these plants under greenhouse conditions. While these four plants are not threatened or endangered species, our results indicate that it is possible to cultivate wild medicinal plants under greenhouse conditions because each plant grew following transplantation. Natural-product chemists are expressing concern that potential therapeutically useful phytopharmaceuticals are at risk of being lost irretrievably due to overexploitation of wild-growing medicinal plants (24,25). Therefore, the second aim was to assess the contribution of different fertilization regimes to antioxidant activity of powders prepared from cultivated medicinal plants as a surrogate marker of potential therapeutic usefulness. In this regard, we found that fertilization regimes influenced the antioxidant activity.
The therapeutic benefit of medicinal plants is often attributed to their antioxidant properties due to the presence of flavonoids, a class of natural polyphenols found in green plant cells (13–15). Wild plants tend to exhibit great variation in their content of aromatic compounds due to environmental and genetic differences (19,26). We showed that fertilizations regimes may alter antioxidant activity of cultivated medicinal plants when compared with the antioxidant activity of wild medicinal plants. More specifically, increasing the amount of fertilizer increased the antioxidant activity of the powders prepared from cultivated T. polium. In contrast, increasing the amount of fertilizer decreased the antioxidant activity of powders prepared from cultivated E. creticum. Although we demonstrated that fertilization regimes influence the antioxidant potential, this finding should not be interpreted to suggest that other therapeutically beneficial plant ingredients may not be affected in the same manner. Accordingly, we conclude that the indices of plant growth cannot be solely used as parameters of successful growth of cultivated plants.
Leaf senescence is a crucial developmental state in the life of plants. It is the time during which compounds synthesized by the plant during its growth phase are mobilized into younger tissues. Leaf senescence also indicates the beginning of the harvest period (27) and has been shown to affect antioxidant activity (28). In our current work, the antioxidant activity of T. polium was measured in powders prepared while the plant was still growing or before the onset of leaf senescence. In contrast, the antioxidant activity of E. creticum was determined when the plant was no longer growing or during leaf senescence because some leaves started wilting. We believe leaf senescence of the two cultivated plants may account for differences in antioxidant activity in response to different fertilization regimes. Wild plants and cultivated species exhibit great variation in content of secondary metabolites as well as their biological activities due to the growth environment (i.e. soil type, nutrients, topographic, salinity, drought, allelochemicals) and genetic differences among species (19,26,29,30). These factors should be considered by ethnopharmacologists before prescribing remedies from plants collected from various regions as well as the pharmaceutical companies dealing with medicinal herbs. This fact is crucial nowadays since traditional medicine and alternative systems of medicine (CAM) is practiced in more than 70% of the developing world's population and there is increase demand in the modern world (1,2,31). Evidence-based CAM therapies have shown remarkable success in healing acute as well as chronic diseases. In addition, the growing popularity of botanical dietary supplements has been accompanied by concern regarding the quality of commercial products and their sources; therefore, health care providers should keep themselves informed regarding product quality in order to be able to appropriately advise patients utilizing both conventional and herbal medicines (3,4,32).
To conclude, we were able to successfully cultivate wild growing medicinal plants. Cultivation per se has several advantages. First, cultivation reduces the possibility of incorrect identification and adulteration. Second, cultivated plants can be irrigated and fertilized to increase their growth rate thereby improving yield. Third, cultivated plants can be grown in areas of similar climate and soil. Our results also showed that cultivation success of medicinal plants should incorporate indices of therapeutic potential. Such an approach can reduce or even eliminate the variation in content of the desired aromatic compounds as well as providing information on the best time to harvest so that the content of the desired aromatic compound(s) is maximal.
Figure 2.
Effect of different fertilization regimes on the average number of green leaves (A), plant height (B) and fresh weight (C) of E. creticum cultivated for 11 weeks under greenhouse conditions. Values presented are mean of 10 plants.
Acknowledgments
This research was supported by grants from the Ministry of Science and Technology, Jerusalem, Israel, The Center for Absorption in Science of the Ministry of Immigrant Absorption, the Committee for Planning and Budgeting of the Council for Higher Education under the framework of the KAMEA and GILADI Programs, and the Technion Vice-President's Research Fund for Promotion for Research at the Technion. Many thanks go to Mr Jonathan Ritter for his help and advice throughout the project.
References
- 1.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States—prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–52. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national study. J Am Med Assoc. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 3.Yankauer A. The recurring popularity of alternative medicine. Perspect Biol Med. 1997;41:132–7. [Google Scholar]
- 4.Druss BG, Rosenheck RA. Association between use of unconventional therapies and conventional medical services. J Am Med Assoc. 1999;282:651–6. doi: 10.1001/jama.282.7.651. [DOI] [PubMed] [Google Scholar]
- 5.Lewis WH, Elvin-Lewis MP. Medicinal plants as sources of new therapeutics. Ann Mol Bot Gard. 1995;82:16–24. [Google Scholar]
- 6.Lambert J, Srivastava J, Vietmeyer N. Medicinal Plants: Rescuing a Global Heritage. Washington, DC: World Bank; 1997. World Bank Technical Paper 355. [Google Scholar]
- 7.Dafni A, Yaniv Z, Palevitch D. Ethnobotanical survey of medicinal plants in northern Israel. J Ethnopharmacol. 1984;10:295–310. doi: 10.1016/0378-8741(84)90017-5. [DOI] [PubMed] [Google Scholar]
- 8.Ali-Shtayeh MS, Yaniv Z, Mahajna J. Ethnobotanical survey in the Palestinain area: a classification of the healing potential of medicinal plants. J Ethnopharmacol. 2000;73:221–32. doi: 10.1016/s0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 9.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J Ethnopharmacol. 2000;72:191–205. doi: 10.1016/s0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 10.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J Ethnopharmacol. 2002;82:131–45. doi: 10.1016/s0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 11.Said O, Khalil K, Fulder S, Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J Ethnopharmacol. 2002;83:251–65. doi: 10.1016/s0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 12.Watson AA, Fleet GWJ, Asano N, Molyneux RJ, Nash RJ. Polyhydroxylated alkaloids—natural occurrence and therapeutic applications. Phytochemistry. 2001;56:265–95. doi: 10.1016/s0031-9422(00)00451-9. [DOI] [PubMed] [Google Scholar]
- 13.Barnes J, Anderson LA, Phillipson JD. Herbal Medicines. London, UK: Pharmaceutical Society; 2002. [Google Scholar]
- 14.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 15.Rice-Evans C. Flavonoids and isoflavones: absorption, metabolism and activity. Free Radic Biol Med. 2004;36:827–8. doi: 10.1016/j.freeradbiomed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Terry N. Limiting factors in photosynthesis. I. Use of iron stress to control photochemical capacity in vivo. Plant Physiol. 1980;65:114–20. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ljubuncic P, Portnaya I, Cogan U, Azaizeh H, Said O, Abu Saleh K, Bomzon A. Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;99:43–47. doi: 10.1016/j.jep.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Vaya J, Belinky PA, Aviram M. Antioxidant constitutes from licorice roots: isolation, structure elucidation and oxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–13. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 19.Palevitch D. Agronomy applied to medicinal plant conservation. In: Akerele O, Heywood V, Synge H, editors. The Conservation of Medicinal Plants. Cambridge, UK: Cambridge University Press; 1991. pp. 168–78. [Google Scholar]
- 20.Shmida A, Fragman A. Endangered and rare plant species of Israel. Ecol Environ. 1999 (in Hebrew) [Google Scholar]
- 21.Palevitch D, Yaniv Z, Dafni A, Fridman Y. Ministry of Science; 1985. Survey of wild plants in Israel as a pharmacological source. (in Hebrew) [Google Scholar]
- 22.Palevitch D, Yaniv Z. Medicinal Plants of the Holy Land. Tel Aviv, Israel: Modan Publishing House; 2000. [Google Scholar]
- 23.Abu-Irmaileh BE, Afifi FU. Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol. 2003;89:193–7. doi: 10.1016/s0378-8741(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 24.Borris RP. Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol. 1996;51:29–38. doi: 10.1016/0378-8741(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 25.Buenz EJ, Schnepple DJ, Bauer BA, Elkin PL, Riddle JM, Motley TJ. Techniques: bioprospecting historical herbal texts by hunting for new leads in old tomes. Trends Pharmacol Sci. 2004;25:494–8. doi: 10.1016/j.tips.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Yaniv D, Palevitch D. Effect of drought on the secondary metabolites of medicinal and aromatic plants, a review. In: In: Atal CK, Kapur BM, editors. Cultivation and Utilization of Medicinal Plants. Jammu-Tawa, India: Regional Research Laboratory, Council of Scientific and Industrial Research; 1982. [Google Scholar]
- 27.Gan S, Amasino RM. Making sense of senescence. Plant Physiol. 1995;113:313–9. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munne-Bosch S, Penuelas J. Photo- and antioxidative protection during summer leaf senescence in Pistacia lentiscus L. grown under Mediterranean field conditions. Ann Bot. 2003;92:385–91. doi: 10.1093/aob/mcg152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Darier SM, Youssef RS. Effect of soil type, salinity, and allelochemicals on germination and seedling growth of a medicinal plant Lepidium sativum L. Ann Appl Biol. 2000;136:273. [Google Scholar]
- 30.Khudsar T, Mahmooduzzafar M, Iqbal M, Sairman RK. Zinc-induced changes in morpho-physiological and biochemical parameters in Artemisia annua. Biologia Plantarum. 2004;48:255–60. [Google Scholar]
- 31.Shaikh BT, Hatcher J. Complementary and alternative medicine in Pakistan: prospects and limitation. Evid Based Complement Alternat Med. 2005;2:139–42. doi: 10.1093/ecam/neh088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krochmal R, Hardy M, Bowerman S, Lu Q-Y, Wang H-J, Elashoff RM, Heber D. Photochemical assays of commercial botanical dietary supplements. Evid Based Complement Alternat Med. 2004;1:305–13. doi: 10.1093/ecam/neh040. [DOI] [PMC free article] [PubMed] [Google Scholar]