Abstract
The zebrafish, with its transparent free-living embryo, is a useful organism for investigating early stages in lymphopoiesis. Previously, we showed that T cells differentiate in the thymus by day 4, but no sites for B cell differentiation were seen until 3 weeks. We report here that on day 4, we detect rearrangements of genes encoding B cell receptors in DNA extracted from whole fish. Also by day 4, rag1 transcripts are seen in the pancreas, an organ not previously associated with lymphopoiesis; by day 10, Igμ transcripts are detected here. Thus, in zebrafish, the pancreas assumes the role of both the liver in fetal mice and the spleen in neonatal mice.
The zebrafish, Danio rerio, offers unique opportunities for investigating early events in vertebrate development. Because the fish remain transparent for about the first week of life, the development of many organ systems can be followed visually. We use this organism to analyze the development of lymphoid cells and organs from their earliest appearance through adulthood.
Knowledge of lymphoid organs in teleosts is based largely on anatomic and histological observation. The thymus and kidney have been considered to be the major primary lymphoid organs; the latter, the equivalent of the bone marrow in adult mammals, the source of B lymphocytes (1, 2). The teleost kidney consists of the pronephros or head kidney and the mesonephros or trunk kidney, both of which are retained throughout life, the former being more active in hematopoiesis and the latter in excretion. Antibodies are produced by both parts of the kidney, especially the pronephros (3). Information about lymphopoiesis and localization of lymphocyte subsets, scarce in adult teleosts, is almost entirely lacking in early developmental stages.
To identify lymphoid organs in zebrafish, we initially cloned the recombination activating genes, rag1 and rag2 (4, 5), which encode the recombinases required for V(D)J rearrangement and are expressed in differentiating B and T lymphocytes (6). Rag1 was found to be expressed in the thymus by the fourth day of development (7). Expression of genes encoding a T cell receptor subunit, TCRα, was also detected in the thymus at day 4,† and lymphoid cells were seen here at this time (9). No other site for rag1 expression was noted, nor were lymphoid cells detected in the pronephros, the presumed site for B cell differentiation, until 3 weeks. These findings suggested that, relative to T lymphopoiesis, B lymphopoiesis may be delayed in zebrafish.
Tools to follow B cell development in zebrafish were obtained by cloning genes encoding Igμ, the heavy chain of IgM, the predominant Ig class in teleosts (11). In the present study, we show that rearrangements of genes encoding Igμ can be detected in DNA extracted from whole zebrafish as early as day 4. Expression of membrane Igμ was detected in RNA derived from whole fish by day 7, suggesting that cells of the B lineage are present well before any lymphocytes can be detected in the pronephros. These findings posed the challenge of identifying sites where B cells are localized in the period between 4 days and 3 weeks, when lymphocytes, presumably B cells, are seen in the pronephros. A resolution was provided when we found, unexpectedly, that rag1 is expressed in the pancreas by day 4, and Igμ is expressed from day 10. The results identify the pancreas as an organ for B lymphopoiesis in this species.
Materials and Methods
Animals.
Zebrafish stocks (wild-type and albino) were obtained from C. Nüsslein-Volhard (Tübingen, Germany) and were maintained as described (12). For some whole-mount in situ hybridization experiments, fish were treated with phenylthiourea to block pigment formation (12).
Preparation of Genomic DNA.
Approximately 30 larvae of different ages or several adults were frozen, pulverized in liquid nitrogen, and treated with 0.1 mg/ml proteinase K in 100 mM NaCl/10 mM Tris·HCl/0.5% SDS/25 mM EDTA for 12 h. DNA was extracted with phenol-chloroform-isoamyl alcohol (25:24:1) and precipitated with ethanol.
Isolation of RNA.
RNA was isolated with Trizol (GIBCO/BRL) from whole fish frozen in liquid nitrogen.
Primers.
VH1 family-specific primers correspond to the sequence of a VH genomic clone (ref. 11; GenBank accession no. AF273897): VHL1, nucleotides 344–364; VHFR1, 385–504; VHFR3, 760–740. VH2 family-specific primers correspond to the sequence of cDNA clone Vh115 (GenBank accession no. AF273879): 5RL, 26–47; 5RFR1, 102–122; 5RFR3r, 388–368. VH3 family-specific primers correspond to the sequence of cDNA clone Vh3 (GenBank accession no. AF273883): vh3L, 30–51; vh3fr1, 85–105; vh3fr3, 383–362. VH4 family-specific primers correspond to the sequence of cDNA clone Vh103 (GenBank accession no. AF273877): vh103L, 14–34; vh103fr1, 72–93; vh103r, 350–330. The FR4 degenerate primer (5′-GTTCCYTTHCCCCAGTAGTCAAA) was based on the sequences of cDNA clones cd6 (GenBank accession no. AF273900), vh114 (GenBank accession no. AF273880), vh3 (GenBank accession no. AF273883), and vh103 (GenBank accession no. AF273877).
Primer Cμ3S (GenBank accession no. AF281480, nucleotides 1191–1209) corresponds to the CH3 domain, which is shared by the secreted and membrane forms of zebrafish Igμ (N.D. and L.A.S., unpublished data). Primer Ctail (GenBank accession no. AF281480, 1580–1562) is specific for the secreted form and primer tm2 (GenBank accession no. AF281479, 532–510) is specific for the membrane form.
Analysis of VDJ Rearrangement.
Genomic DNA was prepared from zebrafish of different ages; 0.2 μg was amplified with 0.6 μM primer corresponding to the leader sequence of zebrafish family VH1 (VHL1), VH2 (5RL), VH3 (vh3L), or VH4 (vh103L) and 1.0 μM of a degenerate primer corresponding to the JH region (FR4). For the VH1, VH3, and VH4 families, 35 cycles were carried out as follows: 94°C for 30 sec, 50°C for 45 sec, and 72°C for 1 min. For the VH2 family, the annealing temperature was 45°C instead of 50°C. The products were applied to a 2% agarose gel; 0.4 μl or 4 μl (adults and embryos, respectively) of the material was eluted from the appropriate gel slice was used as template in nested PCR, with 0.25 μM primers corresponding to FR1 and FR3 of each of the four zebrafish VH families: VH1, VHFR1 and VHFR3; VH2, 5RFR1 and 5RFR3r; VH3, vh3fr1 and vh3fr3; VH4, vh103fr1 and vh103r.
Analysis of IgM Expression by RT-PCR.
Total RNA (1 μg) from whole larvae of different ages was reverse-transcribed by using random hexamer primers; one-sixth of the product was used in PCR to estimate expression of secreted and membrane forms of Igμ. For secreted Igμ, 0.25 μM primers Cμ3S and Ctail were used with cycling conditions: 35 cycles of 30 sec at 95°C, 45 sec at 50°C, and 1 min at 72°C; for membrane Igμ, conditions were the same except the primers were Cμ3S and tm2. A separate reaction with the same cDNA was performed with 0.25 μM primers corresponding to exons 2 and 6 in zebrafish elongation factor 1α (GenBank accession no. L23807l; 86–103, 987–970).
In Situ Hybridization.
Zebrafish were fixed overnight in 4% (wt/vol) paraformaldehyde in PBS. For preparation of cryosections, fish were transferred sequentially to 5%, 15%, and 30% sucrose and frozen in Tissue-Tek OCT embedding medium (Sakura Finetek, Torrance, CA); 8-μm sections were cut and stored at −20°C. For paraffin sections, fixed fish were dehydrated sequentially in ethanol and toluene and embedded in paraffin; sections from 4 to 7 μm thick were prepared. To prepare an Igμ probe, a segment corresponding to restriction sites BamHI/SpeI (nucleotides 463–1432, GenBank accession no. AF281480) was recloned into pBluescript SK(+) (Stratagene). For an antisense probe, the construct was linearized with HindIII and, for a sense probe, with XbaI. RNA labeled with digoxigenin was transcribed according to the manufacturer's instructions (Dig RNA Labeling kit, Roche Molecular Biochemicals) with T3 RNA polymerase to generate the antisense probe and with T7 RNA polymerase to generate the sense probe. Rag1 probes were prepared from a cloned segment (638 bp) of genomic rag1 DNA (4) as described (7). An insulin probe (300 bp) was a gift from Z. Sun and N. Hopkins (Massachusetts Institute of Technology; ref. 13). Probes were not hydrolyzed. In situ hybridization, either on sections or whole larvae, was carried out as described by Harland (14), but omitting 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) from washes and increasing hybridization and washing temperatures to 65°C.
Results
Rearrangement of Genes Encoding Igμ in Developing Zebrafish.
The initial event that must occur before Igμ can be expressed in the maturing B lymphocyte is rearrangement of V, D, and J gene segments that encode the μ chain. To determine when such rearrangements occur, total DNA extracted from fish at different ages was subjected to PCR under conditions such that amplification can occur only if there has been a rearrangement. Upstream primers were chosen to be specific for the leader segment of each of the four previously described VH families; the sequence of the degenerate downstream primer was based on the identified J gene segments (11). If a rearrangement has occurred, the product would contain ≈450 bp; in the absence of a rearrangement, the gene segments corresponding to the primers are expected to be too distant for amplification.
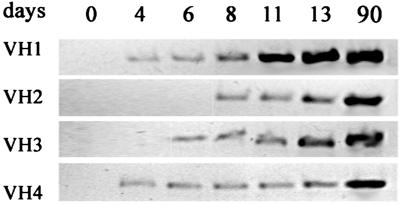
DNA was taken from zebrafish larvae between days 1 and 13, and from adults, as a control. A band corresponding in size to 450 bp was obtained after amplification of DNA from adults. With larval DNA, no amplified products were revealed by staining with ethidium bromide; however, segments corresponding to 450 bp were excised from the gel, and the eluted material was subjected to a second round of amplification with primers corresponding to FR 1 and 3 of the appropriate VH family. As shown in Fig. 1, the products of V(D)J rearrangements involving the VH1 and VH4 families were detected from day 4 and those involving the VH3 and VH2 families from days 6 and 8, respectively. These results suggest that Igμ genes capable of being expressed are already present in 4-day-old zebrafish.
Figure 1.
Time course of Igμ gene rearrangement during development. Total genomic DNA from about 30 larvae was subjected to PCR with primers specific for each of the VH families indicated. Amplification occurred only after VDJ rearrangement of genes in that family.
Expression of Igμ During Development.
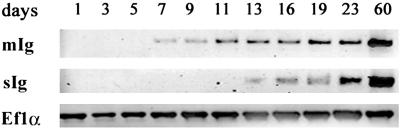
The expression of Igμ during development was initially evaluated by RT-PCR. As in other teleosts (15–17), transcripts encoding the membrane form of zebrafish Igμ are generated by splicing from the 3′ end of the CH3 exon to the membrane exons, thereby excluding the CH4 exon, which is present only in transcripts encoding the secreted form of Igμ (N.D. and L.A.S., unpublished data). Accordingly, the two types of transcript can be selectively amplified by choosing specific downstream primers. Expression of the membrane form of Igμ was detected from day 7, albeit at a low level; expression of the secreted form was detected from day 13 (Fig. 2). Expression of both forms increased slowly with age, but at day 23, expression was still substantially less than at day 60.
Figure 2.
Expression of membrane (mIg) and secreted (sIg) forms of Igμ during development, estimated by RT-PCR. Total RNA was prepared from 50–100 fish at each time point. PCR was performed with an upstream primer, Cμ3S, corresponding to a segment of the CH3 domain, which is found in both the secreted and membrane forms of Igμ; the downstream primers, Ctail and tm2, are specific for the secreted and membrane carboxyl-terminal tail pieces, respectively. The expression of elongation factor 1α served as a standard.
In previous reports, rag1 expression was detected in the thymus by whole-mount in situ hybridization at day 4, presumably reflecting rearrangements in TCR genes (7); indeed, TCRα was found to be expressed in the thymus at this time.† No additional sites for rag1 expression were noted. However, after hybridization with a rag1 probe, we now detected a tiny spot in the right dorsal aspect of the abdomen, in the region where the pancreas has formed (Fig. 3A). This expression could be a consequence of the rearrangement in B lineage cells of genes belonging to the VH1 and VH4 families (Fig. 1).
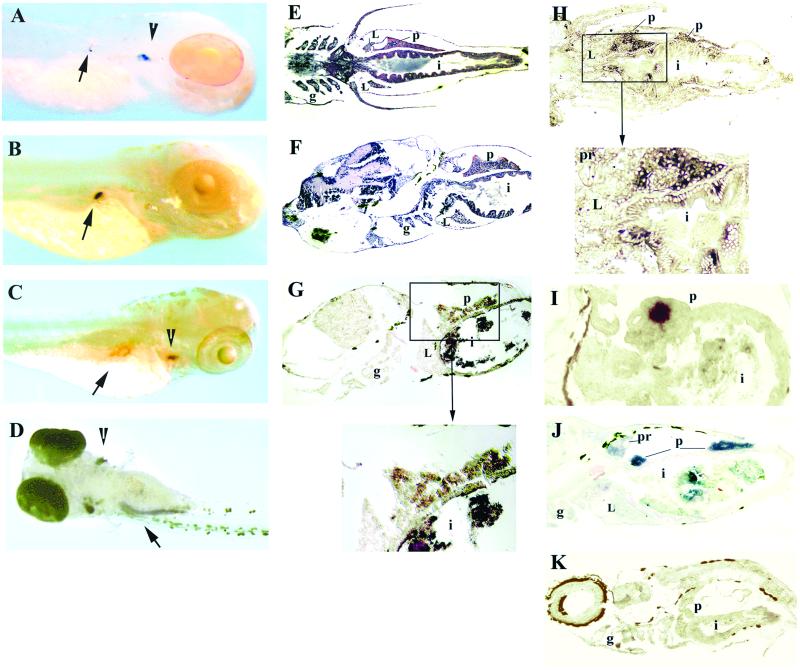
Figure 3.
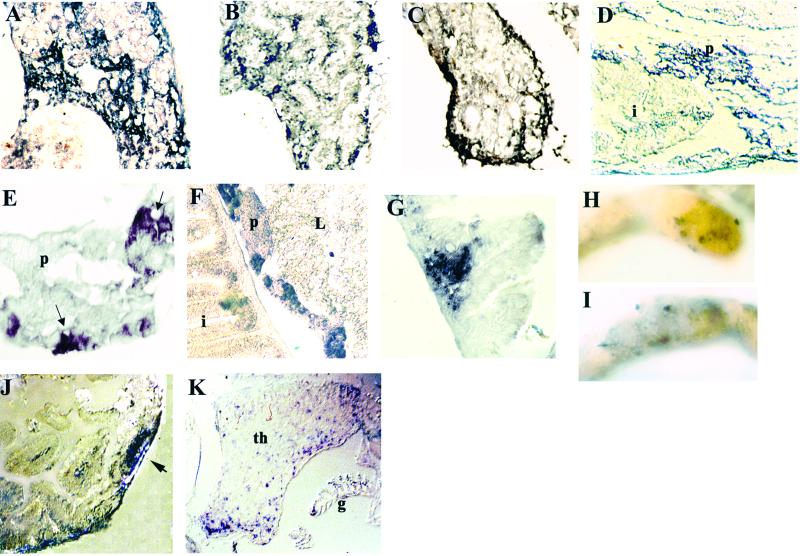
Expression of Igμ and rag1 during development. Whole-mount in situ hybridization of 4-day fish with rag1 (A) and insulin (B) probes. In addition to strong staining with the rag1 probe in the thymus (A, arrowhead), weak staining can be detected in the right dorsal aspect of the abdominal cavity (A, arrow), where the developing pancreas is located; the location of staining with the insulin probe (B, arrow) appears to coincide with that by the rag1 probe. (C) Whole-mount in situ hybridization of 5-day fish with the rag1 probe. The arrowhead points to the thymus and the arrow to the pancreas; the central unstained area within the pancreas corresponds to an islet of Langerhans. (D) Whole-mount in situ hybridization of 8-day fish with rag1 probe, ventral view. Staining is seen in thymus (arrowhead) and pancreas (arrow); the pancreas is now elongated. Horizontal (E) and sagittal (F) paraffin sections through a 10-day-old fish stained with hematoxylin/eosin. The intestine at this age is a straight tube; the pancreas extends along the intestine. In situ hybridization of sagittal (G) or horizontal (H and I) cryosections of 10-day fish with Igμ (G), rag1 (H), or insulin (I) probes. Staining with rag1 and Igμ probes is seen in the pancreas surrounding an islet of Langerhans; with the rag1 probe, a few intraepithelial cells within the intestine also are stained. Intense staining of an islet is seen with the insulin probe. (J) In situ hybridization of a sagittal cryosection of 19-day fish with Igμ probe; most staining is within the pancreas, but some is also seen in the pronephros. (K) In situ hybridization of sagittal cryosection with Igμ sense probe; the brown color is pigment. L, liver; g, gills; i, intestine; p, pancreas; pr, pronephros. The fish in A is albino; those in B–D are wild-type treated with phenylthiourea and the rest are untreated wild-type. The dark material within the intestine in G–J is remnants of food.
To obtain additional evidence regarding the localization of rag1 hybridization, we used an insulin probe, also with 4-day-old fish. As shown in Fig. 3B, the insulin probe hybridized to the same region as the rag1 probe. In another view of rag1 hybridization, at 5 days, a circular ring of stain in the same anatomic region can be seen (Fig. 3C). As shown more clearly in sections (see below), B cells are localized in the exocrine pancreas, thereby accounting for the lack of stain in the central region, which is an islet of Langerhans. At 8 days, the pancreas is an elongated structure on the right side of the abdomen; in the view shown in Fig. 3D, staining of the pancreas as well as the thymi is shown. Whole-mount in situ hybridization with the Igμ probe, which extends from CH1 to CH4 and would hybridize to both membrane and secreted Igμ, did not reveal staining at this time.
To evaluate expression of Igμ, tissue sections were prepared from slightly older fish. Sections of a fish at 10 days, stained with hematoxylin/eosin, are shown in Fig. 3 E and F. The major abdominal organs, liver, pancreas, and intestine, are seen. The liver is anterior and ventral to the elongated pancreas. Igμ as well as rag1 transcripts are seen in the pancreas in tissue surrounding a structure that has the morphology of an islet of Langerhans (Fig. 3 G and H). Rag1, but not Igμ, expression also was detected in a few cells within the intestine. Hybridization with an insulin probe confirmed the identification of the islet; with the insulin probe, there was no stain in the surrounding tissue, supporting the specificity of the rag1 and Igμ hybridization (Fig. 3I). By day 19, weak Igμ staining was seen in the pronephros (Fig. 3J), at approximately the time lymphoid cells are detected in this organ morphologically (9). No staining with either the rag1 or Igμ probe was detected in the liver. There was no staining with sense Igμ (Fig. 3K) and sense rag1 probes (not shown).
Expression of Igμ in the Adult.
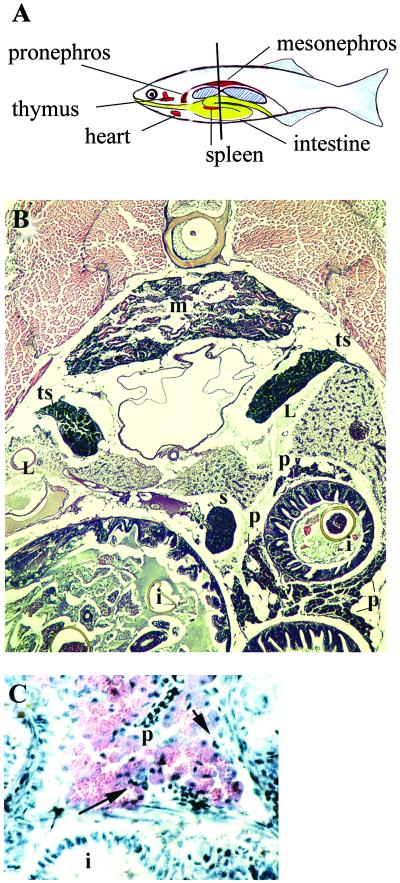
The location of several organs in adult zebrafish is shown schematically in Fig. 4A. Like the carp (18), zebrafish lack a stomach, and the intestine develops as a straight tube until about 3 weeks, when a loop appears (N.D., unpublished observations). Thereafter, the intestine consists of a straight segment posteriorly and a curved or spiral segment anteriorly. An organ similar in appearance and location to the mammalian spleen contains, almost exclusively, erythrocytes. Not shown in this figure, the liver occupies the anterior part of the peritoneal cavity, and bilateral gonads occupy the posterior part.
Figure 4.
Organs of adult zebrafish. (A) Diagram illustrating the position of some of the organs; the liver, pancreas, and gonads are not shown. The liver wraps around the anterior part of the intestine. Pancreatic tissue, intermixed with lymphatic tissue, is dispersed along the intestine. The bilateral gonads extend from the central to the posterior part of the abdominal cavity. The vertical line indicates the position of the section shown in B. (B) Transverse paraffin section, hematoxylin/eosin stain. Three crosssections of intestine (i) are seen; segments of pancreatic tissue (p) are located along the intestine. The mesonephros (m) contains renal tubules and some hematopoietic tissue. The spleen (s) is a compact organ containing, almost exclusively, erythrocytes. Bilateral testes (t) are located adjacent to the swim bladder. Cross sections of liver (L) are also seen. (C) Higher magnification of section similar to that in B, showing wall of intestine and adjacent diffuse pancreatic tissue, which has characteristic morphology. Some lymphoid cells are present (arrow); paraffin section, hematoxylin/eosin.
A transverse cryosection through the abdomen, stained with hematoxylin/eosin, is shown in Fig. 4B. In contrast to the compact appearance of the pancreas in larval zebrafish, in adults, as in other teleosts (19), the pancreas consists of clumps of tissue in the mesentery scattered along the intestine, especially dorsally, between intestinal folds (Fig. 4B; also see ref. 20). The exocrine cells are organized into characteristic acini (Fig. 4 B and C, and ref. 19). Cells with the morphology of lymphocytes can be seen adjacent to pancreatic tissue, often near vessels (Fig. 4C).
Expression of Igμ and rag1 in organs of adult zebrafish was evaluated by in situ hybridization on sections. In the pronephros, clusters of cells between renal tubules hybridized to each probe (Fig. 5 A and B). Igμ+ cells were found along the borders of the mesonephros, with the inner section occupied mostly by excretory tissue (Fig. 5C); cells staining with rag1 were scarce (not shown). Examination of the pancreatic region revealed aggregates of Igμ+ and rag1+ cells (Fig. 5 D–F).
Figure 5.
Expression of Igμ and rag1 in organs of adult (3 months old) zebrafish. In situ hybridization on transverse cryosections (A–C, E–G, J, and K) or sagittal paraffin section (D). Pronephros (A) Igμ and rag1 (B) probes. Stained cells form clusters. (C) Mesonephros, Igμ probe; stained cells are mostly in the periphery, with only a few internally. (D) Intestinal and pancreatic tissue, Igμ probe. Note intense staining in region of pancreas. (E) Higher magnification of pancreatic tissue, Igμ probe; note accumulation of stained cells near blood vessels (arrows). (F) Intestine and pancreas, rag1 probe. Stained cells within pancreas form clusters. Staining is also seen in the lamina propria. (G) Intestine, Igμ probe; note accumulation of Igμ+ cells in lamina propria. Whole-mount in situ hybridization of segment of intestine with Igμ (H) and rag1(I) probes; stained cells form patches. (J) Section across patch shown in H; Igμ+ cells cluster into a follicle-like aggregate (arrow). (K) Section through thymus (th), Igμ probe; note also staining in gills (g).
Cells expressing Igμ (Fig. 5G) ands rag1 (not shown) were found in the lamina propria of the intestine, often in groups, with rare individual cells within the intestinal epithelium. Finding both rag1 and Igμ+ cells in the zebrafish intestine is consistent with reports that B lymphocytes are located in the teleost intestine (2, 28).
The intestine was removed from fish of different ages and examined by whole-mount in situ hybridization. Igμ+ and rag1+ cells were seen to form patches on the wall of the intestine (Fig. 5 H and I) in fish ranging in age from 6 weeks to 6 months, but not in fish older than 1 year. Patches consisting of cells staining with both probes were found mainly in the straight segment (2–7 patches per fish), with a few occasionally seen in the spiral part (1–2 patches). Some of the stained patches were sectioned and were found to have a follicular shape (Fig. 4J), reminiscent of Peyer's patches, which have not been reported in lower vertebrates (2).
We did not find significant levels of either rag1 or Igμ expression in the spleen. Sections through the thymus revealed Igμ expression in both the thymus itself and in the nearby gills (Fig. 4K). This observation is reminiscent of the presence of a subpopulation of B cells in the mouse thymus (21).
Discussion
A key observation in the present study was that genes encoding Igμ in zebrafish begin to rearrange on the fourth day of development. At this time lymphoid cells are present in the thymus (9), where rag1 as well as TCRα are expressed (7, 8). Although we found that expression of membrane Igμ begins at day 7, it is not until 2 weeks later that any lymphoid cells are seen in the pronephros (9). Therefore, it was not clear where B cell development initiates. No organs for B lymphopoiesis other than the pronephros have been described previously in teleosts. There have been hints, however, that some such sites may exist, as expression of cytoplasmic and surface IgM was detected in rainbow trout before any lymphoid cells were found in the kidney (22–24).
Examination of fish for early sites of rag1 expression by whole-mount in situ hybridization revealed a small stained spot in a region consistent in location with the pancreas, not seen in our earlier studies of rag1 expression at this stage (7), presumably because different in situ procedures were used. This spot was first visible at 4 days and gradually became more prominent. The identity of the stained organ as the pancreas was confirmed when sections of 10-day-old fish were examined. At this time, Igμ as well as rag1 were seen expressed in the pancreas, in a region surrounding an islet of Langerhans. These results imply that the pancreas in developing zebrafish is a site for differentiation of B lymphocytes. Both genes also were found expressed in the pronephros beginning at 19 days. Although the expression in the pronephros was expected, as it has been thought to be the bone marrow equivalent in teleosts (1, 2), the expression in the pancreas was surprising, as this organ has not been considered to have a role in lymphopoiesis. We do not know whether the rag1 and Igμ expression in the pancreas after day 19 is the result of B cell differentiation at this site or of migration of cells from the pronephros. We did not see any evidence of rag1 or Igμ expression in the zebrafish liver at any developmental stage. To understand the possible basis for these observations it is necessary to review what is known about the progression and localization of lymphopoiesis in mouse and zebrafish, as well as the development of the pancreas and liver.
In the mouse, B cell progenitors are found in the fetal liver, for several weeks after birth in the spleen, and, subsequently, in bone marrow (25). In zebrafish, the initial location of B cells is in the exocrine pancreas rather than in the liver, and B cells remain associated with the pancreas into young adulthood. It would appear, therefore, that the pancreas in zebrafish, in addition to assuming the lymphopoietic role of the liver in the fetal mouse, also assumes the role of the spleen. The reason for this observation may be related to differences in the organization of the spleen in mammals and in teleosts. The mammalian spleen is a discrete organ consisting of two compartments, red pulp, containing erythroid cells, and white pulp, containing lymphoid cells. In teleosts, the organ that grossly resembles the mammalian spleen contains variable amounts of red and white pulp. In zebrafish (our results), as in carp and trout, this organ consists largely of red pulp, although after antigenic stimulation, increased amounts of white pulp may appear (reviewed in ref. 2; ref. 26). Therefore, in these fish, the bulk of lymphoid tissue must be located elsewhere; indeed, there are more lymphocytes in the pronephros than in the spleen (26, 27).
Rijkers et al. (3) observed that in the carp, lymphatic tissue is interspersed with liver and pancreas, and they considered the splenic white pulp to be fragmented along the intestine. The situation appears to be similar in zebrafish. Tischendorf (29) has suggested that an elongated spleen extending along the entire length of the gut is the ancestral form, the entire dorsal mesentery retaining the potential for forming splenic tissue. Arrest of development can occur at either the caudal or cranial portion, leading to the variation noted in the location of splenic tissue among different species. Thus, the lymphatic tissue in the zebrafish pancreas may be considered to be equivalent to the white pulp of the mouse spleen. In support of a possible relationship between the pancreas and spleen is that in the mouse, both organs are in close proximity in their early development, and the HOX11 gene is expressed in both (30).
In the embryonic mouse, stem cells that generate definitive hematopoietic, including lymphoid, lineages are located in sites ventral to the notochord known as intraembryonic splanchnopleura and aorta-gonad-mesonephros (31–33). At 48 h in zebrafish, a similar region in the ventral wall of the dorsal aorta has been proposed to be an early site of definitive hematopoiesis, based on the expression of the transcription factor c-myb (34). Ikaros, a gene that, in the mouse, is expressed in hematopoietic stem cells as well as in all lymphoid lineages (35), is also expressed in this region in zebrafish from 48 to 72 h (36).
In zebrafish, beginning at about 18 somites, the pancreatic markers, Pdx-1 and insulin, are expressed in a single mass, ventral to the notochord, before the emergence of a morphologically recognizable gut (37, 38). At 48 h, the pancreas lies near the anterior end of the domain of c-myb expression in the dorsal aorta and is closer to this region than is the liver. Proximity may favor migration of B cell progenitors into the pancreas rather than the liver.
In mammals, birds, and amphibians, the pancreas is formed by budding from the foregut, and its location resembles that in zebrafish (8, 10). In the embryonic mouse and in Xenopus laevis, signals from the endothelium of blood vessels such as the dorsal aorta have been shown to induce the expression of insulin by pancreatic endocrine cells, indicating intimate contact between these tissues (28). It would appear, therefore, that B cell progenitors in these species also might have opportunity to migrate into the pancreas. It is not clear what factors promote the differentiation of B cells in the pancreas of zebrafish but not in that of other species.
Acknowledgments
This work was supported in part by National Institutes of Health Grant R01 AI08054.
Abbreviations
- TCR
T cell antigen receptor
- FR
framework
Footnotes
Danilova, N., Hohman, V. S. & Steiner, L. A. (2000) FASEB J. 14, A1187 (abstr.).
References
- 1.Zapata A. Dev Comp Immunol. 1979;3:55–65. doi: 10.1016/s0145-305x(79)80006-3. [DOI] [PubMed] [Google Scholar]
- 2.Zapata A, Amemiya C T. Curr Top Microbiol Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 3.Rijkers G T, Frederix-Wolters E M, van Muiswinkel W B. Immunology. 1980;41:91–97. [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhalgh P, Steiner L A. Immunogenetics. 1995;41:54–55. doi: 10.1007/BF00188438. [DOI] [PubMed] [Google Scholar]
- 5.Willett C E, Cherry J J, Steiner L A. Immunogenetics. 1997;45:394–404. doi: 10.1007/s002510050221. [DOI] [PubMed] [Google Scholar]
- 6.Schatz D G, Oettinger M A, Schlissel M S. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 7.Willett C E, Zapata A G, Hopkins N, Steiner L A. Dev Biol. 1997;182:331–341. doi: 10.1006/dbio.1996.8446. [DOI] [PubMed] [Google Scholar]
- 8.Slack J M. Development (Cambridge, UK) 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 9.Willett C E, Cortes A, Zuasti A, Zapata A G. Dev Dyn. 1999;214:323–336. doi: 10.1002/(SICI)1097-0177(199904)214:4<323::AID-AJA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch G, Jung J, Zheng M, Lora J, Zaret K S. Development (Cambridge, UK) 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 11.Danilova N, Hohman V S, Kim E H, Steiner L A. Immunogenetics. 2000;52:81–91. doi: 10.1007/s002510000255. [DOI] [PubMed] [Google Scholar]
- 12.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. Eugene: Univ. of Oregon Press; 1995. [Google Scholar]
- 13.Sun Z, Hopkins N. Genes Dev. 2001;15:3217–3229. doi: 10.1101/gad946701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harland R M. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson M R, Marcuz A, van Ginkel F, Miller N W, Clem L W, Middleton D, Warr G W. Nucleic Acids Res. 1990;18:5227–5233. doi: 10.1093/nar/18.17.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bengten E, Leanderson T, Pilstrom L. Eur J Immunol. 1992;22:294. doi: 10.1002/eji.1830220145. [DOI] [PubMed] [Google Scholar]
- 17.Hordvik I, Voie A M, Glette J, Male R, Endresen C. Eur J Immunol. 1992;22:2957–2962. doi: 10.1002/eji.1830221130. [DOI] [PubMed] [Google Scholar]
- 18.Davina J H M, Rijkers G T, Rombout J H, Timmermans L P, van Muiswinkel W B. In: Development and Differentiation of Vertebrate Lymphocytes. Horton J D, editor. Amsterdam: Elsevier Biomedical; 1980. [Google Scholar]
- 19.Takashima F, Hibiya T. An Atlas of Fish Histology: Normal and Pathological Features. Stuttgart: Fischer; 1995. [Google Scholar]
- 20.Pack M, Solnica-Krezel L, Malicki J, Neuhauss S C, Schier A F, Stemple D L, Driever W, Fishman M C. Development (Cambridge, UK) 1996;123:321–328. doi: 10.1242/dev.123.1.321. [DOI] [PubMed] [Google Scholar]
- 21.Miyama-Inaba M, Kuma S, Inaba K, Ogata H, Iwai H, Yasumizu R, Muramatsu S, Steinman R M, Ikehara S. J Exp Med. 1988;168:811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grace M F, Manning M J. Dev Comp Immunol. 1980;4:255–264. doi: 10.1016/s0145-305x(80)80029-2. [DOI] [PubMed] [Google Scholar]
- 23.Razquin B E, Castillo A, Lopez Fierro P, Alvarez F, Zapata A, Villena A J. J Fish Biol. 1990;36:159–174. [Google Scholar]
- 24.Castillo A, Sanchez C, Dominguez J, Kaattari S L, Villena A J. Dev Comp Immunol. 1993;17:419–424. doi: 10.1016/0145-305x(93)90033-m. [DOI] [PubMed] [Google Scholar]
- 25.Rolink A, Haasner D, Nishikawa S, Melchers F. Blood. 1993;81:2290–2300. [PubMed] [Google Scholar]
- 26.Rijkers G T. Dev Comp Immunol. 1981;5:527–534. doi: 10.1016/s0145-305x(81)80027-4. [DOI] [PubMed] [Google Scholar]
- 27.Pettersen E F, Bjerknes R, Wergeland H I. Fish Shellfish Immunol. 2000;10:695–710. doi: 10.1006/fsim.2000.0284. [DOI] [PubMed] [Google Scholar]
- 28.Lammert E, Cleaver O, Melton D. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 29.Tischendorf F. Experientia. 1985;41:145–152. doi: 10.1007/BF02002606. [DOI] [PubMed] [Google Scholar]
- 30.Kanzler B, Dear T N. Dev Biol. 2001;234:231–243. doi: 10.1006/dbio.2001.0239. [DOI] [PubMed] [Google Scholar]
- 31.Godin I E, Garcia-Porrero J A, Coutinho A, Dieterlen-Lievre F, Marcos M A. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 32.Medvinsky A L, Samoylina N L, Muller A M, Dzierzak E A. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 33.Cumano A, Dieterlen-Lievre F, Godin I. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 34.Thompson M A, Ransom D G, Pratt S J, MacLennan H, Kieran M W, Detrich H W, III, Vail B, Huber T L, Paw B, Brownlie A J, et al. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 35.Georgopoulos K, Bigby M, Wang J H, Molnar A, Wu P, Winandy S, Sharpe A. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 36.Willett C E, Kawasaki H, Amemiya C T, Lin S, Steiner L A. Dev Dyn. 2001;222:694–698. doi: 10.1002/dvdy.1223. [DOI] [PubMed] [Google Scholar]
- 37.Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Vogel S S, Liu N, Melton D A, Lin S. Mol Cell Endocrinol. 2001;177:117–124. doi: 10.1016/s0303-7207(01)00408-7. [DOI] [PubMed] [Google Scholar]