Abstract
This fourth lecture illustrates the praxis and results of Systemic Medicine (SM) in various therapeutic applications. SM's success has made it popular throughout Venezuela and Puerto Rico. The treatment of over 300 000 patients by 150 orthodox MD's, trained and qualified in SM, in 35 medical establishments with above average results corroborate its effectiveness as an eCAM in chronic degenerative diseases. Herein we provide a synopsis of results obtained in four such pathologies—the journal's necessary space restrictions somewhat limiting content—as well as clinical and photographic evidence. The validity of any medical theory is substantiated by its degree of effectivity and success. The workability of evidence-based SM corroborates Systemic Theory's transcendence.
Keywords: adaptogen, diabetes, negentropy, polycystic ovarian syndrome, psoriasis, synergetics, systemic medicine, systemic theory, varicose ulcer
Past and Present Naturalists … Tomorrow's Systemics?
Recent past and even present successful naturalists and phytotherapeutic practitioners share a long and honorable tradition of knowledge and pride in the cure of illnesses, which goes back to written history and beyond. These qualities have been substantiated by the success of Chinese (1,2), Kampo (3,4), Ayurvedic (5), Chumash (6) or Mayan (7) among many other traditional medicines. These traditional medicines have ‘demonstrated that every culture is capable of understanding and “inventing” the meaning of disease and its cure, even when it is different from our modern medical views’ (7). The variability and extent of cultures to provide answers—traditional medicines—to pathologies are embedded in the curiosity and observational capabilities of the human race. There are collective factors such as ‘a background of extensive family in traditional medicine’ (8) which play an important role in the transmission and survival of medicinal plant knowledge among ethnic groups. A potential issue, though, is the possible curtailment of the wisdom—and therapies—of traditional medicines within geographical and ethnic boundaries. In any case, the amount of plants, potential formulations or properties are a massive concern for any given individual caregiver or group to understand, store and transmit.
But, perhaps, it may be possible to set up a system or periodic table where plants and other natural remedies could, according to their properties, be arranged to produce specific formulae that provide well-being for a given pathology. Some exceptional individuals seem to have come by this ability. One of these gifted health care practitioners was Maurice Messegue, whom Mistinguet and Konrad Adenaur—among his famous patients—swore that only he could treat their illnesses. More recently, both, Dr. Rusudan Lomidze, using the Georgian Kohlkian traditional medicine, and Lonrig Dangar, a Tibetan physician who applied the rich Tibetan traditional medicine have also obtained significant success. These gifted individuals have shown that traditional medicine is a successful medicine. But a question still hangs in the air? Might a theory be devised by which regular practitioners, health care specialists devoid of the naturalists' extensive background, might formulate natural organic therapeutic protocols?
The Systemic Theory is set forth herein to provide an answer to this crucial question.
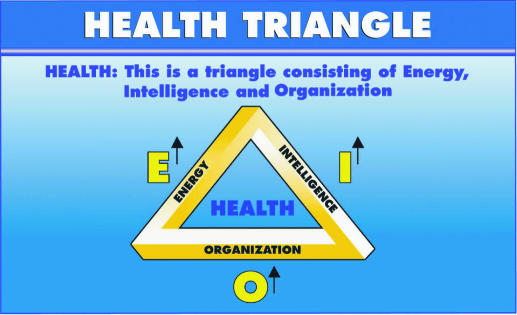
Systemic Theory postulates that Health (H) is directly proportional to the integrity of a living system's Energy (E), Bio-Intelligence (I) and Organization (O) as shown in Fig. 1. Systemic Theory also establishes a common denominator to all sickness (Fig. 2) and ascertains the cause of all disease to be an entropy increase: ‘disorder augmenting within the biologically open system, stemming from energo-informational and organizational impacts, either of external or internal nature’ (9–11). Therapeutics should then include a negentropy supply to enhance the system's energy–work capacity (E), its informational potential (I) intelligence, and finally structure and functional organization (O).
Figure 1.
The Health Triangle is born out of the system's Intelligence that generates Organization and produces Energy.
Figure 2.
Entropy increase brought upon by physical, chemical, biological and emotional impacts bring about the system's collapse.
Systemic Medicine's (SM) treatment strategy is based on identifying and prescribing superior herbs—tonic or adaptogenic—or any nutraceuticals or medicine with potential to strengthen E, I, O by providing energo, informational and organizational aid to the overall network of intelligent cells and cell systems that constitute the body. The main premise proposes that when all three factors are brought back to ideal levels patients' conditions begin recovery to normal health.
Evaluating the Praxis of Systemic Theory: Systemic Medicine
To corroborate the validity of the Systemic approach, we examined the results of its clinical application in chronic degenerative diseases (CDD) through retrospective studies carried out at the Adaptogenic Medical Centres located in Venezuela and Puerto Rico. Also included in the studies, were patients attending the following public hospitals (in Venezuela): Dr Domingo Luciani Hospital, Caracas; Dr Raúl Leoni Hospital, San Félix; and the Rehabilation Center of the Venezuelan Social Security Institute, Caracas. Three parameters were compared, ante and post-SM treatment, and these factors were as follows: Clinical results; Quality of Life (QoL) (12); and Tolerance to treatment. All patients included in these studies had formerly received orthodox treatments without any success in preventing disease progression. Thus, SM became the first choice treatment or even the unique alternative therapy. The complete studies of the pathologies included in this lecture as well as other CDD studies may be found at www.adaptogeno.com.
Outcomes of these as well as other studies have been presented at several scientific events such as 8th International Electrotherapy Congress in Nanning, China, September 2004; First International Neurobiotelekom Congress, in Saint Petersburg, Russian Federation, December 2004; First International Systemic Medicine Congress in Caracas, Venezuela, January 2005; Latin American Center Symposium on Environment and Health: Exploring Natural Products, UCLA, April 2005; First International Congress on Complementary and Alternative Treatments in Cancer, in Madrid, Spain, May 2005; and finally at the Science Information and Spirit Seminar in St Petersburg, Russian Federation, June 2005.
Clinical Study I: Diabetic Foot. Summary of Outcomes and Comparative Photographic Evidence
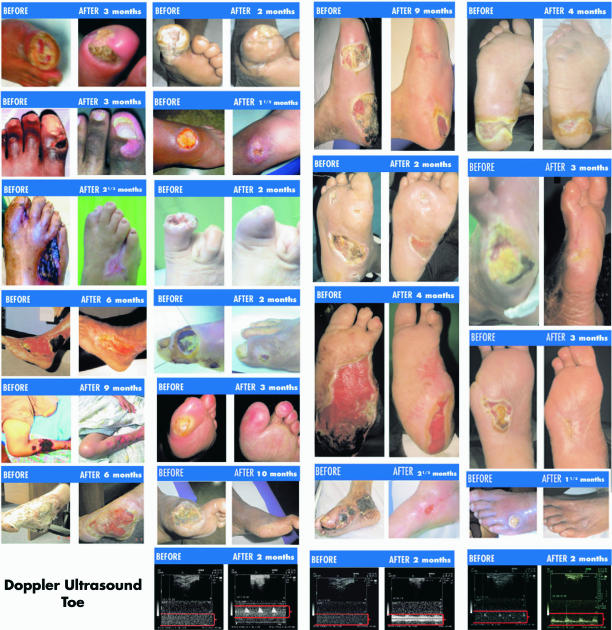
The therapeutic outcome is examined in 110 patients with diverse degrees of diabetic foot (13) through a retrospective, multicenter, descriptive 2 year long study (14). This treatment clinically improved 80.9% of the total diabetic foot population studied (P < 0.00001). SM prevented amputation in 40 patients (80%) of all cases diagnosed for surgical removal of limbs (50 patients). There was a significant improvement in QoL—86.36% of all diabetic foot cases (P < 0.00001). Tolerance to treatment was found to be excellent (Table 1). Results (Fig. 3) suggest that SM is the best therapeutic option for patients affected with diabetic foot.
Table 1.
Synopsis of SM treatment results in diabetic foot
| Number of patients | Clinical improvement | QoL improvement | Treatment tolerance | Other |
|---|---|---|---|---|
| 110 | 80.9% (89 patients) | 86.36% (95 patients) | 97.27% (107 patients) | Amputation avoided in 80% of cases diagnosed for surgery |
Figure 3.
Photographic evidence of diabetic foot remissions, including length of treatment between photos.
Clinical Study II: Severe Psoriasis. Resumé of Results and Illustrative Before and After Case Contrast
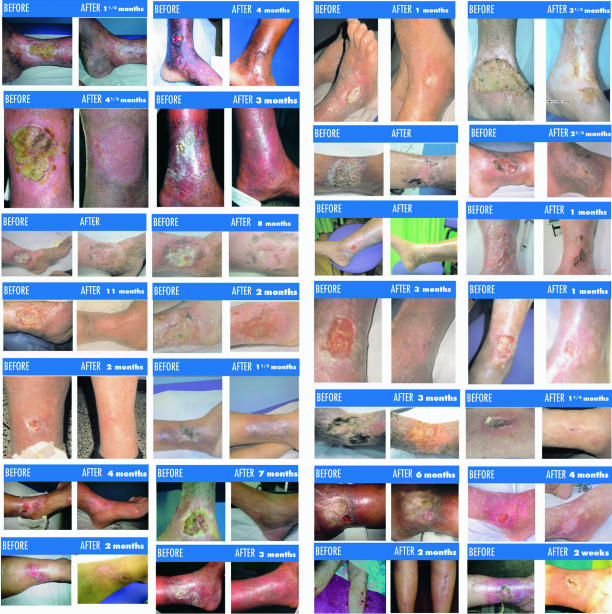
The outcome on the effects of SM in 123 patients with severe psoriasis was examined through a retrospective, multicenter, descriptive 2 year long study (15). Improvement in clinical remission was observed in 77.23% of patients (P < 0.00001). Almost two-thirds of all patients achieved clinical improvement in <46 days. QoL improvement is observed in 82.93% of patients (P < 0.00001). This therapeutic formula was particularly effective in severe varieties of this pathology. Treatment tolerance was excellent (Table 2). Results confirm a high remission rate, without side effects, in patients treated with SM. This suggests that SM is a superior therapeutic tool (Fig. 4).
Table 2.
Synopsis of SM treatment results in severe psoriasis
| Number of patients | Clinical improvement | QoL improvement | Remission time: ≤45 days | Treatment tolerance |
|---|---|---|---|---|
| 123 | 77.23% (95 patients) | 66.3% | 82.93% (102 patients) | 100% |
Figure 4.
Photographic evidence of severe psoriasis remissions, including length of treatment between photos.
Clinical Study III: Varicose Ulcer. Synopsis of Results, Before and After Photo Comparison
SM protocol was evaluated in 129 patients with chronic varicose ulcers through a retrospective, multicenter, descriptive 2 year long study (16). This treatment improved ulcers in 79% of the population. A remission of 21% of all patients was achieved in only 2 months. Systemic treatment also significantly improved the most frequent symptoms (cramps 71.4%, pain 78% and edema 88.7%) (Table 3). About 105 patients had QoL improvement. Some examples of results are seen in Fig. 5. The tolerance was excellent.
Table 3.
Synopsis of SM treatment results in varicose ulcer
| Number of patients | Clinical improvement | QoL improvement | Treatment tolerance | Remission time |
|---|---|---|---|---|
| 129 | 79% (102 patients) P < 0.0001 | 81.35% (105 patients) P < 0.00001 | 99.22% (128 patients) | 2 months in 21% of all patients |
Figure 5.
Photographic evidence of varicose ulcer remissions, including length of treatment between photos.
Clinical Study IV: Polycystic Ovarian Syndrome. Results, Before and After Graphic Differences
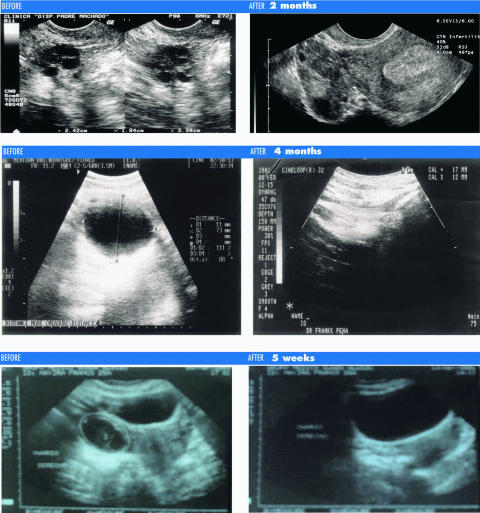
Thirty-five patients with polycystic ovarian syndrome (PCOS) were included in a retrospective, multicenter, descriptive 2 year long study to evaluate their response to a systemic protocol designed to improve their condition and/or obtain remission to the aforementioned pathology (17). SM improved pelvic pain in all 20 symptomatic patients (P < 0.00001); menstrual disorders (amenorrhea, dysmenorrhea, menorrhagia, menometrorrhagia, oligomenorrhea) in all 22 symptomatic patients (P < 0.00001); asthenia and cephalea in all 17 symptomatic patients (P < 0.0001); as well as acne and hirsutism in 8 out of 9 (89%) symptomatic patients (P < 0.0133). Pelvic ecosonograms revealed that 29 patients (82.8%) experienced a total disappearance of cysts, whereas 6 patients (17.2%) showed decrease in cyst size (Table 4). QoL improved in 100% of patients (P < 0.0001). Tolerance to treatment was outstanding (100%). To conclude, evidence-based results in PCOS treatment, with SM, suggest a remarkable CAM therapy (Fig. 6).
Table 4.
Synopsis of SM treatment results in PCOS
| Number of patients | Clinical improvement | Total cyst disappearance | QoL improvement | Treatment tolerance |
|---|---|---|---|---|
| 35 | 100% | 82.85% (29 patients) | 100% | 100% |
Figure 6.
PCOS before/after echosonographic comparison. Interval between echosonograms: 2 months.
E, I, O Classification of Superior Medicines
Adaptogens, tonics and nutraceuticals, in SM, are classified according to their E, I, O potential, i.e. as Energoceuticals, Infoceuticals and Organoceuticals. Examples of these by category are in Table 5.
Table 5.
Superior medicines E, I and O classification
| E | I | O | |||
|---|---|---|---|---|---|
| Energoceuticals that enhance mitochondrial ATP synthesis and resynthesis | Infoceuticals that enhance bio-intelligence on cellular, neuroendocrine and immune levels | Organoceuticals that specifically enhance organ function and structure | |||
| Names | References | Names | References | Names | References |
| Acantopanacis senticosus | Wu et al. (18), Gaffney et al. (19) | Uncaria tomentosa | Sheng et al. (36), Akesson et al. (37) | Glycyrrhiza glabra | Acharya et al. (66) |
| Cornu Cervi pantotrichum | Kim et al. (20), Zhang et al.(21) | Aloe vera | Kim et al. (38) | Curcuma Longa | Chainani-Wu (67) |
| Ilex paraguariensis | Gorgen et al. (22) | Andrographis paniculata | Matsuda et al. (39), Puri et al. (40) | Ulmus fulva | Brown et al. (68) |
| Lepidium meyenii | Lopez-Fando et al. (23) | Astragalus membranaceus | Wang et al. (41), Shao et al. (42) | Angelica sinensis | Mei et al. (69), Yin (70) |
| Ocimum sanctum | Agrawal et al. (24) | Croton lechleri | Risco et al. (43) | Chondroitin/glucosamine | Houpt et al. (71) |
| Panax ginseng | Yang et al. (25) | Echinacea purpurea and E. angustifolia | Randolph et al. (44), Cundell (45) | Chitin fiber | Jing et al. (72) |
| Panax quinquefolius | Wang et al. (26) | Ganoderma lucidum | Kohguchi et al. (46), Jiang et al. (47) | Crataegus oxyacantha | Rigelsky and Sweet (73), Lacaille-Dubois et al. (74) |
| Pfaffia paniculata | Kotsiuruba et al. (27), Tashmukhamedova et al. (28) | Grifola frondosa | Odama et al. (48) Lin et al. (49) | Dioscorea villosa | Shealy (75), Ladriere et al. (76) |
| Ptychopetalum olacoides | Siqueira et al. (29) | Hydrastis canadensis | Rehman et al. (50) | Plants enzymes | Popiela et al. (77) |
| Rhaponticum carthamoides | Kutuzova et al. (30) | Morinda citrifolia | Su et al. (51) | Equisetum arvense | Blumenthal et al. (78), Fleming (79) |
| Rhodiola rosea | Maslova et al. (31), Spasov et al. (32) | Petiveria alliacea | Ruffa et al. (52), Malpezzi et al.(53) | Ginkgo bilova | Kubota et al. (80), Pepe et al. (81) |
| Schizandra chinensis | Antoshechkin (33) | Sutherlandia frutescens | Bence and Crooks (54), Jang et al. (55) | Gotu kola | Incandela et al. (82) |
| l-arginine | Gupta et al. (34) | Tabebuia avellaneda | Planchon et al. (56), Li et al. (57) | Sargassum fusiforme | Ji et al. (83) |
| Ubiquinone (Coenzyme Q10) | Baggio et al. (35) | Valeriana officinalis | Dietz et al. (58) | Harpagophytum procumbens | Chrubasik et al. (84) |
| Vitex agnus castus | Kobayakawa and Sato-Nishimori (59), Ohyama et al. (60) | Vitamins | Carrero et al. (85) | ||
| Lentinus edodes | Borchers et al. (61), Wasser and Weis (62) | Minerals | Hercberg et al. (86) | ||
| Coriolus versicolor | Sun and Zhu (63), Sun et al. (64) | Ptycopetalum olacoides | Bucci (87), Siqueira et al. (29) | ||
| Cordyceps sinensis | Leu et al. (65) | Pygeum africanum | Freeman and Solomon (88), Santa Maria Margalef et al. (89) | ||
| Rhamnus purshiana | Ma et al. (90) | ||||
| Ruscus aculeatus | Redman (91), Bouaziz et al. (92) | ||||
| Salix alba | Chrubasik et al. (93) | ||||
| Sena alejandrina | Franz (94) | ||||
| Serenoa repens | Goldmann et al. (95), Iguchi et al. (96) | ||||
| Silibum marianum | Halim et al. (97), Chrungoo et al. (98) | ||||
| Smilax china | Lee et al. (99) | ||||
| Tribulus terrestris | Hong et al. (100) | ||||
| Vaccinium myrthillus | Zaragoza et al. (101), Savickiene et al. (102) | ||||
| Viburnum spp. | Calle et al. (103) | ||||
| Zingiber officinalis | Young et al. (104) | ||||
Systemic Protocol for Diabetic Foot
A complete description of each systemic protocol exceeds the scope of this article; however, a summarized example for diabetic foot is illustrated below.
E↑:Leuzea carthamoides
Ecdysone phytosteroids activate enzyme synthesis pro-cellular ATP synthesis (27,30).
I↑:Ganoderma lucidum
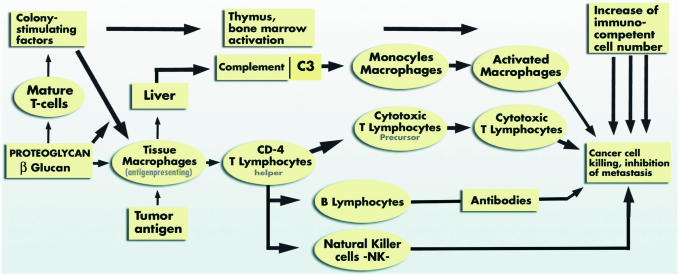
Ganoderan B and dozens of other polysaccharides and beta-glucans stimulate neuroendocrine intelligence and cell immunity (46,47,105,106). Glycans' path for immune enhancement is not certain but Chihara et al. (107) have proposed a likely model modified by Kidd (108) (Fig. 7).
Figure 7.
Mushroom proteoglycans' likely immune enhancement pathway.
O↑Gingko biloba
Flavonolglycosides, bioflavonoids, ginkgolides and bilobalides increase vascular flow (77,78).
The Healing Law of Synergetics
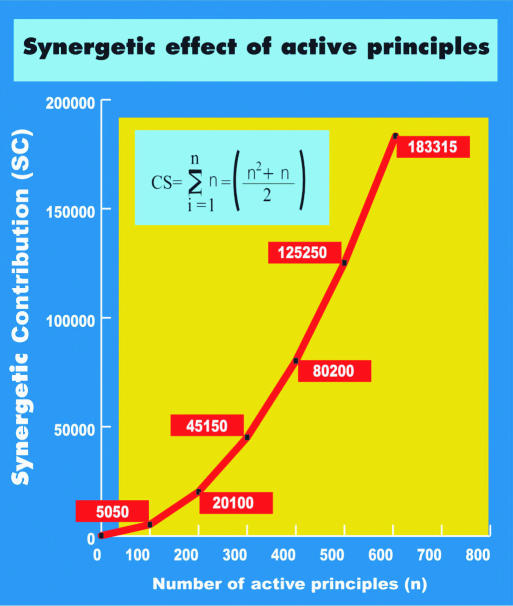
Healing potential, negentropy gain, is directly proportional to synergetic contribution (SC) (11). SC is exponentially proportional to the number of contributive active principles (n) in a formula—ergo in a protocol. The Healing Law of Synergetics is thus derived: Remission in chronic degenerative diseases, ΔS ≫ 0, depends on (n2 + n)/2. Figure 8 demonstrates the exponential number of SC as n increases.
Figure 8.
The Law of Synergetics is depicted by an exponential curve that provides a measure of the healing potential of the contributive active principles.
This law is valid as long as genetic functioning is minimally intact. The greater the SC is, the greater the probability of recovery. Thus all therapeutic formulations should in consequence include as many E, I, O nutraceuticals as possible.
Analysis
There is probably greater potential in developing formulations of synergetic natural supplements than in synthetics for CDD. The potential ‘… to introduce these compounds in the treatment of human diseases in order to raise public awareness on the richness and diversity of natural products that could be carefully harvested for the benefit of mankind’ as Cooper points out, is enormous (109).
Conclusion
Based on the Law of Synergetics future therapeutics should consist of thousands of potentially active E, I, O active principles from all organic sources available. This opens up a huge potential—hitherto ignored—for humanity.
Acknowledgments
We express sincere appreciation and gratitude to Professor Edwin L. Cooper for his invaluable support in making possible the four publications of the Systemic Theory and Praxis.
References
- 1.Wago H, Deng H. Chinese medicine and immunity. In: Cooper EL, Yamaguchi N, editors. Complementary and Alternative Approaches to Biomedicine. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 167–79. [Google Scholar]
- 2.Chen CF, Shum YC, Yang SP. The modernization of traditional Chinese medicine in Taiwan—past, present and future. In: Cooper EL, Yamaguchi N, editors. Complementary and Alternative Approaches to Biomedicine. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 35–42. [DOI] [PubMed] [Google Scholar]
- 3.Terasawa K. Evidence-based reconstruction of Kampo medicine: part I—is Kampo CAM? Evid Based Complement Alternat Med. 2004;1:11–6. doi: 10.1093/ecam/neh003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada H. New scientific approach for natural medicines. In: Cooper EL, Yamaguchi N, editors. Complementary and Alternative Approaches to Biomedicine. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 27–33. [Google Scholar]
- 5.Naik Gh, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohan H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003;1:97–104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 6.Adams JD, Garcia C. The adavantages of traditional Chumash healing. Evid Based Complement Alternat Med. 2005;1:19–23. doi: 10.1093/ecam/neh072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pena JC. The concept of illness and kidney disease in Nahuatl medicine. Synthesis of Mesoamerican and pre-Columbian medicine. Rev Invest Clin. 2002;54:474–81. (in Spanish) [PubMed] [Google Scholar]
- 8.Vandenbroek I, Van Damme P, Van Puyvelde L, Arrazola S, De Kimpe N. A comparison of traditional healer's medicinal plant knowledge in the Bolivian Andes and Amazon. Soc Sci Med. 2004;59:837–49. doi: 10.1016/j.socscimed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Olalde J. The systemic theory of living systems and relevance to CAM: part I: the theory. Evid Based Complement Alternat Med. 2005;1:13–8. doi: 10.1093/ecam/neh068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olalde J. The systemic theory of living systems and relevance to CAM: the theory (part II) Evid Based Complement Alternat Med. 2005;2:129–37. doi: 10.1093/ecam/neh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olalde Rangel JA. The systemic theory of living systems and relevance to CAM. Part III: the theory. Evid Based Complement Alternat Med. 2005;2:267–75. doi: 10.1093/ecam/neh119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grogono AW, Woodgate DJ. Index for measuring health. Lancet. 1971;2:1024–6. doi: 10.1016/s0140-6736(71)90336-9. [DOI] [PubMed] [Google Scholar]
- 13.Wagner FW. The dysvascular foot: a system of diagnosis and treatment. Foot Ankle. 1981;2:64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 14.Olalde JA, Magarici M, Amendola F, del Castillo O. Diabetic Foot Improvement using Systemic Medicine's framework 2005 Jan–Mar. Available from www.adaptogeno.com.
- 15.Olalde JA, Magarici M, Amendola F, del Castillo O. Benefits of Systemic Medicine in patients with Severe Psoriasis 2005 Jan–Mar. Available from www.adaptogeno.com.
- 16.Olalde JA, Magarici M, Amendola F, De Arriba C, del Castillo O. Remision of varicose ulcers with Systemic Medicine. 2005; Jan–Jun. Available from http://www.adaptogeno.com.
- 17.Olalde JA, Magarici M, Amendola F. Effectivity of the Systemic Medicine in patients with Polycystic Ovarian Syndrome 2005 Jan–Jun. Available from http://www.adaptogeno.com.
- 18.Wu Y, Wang X, Li M. Effect of Acanthopanacis senticosus on exercise performance under constant endurance load for elderly. Wei Sheng Yan Jiu. 1998;27:421–4. [PubMed] [Google Scholar]
- 19.Gaffney BT, Hugel HM, Rich PA. Panax ginseng and Eleutherococcus senticosus may exaggerate an already existing biphasic response to stress via inhibition of enzymes which limit the binding of stress hormones to their receptors. Med Hypotheses. 2001;56:567–72. doi: 10.1054/mehy.2000.1163. [DOI] [PubMed] [Google Scholar]
- 20.Kim KS, Choi YH, Kim KH, Lee YC, Kim CH, Moon SH, et al. Protective and anti-arthritic effects of deer antler aqua-acupunture (DAA), inhibiting dihydroorotate dehydrogenase, on phosphate ions-mediated chondrocyte apoptosis and rat collagen-induced arthritis. Int Immunopharmacol. 2004;4:963–73. doi: 10.1016/j.intimp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Wang Y, Wang LZ, Gao XM. Immunopotentiating effect of a ‘Yang’-promoting formula of traditional Chinese medicine on aged female BALB/c mice. Phytother Res. 2004;18:857–61. doi: 10.1002/ptr.1551. [DOI] [PubMed] [Google Scholar]
- 22.Gorgen M, Turatti K, Medeiros AR. Aqueous extract of Ilex paraguariensis decreases nucleotide hydrolysis in rat blood serum. J Ethnopharmacol. 2005;97:73–7. doi: 10.1016/j.jep.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Fando A, Gomez-Serranillos MP, Iglesias I, Lock O, Upamayta UP, Carretero ME. Lepidium peruvianum chacon restores homeostasis impaired by restraint stress. Phytother Res. 2004;18:471–4. doi: 10.1002/ptr.1455. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal P, Rai V, Singh RB. Randomized placebo-controlled, single blind trial of holy basil leaves in patients with noninsulin-dependent diabetes mellitus. Int J Clin Pharmacol Ther. 1996;34:406–9. [PubMed] [Google Scholar]
- 25.Yang M, Wang BX, Jin YL. Effects of ginseng polysaccharides on reducing blood glucose and liver glycogen. Zhongguo Yao Li Xue Bao. 1990;11:520–4. [PubMed] [Google Scholar]
- 26.Wang BX, Yang M, Jin YL. Studies on the mechanism of ginseng polypeptide induced hypoglycemia. Yao Xue Xue Bao. 1990;25:727–31. [PubMed] [Google Scholar]
- 27.Kotsiuruba AV, Bukhanevych OM, Tarakanov SS, Kholodova IuD. Modulation of intracellular pools of cyclic purine nucleotides by biologically active oxysterol-ecdysterone and vitamin D3. Ukr Biokhim Zh. 1993;65:76–83. [PubMed] [Google Scholar]
- 28.Tashmukhamedova MA, Almatov KT, Syrov VN, Sultanov MB, Abidov AA. Comparative study of the effect of ecdysterone, turkesterone and nerobol on the function of rat liver mitochondria in experimental diabetes. Vopr Med Khim. 1986;32:24–8. [PubMed] [Google Scholar]
- 29.Siqueira IR, Fochesatto C, da Silva AL, Nunes DS, Battastini AM, Netto CA, Elisabetsky E. Ptychopetalum olacoides, a traditional Amazonian “nerve tonic”, possesses anticholinesterase activity. Pharmacol Biochem Behav. 2003;75:645–50. doi: 10.1016/s0091-3057(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 30.Kutuzova NM, Filippovich IuB, Kholodova IuD, Miladera K. Ecdysterone induces the activity of multiple forms of acid phosphatase and malate dehydrogenase. Ukr Biokhim Zh. 1991;63:41–5. [PubMed] [Google Scholar]
- 31.Maslova LV, Kondrat'ev BIu, Maslov LN, Lishmanov IuB. The cardioprotective and antiadrenergic activity of an extract of Rhodiola rosea in stress. Eksp Klin Farmakol. 1994;57:61–3. [PubMed] [Google Scholar]
- 32.Spasov AA, Wikman GK, Mandrikov VB. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7:85–9. doi: 10.1016/S0944-7113(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 33.Antoshechkin A. The Primary Adaptogens. Clearwater: Ceptoma Publishing Co.; 2001. [Google Scholar]
- 34.Gupta V, Gupta A, Saggu S, Divekar HM, Grover SK, Kumar R. Anti-stress and adaptogenic activity of l-arginine supplementation. Evid Based Complement Alternat Med. 2005;2:93–7. doi: 10.1093/ecam/neh054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 Drug Surveillance Investigators. Mol Aspects Med. 1994;15:287–94. doi: 10.1016/0098-2997(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 36.Sheng Y, Pero RW, Amiri A. Induction of apoptosis and inhibition of proliferation in human tumor cells treated with extracts of Uncaria tomentosa. Anticancer Res. 1998;18:3363–8. [PubMed] [Google Scholar]
- 37.Akesson C, Lindgren H, Pero RW. An extract of Uncaria tomentosa inhibiting cell division and NF-kappa B activity without inducing cell death. Int Immunopharmacol. 2003;3:1889–900. doi: 10.1016/j.intimp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Kacew S, Lee BM. In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis miller, Lentinus edodes, Ganoderma lucidum and Coriolus versicolor. Carcinogenesis. 1999;20:1637–40. doi: 10.1093/carcin/20.8.1637. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda T, Kuroyanagi M, Sugiyama S. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem Pharm Bull (Tokyo) 1994;42:1216–25. doi: 10.1248/cpb.42.1216. [DOI] [PubMed] [Google Scholar]
- 40.Puri A, Saxena R, Saxena RP. Immunostimulant agents from Andrographis paniculata. J Nat Prod. 1993;56:995–9. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- 41.Wang RT, Shan BE, Li QX. Extracorporeal experimental study on immuno-modulatory activity of Astragalus memhranaceus extracts. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:453–6. [PubMed] [Google Scholar]
- 42.Shao BM, Xu W, Dai H. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–11. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 43.Risco E, Ghia F, Vila R, Iglesias J, Alvarez E, Canigueral S. Immunomodulatory activity and chemical characterisation of sangre de drago (dragon's blood) from Croton lechleri. Planta Med. 2003;69:785–94. doi: 10.1055/s-2003-43208. [DOI] [PubMed] [Google Scholar]
- 44.Randolph RK, Gellenbeck K, Stonebrook K, Brovelli E, Qian Y, Bankaitis-Davis D, Cheronis J. Regulation of human immune gene expression as influenced by a commercial blended Echinacea product: preliminary studies. Exp Biol Med (Maywood) 2003;228:1051–6. doi: 10.1177/153537020322800910. [DOI] [PubMed] [Google Scholar]
- 45.Cundell DR. The effect of aerial parts of Echinacea on the circulating white cell levels and selected immune functions of the aging male Sprague-Dawley rat. Int Immunopharmacol. 2003;3:1041–8. doi: 10.1016/S1567-5769(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 46.Kohguchi M, Kunikata T, Watanabe H, Kudo N, Shibuya T, Ishihara T, et al. Immuno-potentiating effects of the antler shaped fruiting body of Ganoderma lucidum. Biosci Biotechnol Biochem. 2004;68:881–7. doi: 10.1271/bbb.68.881. [DOI] [PubMed] [Google Scholar]
- 47.Jiang J, Slivova V, Valachovicova T, Harvey K, Sliva D. Ganoderma lucidum inhibits proliferation and induces apoptosis in human prostate cancer cells PC-3. Int J Oncol. 2004;24:1093–9. [PubMed] [Google Scholar]
- 48.Odama N, Murata Y, Nanba H. Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. J Med Food. 2004;7:141–5. doi: 10.1089/1096620041224012. [DOI] [PubMed] [Google Scholar]
- 49.Lin H, She YH, Cassileth BR, Sirotnak F. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int Immunopharmacol. 2004;4:91–9. doi: 10.1016/j.intimp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Rehman J, Dillow JM, Carter SM, Chou J, Le B, Maisel AS. Increased production of antigen-specific immunoglobulins G and M following in vivo treatment with the medicinal plants Echinacea angustifolia and Hydrastis canadensis. Immunol Lett. 1999;68:391–5. doi: 10.1016/s0165-2478(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 51.Su C, Wang MY, Nowicky D, Jensen CJ, Anderson G. Selective COX-2 inhibition of Morinda citrifolia (Noni) in vitro. Proceedings of the 7th Annual Conference Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation and Related Disease; Nashville USA. 2001. [Google Scholar]
- 52.Ruffa MJ, Ferraro G, Wagner ML, Calcagno ML, Campos RH, Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J Ethnopharmacol. 2002;79:335–9. doi: 10.1016/s0378-8741(01)00400-7. [DOI] [PubMed] [Google Scholar]
- 53.Malpezzi EL, Davino SC, Costa LV, Freitas JC, Giesbrecht AM, Roque NF. Antimitotic action of extracts of Petiveria alliacea on sea urchin egg development. Braz J Med Biol Res. 1994;27:749–54. [PubMed] [Google Scholar]
- 54.Bence AK, Crooks PA. The mechanism of l-canavanine cytotoxicity: arginyl tRNA synthetase as a novel target for anticancer drug discovery. J Enzyme Inhib Med Chem. 2003;18:383–94. doi: 10.1080/1475636031000152277. [DOI] [PubMed] [Google Scholar]
- 55.Jang MH, Jun do Y, Rue SW. Arginine antimetabolite l-canavanine induces apoptotic cell death in human Jurkat T cells via caspase-3 activation regulated by Bcl-2 or Bcl-xL. Biochem Biophys Res Commun. 2002;295:283–8. doi: 10.1016/s0006-291x(02)00650-2. [DOI] [PubMed] [Google Scholar]
- 56.Planchon SM, Wuerzberger S, Frydman B. β-Lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res. 1995;55:3706–11. [PMC free article] [PubMed] [Google Scholar]
- 57.Li CJ, Wang C, Pardee AB. Induction of apoptosis by β-lapachone in human prostate cancer cells. Cancer Res. 1995;55:3712–5. [PubMed] [Google Scholar]
- 58.Dietz BM, Mahady GB, Pauli GF, Farnsworth NR. Valerian extracts and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res. 2005;138:191–7. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayakawa J, Sato-Nishimori F. G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifolia Linne fil.) Cancer Lett. 2004;208:59–64. doi: 10.1016/j.canlet.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Ohyama K, Akaike T, Hirobe C. Cytotoxicity and apoptotic inducibility of Vitex agnus-castus fruit extract in cultured human normal and cancer cells and effect on growth. Biol Pharm Bull. 2003;26:10–8. doi: 10.1248/bpb.26.10. [DOI] [PubMed] [Google Scholar]
- 61.Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999;221:281–93. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- 62.Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol. 1999;19:65–96. [PubMed] [Google Scholar]
- 63.Sun T, Zhu Y. The effect of PSP on immune function and living quality in patients receiving chemotherapy for gynecological malignancies. In: Yang Q, editor. Advanced Research in PSP, 1999. Hong Kong: Hong Kong Association for Health Care Ltd; 1999. pp. 308–9. [Google Scholar]
- 64.Sun Z, Yang Q, Fei H. The ameliorative effect of PSP on the toxic and side reactions of chemo- and radiotherapy of cancers. In: Yang Q, editor. Advanced Research in PSP, 1999. Hong Kong: Hong Kong Association for Health Care Ltd; 1999. pp. 304–7. [Google Scholar]
- 65.Leu SF, Chien CH, Tseng CY, Kuo YM, Huang BM. The in vivo effect of Cordyceps sinensis mycelium on plasma corticosterone level in male mouse. Biol Pharm Bull. 2005;28:1722–5. doi: 10.1248/bpb.28.1722. [DOI] [PubMed] [Google Scholar]
- 66.Acharya SK, Dasarathy S, Tandon A, Joshi YK, Tandon BN. A preliminary open trial on interferon stimulator (SNMC) derived from Glycyrrhiza glabra in the treatment of subacute hepatic failure. Indian J Med Res. 1993;98:69–74. [PubMed] [Google Scholar]
- 67.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 68.Brown AC, Hairfield M, Richards DG, McMillin DL, Mein EA, Nelson CD. Medical nutrition therapy as a potential complementary treatment for psoriasis—five case reports. Altern Med Rev. 2004;9:297–307. [PubMed] [Google Scholar]
- 69.Mei QB, Tao JY, Cui B. Advances in the pharmacological studies of radix Angelica sinensis (Oliv.) Diels (Chinese danggui) Chin Med J. 1991;104:776–81. [PubMed] [Google Scholar]
- 70.Yin ZZ, Zhang LY, Xu LN. The effect of dang-gui (Angelica sinensis) and its ingredient ferulic acid on rat platelet aggregation and release of 5-HT. Yao Xue Xue Bao. 1980;15:321–6. [PubMed] [Google Scholar]
- 71.Houpt JB, Mc Millan R, Wein C, Paget-Dellio SD. Effect of Glucosamine Hydrochloride in the treatment of pain of osteoarthritis of the knee. J Rheumatol. 1999;26:2423–30. [PubMed] [Google Scholar]
- 72.Jing SB, Li L, Ji D, Takiguchi Y, Yamaguchi T. Effect of chitosan on renal function in patients with chronic renal failure. J Pharm Pharmacol. 1997;49:721–3. doi: 10.1111/j.2042-7158.1997.tb06099.x. [DOI] [PubMed] [Google Scholar]
- 73.Rigelsky JM, Sweet BV. Hawthorn: pharmacology and therapeutic uses. Am J Health Syst Pharm. 2002;59:417–22. doi: 10.1093/ajhp/59.5.417. [DOI] [PubMed] [Google Scholar]
- 74.Lacaille-Dubois, Franck U, Wagner H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine. 2001;8:47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- 75.Shealy CN. Natural Progesterone. Safe and Natural Hormone Replacement. Los Angeles: Keats Publishing; 1999. [Google Scholar]
- 76.Ladriere L, Laghmich A, Malaisse-Lagae F, Malaisse WJ. Effect of dehydroepiandrosterone in hereditarily diabetic rats. Cell Biochem Funct. 1997;15:287–92. doi: 10.1002/(SICI)1099-0844(199712)15:4<287::AID-CBF753>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 77.Popiela T, Kulig J, Hanisch J, Bock PR. Influence of a complementary treatment with oral enzymes on patients with colorectal cancers—an epidemiological retrolective cohort study. Cancer Chemother Pharmacol. 2001;47(Suppl):S55–63. doi: 10.1007/s002800170010. [DOI] [PubMed] [Google Scholar]
- 78.Blumenthal M, Busse WR, Goldberg A, Gruenwald J, editors. The Complete German Commission E Monographs. Austin: American Botanical Council; 1998. [Google Scholar]
- 79.Fleming T, editor. PDR for Herbal Medicines. Montvale: Medical Economics Company; 2000. [Google Scholar]
- 80.Kubota Y, Tanaka N, Umegaki K, Takenaka H, Mizuno H, Nakamura K, et al. Gingko biloba extract induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001;69:2327–36. doi: 10.1016/s0024-3205(01)01303-0. [DOI] [PubMed] [Google Scholar]
- 81.Pepe C, Rozza A, Veronesi G. The evaluation by video capillaroscopy of the efficacity of a Gingko biloba extract with l-arginine and magnesium in the treatment of trophic lesions in patients with stage-IV chronic obliterating arteriopathy. Minerva Cardioangiol. 1999;47:223–30. [PubMed] [Google Scholar]
- 82.Incandela L, Cesarone MR, Cacchio M. Total triterpenic fraction of Centella asiatica in chronic venous insufficiency and in high-perfusion microangiopathy. Angiology. 2001;52(Suppl 2):S9–13. [PubMed] [Google Scholar]
- 83.Ji YB, Gao SY, Zhang XJ. Influence of Sargassum fusiforme polysaccharide on apoptosis of tumor cells. Zhongguo Zhong Yao Za Zhi. 2004;29:245–7. [PubMed] [Google Scholar]
- 84.Chrubasik S, Model A, Black A, Pollack S. A randomized double blind pilot study comparing Doloteffin and Vioxx in the treatment of lower back pain. Rheumatology (Oxford) 2003;42:141–8. doi: 10.1093/rheumatology/keg053. [DOI] [PubMed] [Google Scholar]
- 85.Carrero JJ, Lopez-Huertas E, Salmeron LM, Baro L, Ros E. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B-6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J Nutr. 2005;135:1393–9. doi: 10.1093/jn/135.6.1393. [DOI] [PubMed] [Google Scholar]
- 86.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 87.Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr. 2000;72:624S–36S. doi: 10.1093/ajcn/72.2.624S. [DOI] [PubMed] [Google Scholar]
- 88.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 89.Santa Maria Margalef A, Paciucci Barzanti R, Reventos Puigjaner J. Antimitogenic effect of Pygeum africanum extracts on human prostatic cancer cell lines and explants from benign prostatic hyperplasia. Arch Esp Urol. 2003;56:369–78. [PubMed] [Google Scholar]
- 90.Ma T, Qi QH, Xu J, Dong ZL, Yang WX. Signal pathways involved in emodin-induced contraction of smooth muscle cells from rat colon. World J Gastroenterol. 2004;10:1476–9. doi: 10.3748/wjg.v10.i10.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Redman DA. Ruscus aculeatus (butcher's broom) as a potential treatment for orthostatic hypotension, with a case report. J Altern Complement Med. 2000;6:539–49. doi: 10.1089/acm.2000.6.539. [DOI] [PubMed] [Google Scholar]
- 92.Bouaziz N, Michiels C, Janssens D. Effects of Ruscus extract and hesperidin methylchalcone on hypoxia-induced activation of endothelial cells. Int Angiol. 1999;18:306–12. [PubMed] [Google Scholar]
- 93.Chrubasik S, Kunzel O, Model A, Conradt C, Black A. Treatment of low back pain with a herbal or synthetic anti-rheumatic: a randomized controlled study. Willow bark extract for low back pain. Rheumatology (Oxford) 2001;40:1388–93. doi: 10.1093/rheumatology/40.12.1388. [DOI] [PubMed] [Google Scholar]
- 94.Franz G. The senna drug and its chemistry. Pharmacology. 1993;47(Suppl 1):2–6. doi: 10.1159/000139654. Review. [DOI] [PubMed] [Google Scholar]
- 95.Goldmann WH, Sharma AL, Currier SJ, Johnston PD, Rana A, Sharma CP. Saw palmetto berry extract inhibits cell growth and Cox-2 expression in prostatic cancer cells. Cell Biol Int. 2001;25:1117–24. doi: 10.1006/cbir.2001.0779. [DOI] [PubMed] [Google Scholar]
- 96.Iguchi K, Okumura N, Usui S, Sajiki H, Hirota K, Hirano K. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate. 2001;47:59–65. doi: 10.1002/pros.1047. [DOI] [PubMed] [Google Scholar]
- 97.Halim AB, el-Ahmady O, Hassab-Allah S, Abdel-Galil F, Hafez Y, Darwish D. Biochemical effect of antioxidants on lipids and liver function in experimentally-induced liver damage. Ann Clin Biochem. 1997;34:656–63. doi: 10.1177/000456329703400610. [DOI] [PubMed] [Google Scholar]
- 98.Chrungoo VJ, Singh K, Singh J. Silymarin mediated differential modulation of toxicity induced by carbon tetrachloride, paracetamol and d-galactosamine in freshly isolated rat hepatocytes. Indian J Exp Biol. 1997;35:611–7. [PubMed] [Google Scholar]
- 99.Lee SE, Ju EM, Kim JH. Free radical scavenging and antioxidant enzyme fortifying activities of extracts from Smilax china root. Exp Mol Med. 2001;33:263–8. doi: 10.1038/emm.2001.43. [DOI] [PubMed] [Google Scholar]
- 100.Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA, Lee SK. Evaluation of natural products on inhibition of inducible cyclooxigenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol. 2002;83:153–9. doi: 10.1016/s0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 101.Zaragoza F, Iglesias I, Benedi J. Comparative study of the anti-aggregation effects of anthocyanosides and other agents. Arch Farmacol Toxicol. 1985;11:183–8. [PubMed] [Google Scholar]
- 102.Savickiene N, Dagilyte A, Lukosius A. Importance of biologically active components and plants in the prevention of complications of diabetes mellitus. Medicina (Kaunas) 2002;38:970–5. [PubMed] [Google Scholar]
- 103.Calle J, Toscano M, Pinzon R, Baquero J, Bautista E. Antinociceptive and uterine relaxant activities of Viburnum toronis alive (Caprifoliaceae) J Ethnopharmacol. 1999;66:71–3. doi: 10.1016/s0378-8741(98)00208-6. [DOI] [PubMed] [Google Scholar]
- 104.Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96:207–10. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Miller S. Echinacea: a miracle herb against aging and cancer? Evidence in vivo in mice. Evid Based Complement Alternat Med. 2005;3:309–14. doi: 10.1093/ecam/neh118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takeda K, Okomura K. CAM and NK cells. Evid Based Complement Alternat Med. 2004;1:17–27. doi: 10.1093/ecam/neh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chihara G, Hamuro J, Maeda YY, Shio T, Suga T, Takasuka N, Sasaki T. Antitumor and metastasis-inhibitory activities of lentinan as an immunomodulator: and overview. Cancer Detect Prev Suppl. 1987;1:423–43. [PubMed] [Google Scholar]
- 108.Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. Review. [PubMed] [Google Scholar]
- 109.Cooper EL. CAM, eCAM, bioprospecting: The 21st century pyramid. Evid Based Complement Alternat Med. 2005;2:1–3. doi: 10.1093/ecam/neh094. [DOI] [PMC free article] [PubMed] [Google Scholar]